Summary
The exact prevalence and nature of cardiac involvement in facioscapulohumeral muscular dystrophy (FSHD) is unknown. Nevertheless, the current opinion is that symptomatic cardiac disease is rare.
We performed a cardiac screening [electrocardiogram (ECG) and echocardiography in the event of ECG abnormalities] in 75 genetically confirmed, ambulant FSHD patients without cardiac symptoms, with an eight-year follow-up of 57 patients, and compared the findings with results of previously performed cardiac screenings in the normal population. Baseline ECG demonstrated incomplete right bundle branch block (RBBB) in 33%, complete RBBB in 4%, and other minor abnormalities in 16%. Echocardiography showed no abnormalities. No significant changes were found after eight years of follow-up. Comparison with ECG abnormalities in the normal population showed a higher prevalence of incomplete RBBB (9.7 times higher) and of complete RBBB (4.8 times higher) in FSHD patients. This study in cardiac asymptomatic FSHD patients shows i) increased prevalence of incomplete RBBB in the absence of cardiomyopathy; ii) no progression of these abnormalities during eight years of follow-up. We conclude that FSHD patients without cardiac complaints do not need specific cardiac screening or surveillance. Furthermore, the increased prevalence of incomplete RBBB in the absence of cardiomyopathy suggests a selective involvement of the His-Purkinje system in FSHD.
Keywords: bundle branch block (BBB), facioscapulohumeral muscular dystrophy (FSHD), electrocardiography (ECG)
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is the third most common muscular dystrophy (after Duchenne muscular dystrophy and myotonic dystrophy) with a prevalence estimated at one per 20,000 in the adult European population (Padberg, 1982; Padberg et al., 1991). Cardiomyopathy or cardiac conduction abnormalities are well-known features of various hereditary myopathies. By contrast, cardiac involvement in FSHD has only incidentally been reported (Caponetto et al., 1968; Stevenson et al., 1990; Berlit and Stegaru-Hellring, 1991; Laforêt et al., 1998; Faustmann et al., 1996; de Visser et al., 1992; Galetta et al., 2005; Trevisan et al., 2006) and is not included in the diagnostic criteria of FSHD (Padberg et al., 1991). Table I summarizes the results of these previous studies on cardiac involvement in FSHD. Permanent atrial paralysis has been reported in three patients supposed to have FSHD, but these patients were more likely affected by Emery-Dreifuss muscular dystrophy (Caponetto et al., 1968; Laforêt et al., 1998; Bloomfield and Sinclair-Smith, 1965). More convincing data emerged from the report of Stevenson et al. (1990), which described a series of 30 clinically defined patients with a high susceptibility to inducible atrial arrhythmia and sinus node dysfunction. However, subsequent studies showed inconsistent results (Berlit and Stegaru-Hellring, 1991; de Visser et al., 1992), and only two more recent studies were performed on genetically defined patients (Laforêt et al., 1998; Galetta et al., 2005) (Table I). Since none of these studies included a long-term follow-up, clinical recommendations about the frequency of cardiac screening are difficult to make.
Table I.
Review of literature on cardiac involvement in facioscapulohumeral muscular dystrophy.
