Abstract
The requirement for transcription during development of the stalked bacterium, Caulobacter crescentus, was studied with synchronous cultures of swarmer cells. The developmental pattern in these bacteria was first established by determination of the times at which specific changes in cell structure and function occurred. These changes could be divided into those characteristic of (a) development of the swarmer cell into the stalked cell: loss of motility and synthesis of the stalk, and (b) development of the stalked cell into the asymmetric dividing cell: chromosome replication, synthesis of the flagellum, motility, and division. The effect of rifampin in blocking several of these steps—loss of motility, initiation of chromosome replication, and cell division—indicates that RNA synthesis is required throughout the cell cycle for normal differentiation.
Keywords: stalk, rifampin, RNA synthesis, DNA synthesis, motility
Full text
PDF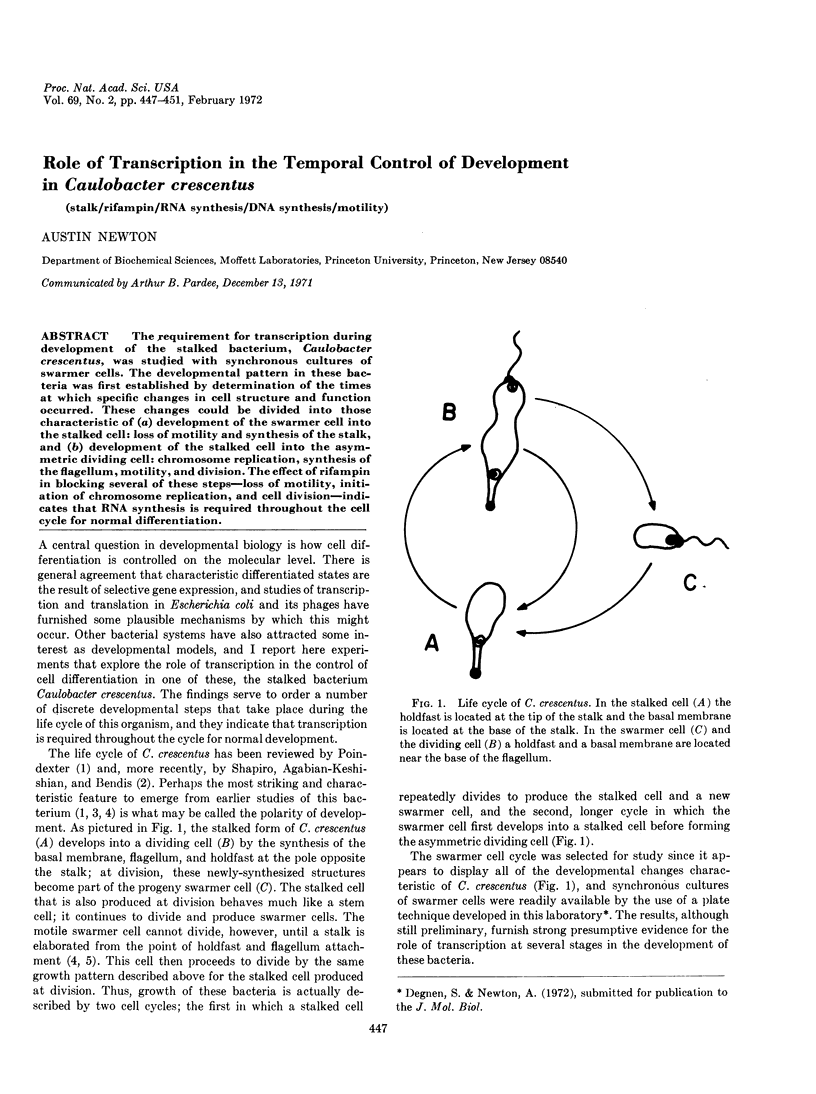




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Transcription of the tryptophan operon in Escherichia coli: rifampicin as an inhibitor of initiation. J Mol Biol. 1970 Mar;48(3):525–531. doi: 10.1016/0022-2836(70)90064-1. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Bendis I. Bacterial differentiation. Science. 1971 Sep 3;173(4000):884–892. doi: 10.1126/science.173.4000.884. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N. Specific Assay for Differentiation in the Stalked Bacterium Caulobacter crescentus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):200–203. doi: 10.1073/pnas.67.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]



