Abstract
The flagellum of Trypanosoma brucei is an essential and multifunctional organelle that drives parasite motility and is receiving increased attention as a potential drug target. In the mammalian host, parasite motility is suspected to contribute to infection and disease pathogenesis. However, it has not been possible to test this hypothesis owing to lack of motility mutants that are viable in the bloodstream life cycle stage that infects the mammalian host. We recently identified a bloodstream-form motility mutant in 427-derived T. brucei in which point mutations in the LC1 dynein subunit disrupt propulsive motility but do not affect viability. These mutants have an actively beating flagellum, but cannot translocate. Here we demonstrate that the LC1 point mutant fails to show enhanced cell motility upon increasing viscosity of the surrounding medium, which is a hallmark of wild type T. brucei, thus indicating that motility of the mutant is fundamentally altered compared to wild type cells. We next used the LC1 point mutant to assess the influence of trypanosome motility on infection in mice. We surprisingly found that disrupting parasite motility has no discernible effect on T. brucei bloodstream infection. Infection time-course, maximum parasitemia, number of waves of parasitemia, clinical features and disease outcome are indistinguishable between motility mutant and control parasites. Our studies provide an important step toward understanding the contribution of parasite motility to infection and a foundation for future investigations of T. brucei interaction with the mammalian host.
Keywords: Trypanosome, flagellum, dynein, motility, pathogenesis
Introduction
African trypanosomes, i.e. Trypanosoma brucei and related species, are protozoan parasites that cause sleeping sickness in humans and related diseases in wild and domestic animals. These parasites cause significant human mortality and limit economic development over vast regions of sub-Saharan Africa. Trypanosome infection of a mammalian host is a multistep process. Following transmission via the bite of an infected tsetse fly, the parasites must first establish and then maintain an infection in the bloodstream despite being extracellular and constantly exposed to the host immune system. Antigenic variation of the parasite surface coat and active clearance of host immunoglobulin bound to the parasite surface enable the trypanosome population to evade immune destruction and persist in the bloodstream indefinitely (Barry and McCulloch 2001; Borst 2002; Pays 2005). Bloodstream infection represents stage one of the disease and manifests clinically as flu-like symptoms with recurrent waves of fever and development of lymphadenopathy (Stich et al., 2002; Rodgers 2010). After a period of weeks to months, parasites penetrate the blood vessel endothelium and invade the central nervous system (CNS) (Stich et al., 2002; Rodgers 2010) bringing on stage two of the disease. During this stage, patients develop chronic meningoencephalitis with headaches and neurological changes that severely disturb sleep. Coma and death follow if the disease is left untreated (Stich et al., 2002; Rodgers 2010).
Trypanosomes are highly motile and the contribution of parasite motility to infection and pathogenesis is a long-standing question. Cell motility in T. brucei is driven by a single flagellum that runs alongside the cell and is laterally connected to the cell body (Ralston et al., 2009). The T. brucei flagellum possesses a canonical “9 + 2” axoneme as the platform for assembly of dynein motors that drive flagellum beating (Supplemental Figure S1A–D) (Ralston et al., 2009). The prominent influence of the flagellum on trypanosome biology is apparent even in original descriptions of these organisms, which centered on their distinctive, helical cell locomotion as observed in blood samples from infected amphibians (Gruby 1843). In those studies the parasite’s auger-like movement, twisting and rotating around its long axis as it moved forward, led to the genus name “Trypanosoma”, which combines the Greek words trypanon (auger) and soma (body). Since then, parasite motility has captured the attention of many scientists and an “undulating membrane” (now known to correspond to the flagellum) is a prominent feature in most descriptions of these organisms. Early work by Walker (Walker 1961) demonstrated that motility is driven by a tractile flagellar beat that initiates at the tip of the flagellum and travels to the flagellum base, the opposite of what is seen in most other eukaryotic flagella. More recent analyses of Trypanosoma brucei motility using high-speed, high-resolution video microscopy provided adjustments to the original “auger-like” description and further emphasized that parasite motility is a prominent feature of these pathogens in the blood (Rodriguez et al., 2009; Uppaluri et al., 2011; Heddergott et al., 2012; Weisse et al., 2012).