| Authors, year | Patients | Design | Investigations (number of patients in whom test is performed if not in all) | Main findings |
|---|---|---|---|---|
| Caponnetto et al., 1968 | n=1, clinically defined | Case report | ECG Echocardiography |
Persistent atrial standstill |
| Stevenson et al., 1991 | n=30, clinically defined | Cross-sectional | ECG Echocardiography (n=22) 24-hr Holter (n=15) Intracardiac studies |
Left atrial, right atrial or bi-atrial P wave abnormalities in 60%; evidence of abnormal AV node or infranodal conduction in 27%; sinus node function was abnormal in 10%; atrial flutter or fibrillation was induced in 10 of the 12 intracardiac electrophysiological studies. |
| Berlit and Stegaru-Hellring, 1991 | n=4, clinically defined | Cross-sectional | ECG Echocardiography |
Hypertrophic cardiomyopathy (n=2); bradycardia (n=1); prolonged QT interval (n=2) |
| De Visser et al., 1992 | n=31, clinically defined | Cross-sectional | ECG Echocardiography |
Mitral valve prolapse (n=1) |
| Faustmann et al., 1996 | n=15 (related), clinically / genetically defined | Cross-sectional | ECG at rest/stress Echocardiography Cardiac SPECT |
Abnormal reduced Tl-201 uptake in cardiac SPECT in the affected members of the family (n=7). |
| Laforêt et al., 1998 | n=100, clinically / genetically defined | Cross-sectional | ECG Echocardiography (n=20) 24-hr Holter (n=16) |
ECG abnormalities in 27%: incomplete RBBB in 19%, left ventricular hypertrophy in 2%, and more complex abnormalities in 5%. |
| Galetta et al., 2005 | n=24, clinically defined | Cross-sectional | ECG Echocardiography |
Patients had a subclinical cardiac involvement, which can represent a substrate for ventricular arrhythmias and heart failure. No signs of conduction abnormalities. |
| Trevisan et al., 2006 | n=83, clinically defined | Cross-sectional | ECG Echocardiography (most) 24-hr Holter |
Cardiac involvement of mainly arrhythmic origin in 12% of patients. |
Abbreviations: ECG=electrocardiogram; SPECT=single-photon emission computed tomography; RBBB= right bundle branch block, AV=atrial ventricle; QT interval= time between the start of the Q wave and the end of the T wave in ECG.
The aim of this study was to determine the presence and delineate the characteristics of cardiac involvement in a large group of genetically confirmed ambulant FSHD patients. We excluded patients with overt cardiac disease to prevent possible confusion about the cause of the cardiac symptom(s). Furthermore, we compared the results with findings of previously performed studies in a normal population and investigated the progression of cardiac abnormalities in FSHD over time.
Materials and methods
Patients
The FSHD patients in this study were originally recruited for our trial on the efficacy of strength training and albuterol in FSHD (van der Kooi et al., 2004). Exclusion criteria with possible implications for the interpretation of the findings in this subsequent study on cardiac involvement were: inability to walk independently (thereby excluding the most severely affected patients who might have secondary deconditioning); a history of (treated) hypertension, heart failure, ischemic heart disease, arrhythmias, predominantly atrial fibrillation, diabetes mellitus, or thyrotoxicosis; use of sympathicomimetics or beta-blockers. Written informed consent was obtained from all participants.
Cardiac screening
A general medical and cardiac history was taken at baseline (1998–1999) and follow-up (2005–2006). Physical examination comprised inspection of the thorax, especially whether there was a pectus excavatum.
Electrocardiography
A standard 12-lead electrocardiogram (ECG) with standard limb and chest leads in supine position at rest was carried out at baseline and follow-up. All ECG recordings were blindly and independently analyzed by two cardiologists. The outcomes were compared and the ECG was analyzed by a third cardiologist in the event of discrepancy. The Minnesota Code Classification System for ECG Findings was used with manual coding to detect changes suggestive of cardiac involvement (Blackburn et al., 1960). The two subsequent ECG recordings of each patient were assessed by the same cardiologists to identify progression in cardiac abnormalities.
Echocardiography
At baseline, nine patients who showed major ECG abnormalities, such as ST segment abnormalities, complete right bundle branch block (RBBB) and left bundle branch block (LBBB), underwent an echocardiographic examination. The echocardiography in these patients was repeated at follow-up. The examination focused on atrial dimensions, ventricular dimensions, wall thickness and left ventricular ejection fraction. A comprehensive transthoracic Doppler echocardiogram with color flow mapping was performed in standard views, using a Vingmed System V (General Electric Medical Systems, Horten, Norway), with second harmonic imaging and transducer frequencies of 1.6–3.4 MHz at baseline and a Vivid 7 (General Electric Medical Systems, Horten, Norway) at follow-up. All exams were performed by an experienced echocardiologist who was blinded for the ECG evaluation. The echocardiograms were evaluated independently by two cardiologists according to the recommendations of the American Society of Echocardiography (Sahn et al., 1978).
Comparison of findings with previously performed population studies
The prevalence of ECG abnormalities at baseline was compared with previously published prevalence data on ECG abnormalities in the normal population (control subjects of various studies identified using the PubMed search terms: prevalence, bundle branch block and population) (Rotman and Triebwasser, 1975; Fahy et al., 1996; Ashley et al., 2000; Jensen et al., 2003; Canaveris and Halpbern, 1988; Duraković and Mimica, 1983; Eriksson et al., 1998). We assumed that ECG abnormalities are not related to cultural or ethnic background, and that these studies are representative of the Dutch population.
Statistical analysis
Numerical data were expressed as mean ± standard deviation (SD). The Fisher’s exact test was used to test differences in prevalence of clinical cardiac features and ECG characteristics at baseline and follow-up. To compare the prevalence of ECG abnormalities in FSHD patients and the general population, 95% confidence intervals were used. All statistical procedures were performed using the Statistical Package for the Social Sciences 17.0 (SPSS, IBM, Chicago).
Results
Patients
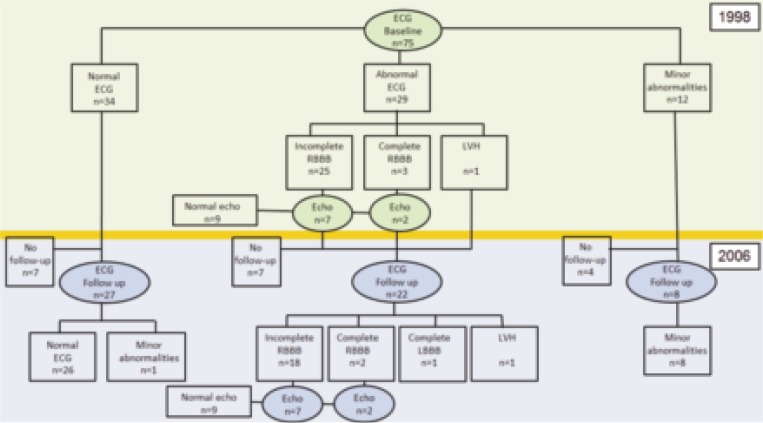
Ninety-seven patients were recruited to participate. Nineteen patients were excluded, because of cardiovascular events in their history (n=10), inability to walk independently (n=7), or other exclusion criteria (n=2). Three patients withdrew for personal reasons (n=3). Hence, 75 patients were included in the training and albuterol study, and in this study on cardiac involvement. Fifty-seven patients were screened for follow-up after eight years. The other 18 patients were not assessed for various reasons: lost to follow-up (n=10), no informed consent (n=6), and personal reasons (n=2) (Fig. 1). Seven of them had a normal ECG at baseline, six patients had an incomplete RBBB and one a complete RBBB at baseline, and four had minor ECG abnormalities. None of these 18 patients had symptoms related to chronic cardiac failure.
Figure 1.
Flowchart showing ECG and echo data in patients with facioscapulohumeral muscular dystrophy in 1998 and 2006. RBBB=right bundle branch block; LBBB=left bundle branch block; LVH=left ventricular hypertrophy.
Table II shows the demographic features of the patients included. All patients had clinical signs compatible with the diagnosis of FSHD, which was genetically confirmed. This cohort comprises 10% of the estimated Dutch FSHD population.
Table II.
Cardiac signs and symptoms at baseline and follow-up of 75 facioscapulohumeral muscular dystrophy patients without cardiac symptoms.
| Characteristics | Baseline | Follow-up | p-value of difference | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | ||
| Number (%) | 48 (64) | 27 (36) | 75 (100) | 34 (59.6) | 23 (40.4) | 57 (100) | |
| Mean age in years | 37.1±10.7 | 40.2±11.5 | 38.2±11.0 | 45.6±10.9 | 48.6±11.3 | 46.7±11.1 | |
|
| |||||||
| Chest pain (%) | 4 (8.3) | 1 (3.7) | 5 (6.7) | 6 (17.6) | 3 (13.0) | 9 (15.8) | ns2 |
| Exertional dyspnea (%) | 3 (6.3) | 0 | 3 (4.0) | 7 (20.6) | 5 (21.7) | 12 (21.1) | 0.0032 |
| Dyspnea at rest (%) | 0 | 1 (3.7) | 1 (1.3) | 1 (2.9) | 1 (4.3) | 2 (3.5) | ns2 |
| Palpitations (%) | 4 (8.3) | 1 (3.7) | 5 (6.7) | 6 (17.6) | 6 (26.1) | 12 (21.1) | 0.0192 |
| Edema (%) | 2 (4.2) | 4 (14.8) | 6 (8.0) | 5 (14.7) | 6 (26.1) | 11 (19.3) | ns2 |
| Hypertension (%) | 8 (16.7) | 5 (18.5) | 13 (17.3) | 8 (23.5) | 3 (13.0) | 11 (19.3) | ns2 |
| Systolic BP (mmHg)1 | 140±23.2 | 136±11.4 | 138±19.7 | 130±10.9 | 139±23.3 | 134±17.7 | ns3 |
| Diastolic BP (mmHg)1 | 89±7.9 | 87±6.9 | 88±7.6 | 84±9.2 | 85±8.5 | 84.5±8.8 | ns3 |
| Heart rate (bpm) 1 | 70±11.4 | 70±8.3 | 70±10.3 | 72±13.9 | 74±9.7 | 73±12.3 | ns3 |
blood pressure (BP) and heart rate: mean±1 SD, heart rate in beats per minute (bpm);
Fisher’s exact test;
Independent t-test; ns=not statistically significant
Results of cardiac screening
Results of history taking and physical examination at baseline and follow-up are shown in table II. Three male patients had pectus excavatum. At follow-up, exertional dyspnea and palpitations were reported statistically significantly more frequently than at baseline (Table II).