Important recent studies have demonstrated a requirement for trypanosome propulsive motility for infection of the tsetse fly (Rotureau et al., 2013). Parasite motility is also widely considered to be important for infection and pathogenesis in the mammalian host and motility functions of the trypanosome flagellum are receiving increasing attention as potential targets for therapeutic intervention (Broadhead et al., 2006; Ralston and Hill 2006; Ginger et al., 2008; Ralston et al., 2009). Specific requirements for motility in the mammalian host are not clear, but motility has been hypothesized to participate in parasite immune evasion and for penetration of the blood brain barrier and other extravascular tissues. For example, parasite forward movement has been suggested to drive movement of surface-bound immunoglobulin to the posterior end of the cell, where it is internalized, thereby allowing the parasite to resist opsonization and immune destruction (Engstler et al., 2007). As an extracellular pathogen, T. brucei presumably depends on its own motility for penetration of the vascular endothelium and entry into the CNS. Recent studies have even suggested that T. brucei motility is adapted specifically to the bloodstream environment (Heddergott et al., 2012). Unfortunately, direct investigation of a requirement for parasite motility in any aspect of host infection or pathogenesis has not been possible, because of the lack of viable motility mutants in the bloodstream life cycle stage that infects the mammalian host (Ginger et al., 2008; Ralston and Hill 2008).
Parasite “motility” refers to propulsive translocation of the cell away from the point of origin, rather than simple writhing of the cell body, which may be caused by uncontrolled flagellum beating. Indeed, all T. brucei motility mutants so far described retain a beating flagellum despite defects in sustained propulsive translocation of the cell (Hutchings et al., 2002; Branche et al., 2006; Broadhead et al., 2006; Ralston and Hill 2006; Ralston and Hill 2008). Most T. brucei motility mutants have been generated by RNAi knockdown of specific flagellar proteins and these knockdowns were lethal in bloodstream form cells, leading to the suggestion that disrupting motility is lethal (Branche et al., 2006; Broadhead et al., 2006; Ralston and Hill 2006; Ralston and Hill 2008). However, most if not all, T. brucei flagellar protein knockdowns have known or suspected structural defects, including some that are far more substantial than just loss of the targeted protein (Ralston and Hill 2008). Thus, it is not possible with RNAi alone to distinguish between phenotypes arising from defective motility versus pleotropic consequences of ablating target gene expression.
We recently developed a system to study flagellum protein function that employs loss of function point mutants to replace an endogenous protein, rather than simply depleting proteins via RNAi (Ralston et al., 2011). As such, the system enables one to perturb protein function, while avoiding pleotropic structural defects that might arise when depleting building blocks of axonemal protein complexes. Using this system, we identified a point mutation, K203A/R210A, in the LC1 subunit of outer arm dynein (Supplemental Figure S1A–D) that disrupts cell motility, but does not disrupt the structure of outer arm dynein (Ralston et al., 2011). Bloodstream form LC1 K203A/R210A mutants, henceforth referred to as “K/R” mutants, were generated by introducing a Tet-inducible copy of the mutant LC1 gene into a Tet-inducible LC1 knockdown line. Tet-induction in the parental LC1 knockdown is lethal, while tet-induction of the K/R mutant yields motility mutants that are viable, thereby offering a unique opportunity to examine the influence of motility on trypanosome infection (Ralston et al., 2011).
In the current work, we analyze parasite motility in multiple environments and demonstrate that motility in the K/R mutant is fundamentally altered compared to wild type cells. The motility defect of the LC1 K/R point mutant is at least as severe as that caused by LC1 knockdown, supporting the idea that lethality in the knockdown is not simply due to defective motility. We also exploit the availability of a viable T. brucei bloodstream-form motility mutant to directly test the requirement for parasite motility in mouse infection and pathogenesis. We find that, contrary to the prevailing notion, establishment and maintenance of infection in the bloodstream, as well as gross pathology and lethal outcome are indistinguishable between wild type and motility mutant parasites.