Electrocardiographic findings at baseline
ECG abnormalities were detected in 29 patients, consisting of an incomplete RBBB in 25 patients (33%), with a mean age of 38 years (range 18 to 53 years); a complete RBBB in three patients (3.8%) aged 28, 55 and 59 years; and left ventricular hypertrophy in one patient (aged 34 years). In addition, minor ECG abnormalities were detected in 12 patients (16%): sinus bradycardia (n=4), right ventricular abnormalities (n=5), left axis deviation (n=1), right axis deviation (n=1) and signs of right atrial dilatation (n=1) (Fig. 1). The clinical characteristics of patients with ECG abnormalities are shown in table III. overall, (in)complete RBBB was more common in male patients (p<0.001). All three patients with pectus excavatum showed an incomplete RBBB on ECG. This is in line with the fact that a pectus excavatum and similar deformities can influence the ECG configuration due to the altered position of the heart, thus mimicking incomplete RBBB (Padberg et al., 1991; Caponnetto et al., 1968).
Table III.
Clinical characteristics of facioscapulohumeral muscular dystrophy patients with ECG abnormalities at baseline after eight years of follow-up.
| Follow-up | ||||
|---|---|---|---|---|
| Patients with right bundle branch block | Patients with normal ECG or only minor abnormalities | |||
| Incomplete | Complete | Total | ||
| Number | 18 | 2 | 20 | 37 |
| Age (years) | 46.4 | 54.5 | 47.2 | 46.6 |
| Male | 17 | 2 | 19 | 15 |
| Female | 1 | 0 | 1 | 22 |
| EcoRI fragment (Kb) | 22.3 | 28.0 | 22.8 | 25.3 |
| Age at onset (years) | 15.7 | 38.5 | 18.0 | 20.8 |
| Age at diagnosis (years) | 28.4 | 41.5 | 29.7 | 28.6 |
Electrocardiographic findings at follow-up
ECG abnormalities at follow-up were observed in 22 patients (39%); incomplete RBBB was observed in 18 patients (32%) with a mean age of 46.4 years (range 24 to 63 years); complete RBBB in two patients (3.5%; aged 36 and 44), left ventricular hypertrophy in one patient (1.7%; aged 42) and LBBB also in one patient (1.7%; aged 55).
Minor ECG abnormalities were detected in 16 patients (28%), seven of whom also had other ECG abnormalities [(in)complete RBBB, LBBB or left ventricular hypertrophy]. These consisted of medication-induced sinus bradycardia (n=2), right ventricular abnormalities (n=5), left axis deviation (n=1), right axis deviation (n=1), left atrium dilatation (n=2), intraventricular conduction disorder (n=2), elevated pressure in the right atrium (n=2), abnormal repolarization (n=1), and ventricular extra systole (n=1) (Fig. 1). (In)complete RBBB was again more common in male patients (p<0.001) (Table III).
Progression of ECG abnormalities
The ECG showed mild changes at follow-up in four patients, and did not change in the other patients. one patient with a normal ECG at baseline showed abnormal repolarization in V2 at follow-up. In another patient the incomplete RBBB became less pronounced at follow-up and no longer fulfilled the criteria for incomplete RBBB. In the third patient the incomplete RBBB became more pronounced, but remained incomplete. The fourth patient had incomplete RBBB which converted to LBBB.
Echocardiographic findings at baseline and follow-up
Echocardiography performed in nine patients showed normal atrial and ventricular dimensions and function, and no abnormalities of heart valves, wall motion and wall thickness were seen at baseline or follow-up. These patients included the three males with pectus excavatum, and six patients with major abnormalities on ECG. Hence, based on these echocardiographic findings, no signs of cardiomyopathy or other structural heart disease were seen.
Comparison of findings with previously performed population studies
We compared the prevalence of ECG abnormalities in this FSHD group with the prevalence in a healthy population (Table IV) (Blackburn et al., 1960; Rotman and Triebwasser, 1975; Fahy et al., 1996; Ashley et al., 2000; Jensen et al., 2003; Canaveris and Halpbern, 1988; Duraković and Mimica, 1983; Eriksson et al., 1998). The prevalence of incomplete RBBB was significantly higher in patients with FSHD (33%; 95% CI 22.6–44%) than in the normal population (1.2–3.4%) (Canaveris and Halpbern, 1988). In addition, the prevalence of complete RBBB in FSHD (3.8%; 95% CI –0.4–8.4) tended to be higher than in the normal population (0.16–0.8%) (Eriksson et al., 1998). The other minor ECG abnormalities detected could be related to age and had a prevalence equivalent to that found in the normal population.
Table IV.
Comparison of findings with previously performed population studies.
| Authors, year | Country | Number of patients | Prevalence of | |
|---|---|---|---|---|
| Incomplete RBBB (%) | Complete RBBB (%) | |||
| Rotman and Triebwasser, 1975 | USA | 237,000 | - | 0.17 |
| Duraković and Mimica, 1983 | Yugoslavia | 4,210 | 3.0 | 0.24 |
| Fahy et al., 1996 | Ireland | 110,000 | - | 0.28 |
| Canaveris and Halpern, 1998 | Argentina | 7,685 | 3.4 | 0.16 |
| Eriksson et al., 1998 | Sweden | 855 | - | 0.8 |
| Jensen et al., 2003 | Denmark | 902 | 1.2 | - |
Discussion
The results of this cardiac screening study in ambulant FSHD patients without a cardiac history show frequent ECG abnormalities, consisting of incomplete RBBB in 33% and complete RBBB in 3.8% of patients (at follow-up). overall, ECG abnormalities were significantly more common in male patients, which is similar to what is observed in population studies. Echocardiography in the patients with clinically important ECG changes showed no abnormalities. After eight years of follow-up, 95% of the ECG recordings were unchanged. The prevalence of incomplete RBBB was statistically significantly higher than in the normal population.
This study confirms the previously reported high prevalence of ECG conduction disturbances in FSHD patients (Stevenson et al., 1990; Laforêt et al., 1998; Trevisan et al., 2006). We have now identified these as incomplete RBBB without signs of cardiomyopathy. Furthermore, this is the first prospective study investigating the prevalence and natural course of ECG abnormalities in FSHD.
The prevalence of cardiac conduction abnormalities in our study was higher than previously reported in FSHD (Stevenson et al., 1990; Berlit and Stegaru-Hellring, 1991; Laforêt et al., 1998; Faustmann et al., 1996; de Visser et al., 1992; Galetta et al., 2005; Trevisan et al., 2006). A recent French study showed incomplete RBBB in 19% of its genetically confirmed FSHD patients. This was assumed to be similar to the prevalence in the general population and therefore not considered a consequence of FSHD (Laforêt et al., 1998). our study found a higher prevalence (of incomplete RBBB), which may be partly related to the higher percentage of male patients in our sample. Furthermore, three of our male patients had pectus excavatum. This condition, due to the altered position of the heart, may influence the ECG configuration, which may therefore mimic incomplete RBBB (Padberg et al., 1991; Caponnetto et al., 1968). Trevisan et al. (2006) investigated 83 patients with genetically confirmed FSHD. They reported arrhythmic disturbances, consisting of supraventricular paroxysmal tachycardia, in 10% of the patients. (In)complete BBBs were not reported in this study, which might be due to an absence of BBB or differences in the criteria for evaluation of the ECG (Trevisan et al., 2006). However, as shown in table IV, the prevalence of incomplete RBBB in the general population is much lower than that found in FSHD patients, both in our study and in the French study by Laforêt et al. (1998).