Results
K/R mutant parasites exhibit similar extent of motility defect as knockdown parasites
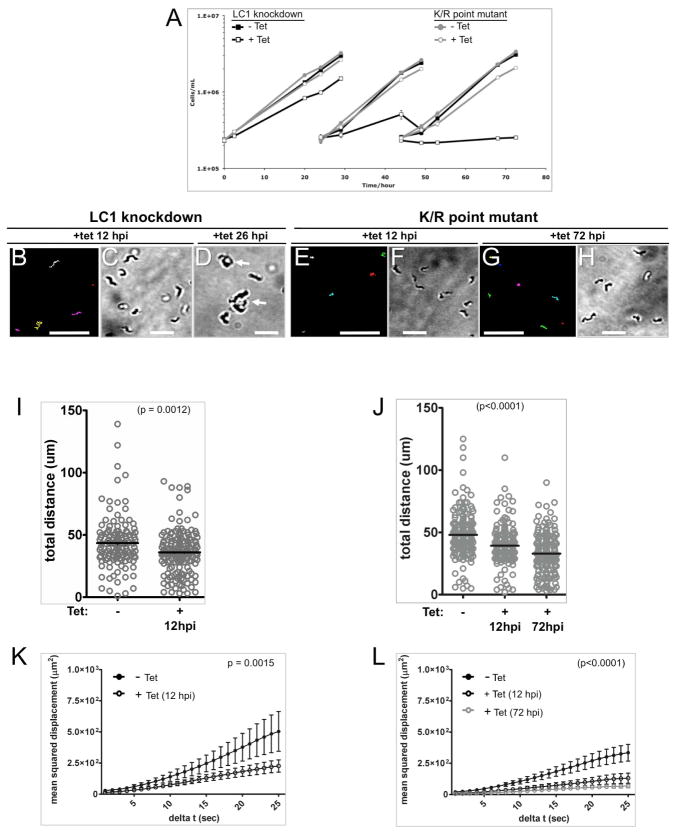
The contribution of parasite motility to T. brucei infection and pathogenesis in the mammalian host has not been studied. To address this question, we used the T. brucei K/R motility mutant, a conditional mutant that has normal motility in the absence of tetracycline, but defective motility in the presence of tetracycline (Ralston et al., 2011). As part of the current study, we performed an in-depth analysis of cell motility in the K/R mutant compared to parental LC1 knockdowns to assess the extent of the motility defect. We previously analyzed motility in K/R point mutants versus LC1 knockdowns at 24 hours post induction (hpi), but at this time point lethal cell division failure was already evident in the parental line and interfered with motility analysis (Ralston et al., 2011). We therefore examined motility at 12 hpi because at this time point a clear motility defect was evident in the parental line and cell division defects were not yet apparent (Figure 1). As shown in Figure 1 and Supplemental Videos 1, 2, and 4, motility K/R mutants and LC1 knockdown parasites at 12 hpi both have erratic flagellum beating that does not drive cell propulsion. Knockdown parasites are inviable owing to cell division failure that is evident within 26 hpi (Figure 1A, D and Supplemental Video 3), while K/R mutants remain viable. By 72 hpi, the motility defect is even more pronounced in K/R mutants (Figure 1J and supplemental video 5). In all cases, the flagellum beats rapidly, but the cells lack cell propulsion (Supplemental Video 5). We also examined flagellar beating using high speed, 1000 frames per second (fps), video microscopy (Supplemental Video 6). This analysis revealed slower flagellar beating and flagellum tip movements that did not propagate along the cell in knockdown and K/R mutant cells compared to wild type. However, we could not discern a specific beat feature that distinguished LC1 knockdown versus point mutant cells.
Figure 1. LC1 knockdown and LC1 K/R point mutant parasites show similar motility defects but the knockdown is lethal, while the point mutant is viable.
(A) Growth rates of LC1-knockdown cells (black lines) and K/R point mutants (gray lines). At time zero cultures were incubated with (open symbols) or without (closed symbols) tetracycline and diluted back to the starting density at 24 h and 48 h. (B, E and G) Motility traces of LC1 knockdown and LC1 K/R point mutant parasites grown with Tet for the indicated hours post-induction (hpi). Each line traces the path of a single cell for 30 seconds. (C, D, F, and H) DIC images of cultures grown with tetracycline for the indicated hpi. Arrows in panel D show cells accumulating as amorphous masses, indicating cell division failure by 26hpi for the LC1 knockdown. Scale bar is 95 um (panels B, E and G) or 25 μm (panels C, D, F and H). (I and J) Quantification of distance traveled in 30 seconds by individual LC1 knockdown (I) and K/R point mutant (J) cells grown in the absence (−) or presence (+) of Tet for the indicated times. For the LC1 knockdown, n=126 for −Tet and 159 for +Tet. For the K/R point mutant, n=180 for −Tet, 167 for +Tet(12hpi) and 190 for +Tet(72hpi). Horizontal lines indicate the mean of each data set, with bars showing the 95% confidence interval. (K and L) Mean squared displacement plotted as a function of time interval for LC1 knockdown (K) and K/R point mutant (L) trypanosomes grown without (closed circles) or with (open circles) Tet for the indicated time. n=52 for all samples and error bars show standard error of the mean.