The eight-year follow-up results show that in the great majority of FSHD patients ECG abnormalities remain basically unchanged during this period. This suggests that FSHD patients without cardiological symptoms and a normal ECG require the same follow-up as individuals in the normal population. Complete and incomplete BBB also occur in other muscular dystrophies, such as Duchenne and Becker muscular dystrophy and myotonic dystrophy type 1 (Groh et al., 2008; Finsterer and Stöllberger, 2008; Muntoni, 2003). In Duchenne muscular dystrophy the BBB is often accompanied by dilated cardiomyopathy, and myotonic dystrophy type I patients show all kinds of blocks merely caused by conduction delay. The differences between this progressive cardiomyopathy and our findings suggest that in FSHD there is predominant involvement of the conduction system, and more specifically the His-Purkinje fibers, without signs of cardiomyopathy (Pennisi et al., 2002).
Incomplete RBBB in FSHD seems to be one of the extra-muscular features of FSHD, which also include retinal vasculopathy and deafness. These extra-muscular features may have a common pathophysiological basis related to abnormal vascular smooth muscle differentiation and/or dysfunction; this might play an important role in the pathogenesis of FSHD (Osborne et al., 2007). Selective involvement of the His-Purkinje fibers might be compatible with this hypothesis, since during cardiac morphogenesis, embryonic myocytes are induced to be differentiated into Purkinje fibers (Pennisi et al., 2002; Gourdie et al., 2003).
The inclusion criterion of ambulant patients without overt cardiac disease introduced a selection bias for mildly affected patients. Hence, the prevalence of ECG conduction disturbances might even be higher in the overall FSHD population. Another limitation was the loss to follow-up, because of withdrawal due to personal reasons and loss of contact, as shown in figure 1.
More research investigating pre-symptomatic cardiological abnormalities in patients with FSHD is needed to determine whether they show an increased prevalence of myocardial abnormalities. Furthermore, future research should focus on extensive follow-up of all FSHD patients, including those with prior cardiological symptoms, to assess the cardiological course and whether there are arrhythmias.
In conclusion, ambulant FSHD patients without a cardiac history have a high prevalence of incomplete RBBB (33%), which does not progress during eight years of follow-up, and which is not accompanied by cardiomyopathy. These findings suggest a selective involvement of the His-Purkinje system in FSHD without disease progression. We therefore suggest that if an initial ECG in FSHD patients without a cardiac history is normal or shows only incomplete or complete RBBB, then there is no need for a systematic cardiac work-up.
Acknowledgments
S.M. van der Maarel was supported by the Fields Center for FSHD and Neuromuscular Research. N.C. Voermans was supported by the Prinses Beatrix Foundation.
References
- Ashley EA, Raxwal VK, Froelicher VF. The prevalence and prognostic significance of electrocardiographic abnormalities. Curr Probl Cardiol. 2000;25:1–72. doi: 10.1016/s0146-2806(00)70020-x. [DOI] [PubMed] [Google Scholar]
- Berlit P, Stegaru-Hellring B. The heart in muscular dystrophy: an electrocardiographic and ultrasound study of 20 patients. Eur Arch Psychiatry Clin Neurosci. 1991;241:177–180. doi: 10.1007/BF02219718. [DOI] [PubMed] [Google Scholar]
- Blackburn H, Keys A, Simonson E, et al. The electrocardiogram in population studies. A classification system. Circulation. 1960;21:1160–1175. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- Bloomfield DA, Sinclair-Smith BC. Persistent atrial standstill. Am J Med. 1965;39:335–340. doi: 10.1016/0002-9343(65)90059-8. [DOI] [PubMed] [Google Scholar]
- Canaveris G, Halpern MS. Intraventricular conduction disturbances in flying personnel: incomplete right bundle branch block. Aviat Space Environ Med. 1988;59:960–964. [PubMed] [Google Scholar]