The K/R mutant motility defect manifests in the bloodstream environment
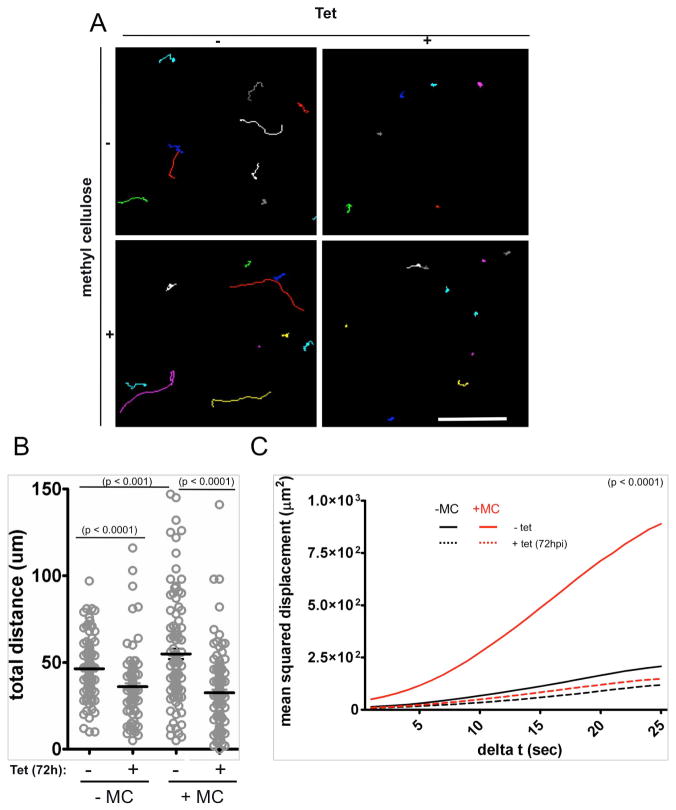
The environment encountered by parasites in an animal host differs from that of culture medium and these differences can influence parasite motility. For example, the viscosity of blood is significantly higher than that of culture medium and a hallmark of T. brucei motility is that cell velocity and propulsive motility increase with increasing viscosity of the medium (Heddergott et al., 2012). The ability of the parasite to increase motility when viscosity is raised is a consequence of the distinctive auger-like motility of the organism and is also observed for other microbes, such as spirochetes, that move via spiral cell motility (Berg and Turner 1979). As a context for mouse infection experiments, we therefore considered the possibility that increased viscosity might rescue the motility defect of K/R mutants. To test this, we examined parasite motility in normal medium versus medium in which viscosity was increased by adding 0.4 % methyl cellulose to approximate the viscosity of blood (Heddergott et al., 2012). In the presence of methyl cellulose, induced K/R cells exhibited a pronounced motility defect relative to uninduced controls (Figure 2 and Supplemental Figure S1E). This defect was evident when assessed by motility traces and total distance traveled over time, i.e. velocity (Figure 2A–B), but is most clearly evident when assessed by mean squared displacement, which measures propulsive motility, i.e. translocation away from the point of origin (Figure 2C). Moreover, while addition of methylcellulose increased motility of uninduced cells, it did not significantly increase motility of induced cells. Therefore, rather than being rescued, the motility defect of induced K/R mutants relative to control cells was more pronounced in the presence of methylcellulose.
Figure 2. Increasing viscosity improves motility of control (−Tet) but not of K/R mutant parasites in culture.
(A) Motility traces of K/R parasites grown with or without tetracycline for 72 hours as indicated and with or without methyl cellulose (0.4%, w/v) as indicated. Each line traces the path of a single cell for 30 seconds. Scale bar is 100 um. (B) Quantification of distance traveled in 30 seconds by uninduced (−) and Tet-induced (+) K/R mutants measured in the absence (−MC) or presence (+MC) of methyl cellulose. For samples in the absence of MC, n=112 for −Tet and 96 for +Tet. For samples with MC, n=102 for −Tet and 126 for +Tet). (C) Mean squared displacement plotted as a function of time interval for K/R point mutants maintained in the absence (solid lines) or presence (dashed lines) of Tet and measured without (black lines) or with (red lines) methyl cellulose (MC). n = 52 cells for each culture. For clarity, error bars are not shown, but are provided in supplemental figure S1E.