- Caponnetto S, Pastorini C, Tirelli G. Persistent atrial standstill in a patient affected with facioscapulohumeral dystrophy. Cardiologia. 1968;53:341–350. doi: 10.1159/000166205. [DOI] [PubMed] [Google Scholar]
- de Visser M, de Voogt WG, la Rivière GV. The heart in Becker muscular dystrophy, facioscapulohumeral dystrophy, and Bethlem myopathy. Muscle Nerve. 1992;15:591–596. doi: 10.1002/mus.880150510. [DOI] [PubMed] [Google Scholar]
- Duraković Z, Mimica M. Right bundle branch block in a prospective population study. Cor Vasa. 1983;25:185–190. [PubMed] [Google Scholar]
- Eriksson P, Hansson Po, Eriksson H, et al. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98:2494–2500. doi: 10.1161/01.cir.98.22.2494. [DOI] [PubMed] [Google Scholar]
- Fahy GJ, Pinski SL, Miller DP, et al. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190. doi: 10.1016/s0002-9149(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Faustmann PM, Farahati J, Rupilius B, et al. Cardiac involvement in facio-scapulo-humeral muscular dystrophy: a family study using Thallium-201 single-photon-emission-computed tomography. J Neurol Sci. 1996;144:59–63. doi: 10.1016/s0022-510x(96)00145-1. [DOI] [PubMed] [Google Scholar]
- Finsterer J, Stöllberger C. Cardiac involvement in Becker muscular dystrophy. Can J Cardiol. 2008;24:786–792. doi: 10.1016/s0828-282x(08)70686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetta F, Franzoni F, Sposito R, et al. Subclinical cardiac involvement in patients with facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2005;15:403–408. doi: 10.1016/j.nmd.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, et al. His-Purkinje line-ages and development. Novartis Found Symp. 2003;250:110–122. [PubMed] [Google Scholar]
- Groh WJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Thomsen JL, Jensen SE, et al. Low prevalence of ischemic electrocardiographic findings in a Danish population. Scand Cardiovasc J. 2003;37:49–57. doi: 10.1080/14017430310007036. [DOI] [PubMed] [Google Scholar]
- Laforêt P, de Toma C, Eymard B, et al. Cardiac involvement in genetically confirmed facioscapulohumeral muscular dystrophy. Neurology. 1998;51:1454–1456. doi: 10.1212/wnl.51.5.1454. [DOI] [PubMed] [Google Scholar]
- Muntoni F. Cardiomyopathy in muscular dystrophies. Curr opin Neurol. 2003;16:577–583. doi: 10.1097/01.wco.0000093100.34793.81. [DOI] [PubMed] [Google Scholar]
- Osborne RJ, Welle S, Venance SL, et al. Expression profile of FSHD supports a link between retinal vasculopathy and muscular dystrophy. Neurology. 2007;68:569–577. doi: 10.1212/01.wnl.0000251269.31442.d9. [DOI] [PubMed] [Google Scholar]
- Padberg GW. 1982. 1982. Fascioscapulohumeral disease [thesis] Leiden, Leiden University. [Google Scholar]
- Padberg GW, Lunt PW, Koch M, et al. Diagnostic criteria for facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 1991;1:231–234. doi: 10.1016/0960-8966(91)90094-9. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Rentschler S, Gourdie RG, et al. Induction and patterning of the cardiac conduction system. Int J Dev Biol. 2002;46:765–775. [PubMed] [Google Scholar]
- Rotman M, Triebwasser JH. A clinical and follow-up study of right and left bundle branch block. Circulation. 1975;51:477–484. doi: 10.1161/01.cir.51.3.477. [DOI] [PubMed] [Google Scholar]
- Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Stevenson WG, Perloff JK, Weiss JN, et al. Facioscapulohumeral muscular dystrophy: evidence for selective, genetic electrophysiologic cardiac involvement. J Am Coll Cardiol. 1990;15:292–299. doi: 10.1016/s0735-1097(10)80052-x. [DOI] [PubMed] [Google Scholar]
- Trevisan CP, Pastorello E, Armani M, et al. Facioscapulohumeral muscular dystrophy and occurrence of heart arrhythmia. Eur Neurol. 2006;56:1–5. doi: 10.1159/000094248. [DOI] [PubMed] [Google Scholar]
- van der Kooi EL, Vogels OJ, van Asseldonk RJ, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology. 2004;63:702–708. doi: 10.1212/01.wnl.0000134660.30793.1f. [DOI] [PubMed] [Google Scholar]