Another difference in blood versus culture medium is that blood presents a heterogeneous environment, owing to the presence of red blood cells and other cell types. These external obstacles influence microbial cell motility (Berg and Turner 1979; Heddergott et al., 2012). We therefore examined motility of induced and uninduced K/R parasites in whole blood. The density of red blood cells made it impossible to reliably trace individual trypanosomes using dark field illumination as was done for parasites in culture medium. To overcome this, we generated a K/R mutant cell line that constitutively expresses mCherry fluorescent protein, thereby allowing us to track trypanosome cell movements using fluorescent microscopy. We then examined motility of parasites in whole blood. Both uninduced and induced cells showed increased motility in blood than was observed for parasites in culture medium. Nonetheless, induced K/R cells exhibited a clear motility defect relative to uninduced controls even in whole blood (Figure 3 and Supplemental Videos 7).
Figure 3. K/R mutant parasites exhibit defective motility in whole blood.
(A) Motility traces of K/R point mutants expressing mCherry and maintained in the absence (−Tet) or presence (+Tet) of tetracycline for 72 hours. Motility traces were done in undiluted whole blood. Each line traces the path of single cell for 11 seconds. Scale bar, 50 μm. (B) Quantification of distance traveled in 11 seconds by uninduced (−) and or Tet-induced (+, 72hpi) mCherry K/R mutants measured in whole blood. n = 66 for −Tet and n=50 for +Tet. (C) Mean squared displacement is plotted as a function of time interval for uninduced (−Tet, closed symbols) and Tet-induced (+Tet 72hpi, open symbols) mCherry K/R point mutants measured in blood. n = 52 cells for each culture. n=52 for −Tet and 50 for +Tet and error bars show standard error of the mean, p <0.0001. (D) Comparison of the mean squared displacement analyses for LC1 knockdown (KD) and K/R point mutants (K/R) grown in the absence (−) or presence (+) of tet for the indicated hours post induction (hpi). MSD analyses are from figures 1K-L, 2C and 3C, done in culture medium with (+MC) or without (−MC) methyl cellulose, or in whole blood (blood) as indicated. The culture medium –MC slope data are the average from figure 1L and 2C msd analyses. For each condition, the slope of the best fit line through the msd data is plotted as a percentage of the slope of the −Tet sample for that condition. A decrease in slope indicates decreased propulsive motility.
To compare the extent to which cell motility is disrupted in different environments, we determined the mean squared displacement slope for induced relative to uninduced cells in each condition, culture medium with or without methylcellulose, and whole blood (Figure 3D). The combined data show that the LC1 K/R mutant exhibits a motility defect that is at least as pronounced as that of the parental LC1 knockdown and further, that this defect is manifest in culture medium with or without methyl cellulose, as well as in whole blood (Figure 3D). Given that this motility defect is penetrant even in whole blood, the K/R mutant presents a unique opportunity for assessing the impact of parasite motility on mouse infection.
Motility mutants establish bloodstream infection and cause trypanosomiasis pathology in mice
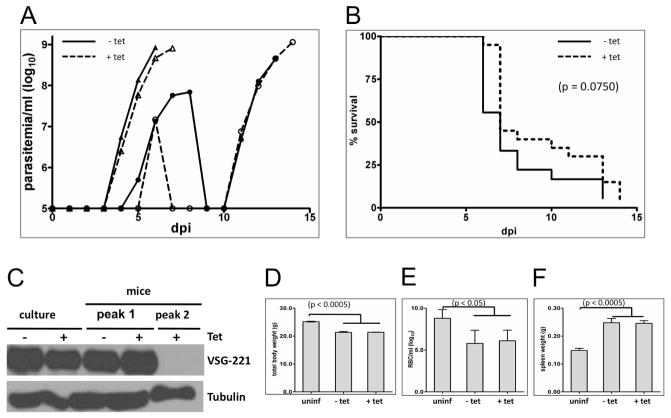
K/R mutants that were uninduced or induced for 72 hours with tetracycline were used to infect mice as described in Methods. Following infection, mice were examined and parasitemia was determined daily. For - Tet controls, parasitemia was first detected by four days post infection and mice usually developed a single wave of unremitting parasitemia with terminal outcome by 7 – 8 days post-infection (Figure 4A–B). One mouse in each group exhibited two waves of parasitemia, but in no case did infected mice clear the second wave or survive longer than 14 days. These infection parameters mimic what we observed with the parental blood stream single marker (BSSM) trypanosome cell line (not shown) and are consistent with previous studies using BSSM T. brucei (Emmer et al., 2010). In + Tet mice the infection time-course, maximum parasitemia, number of waves of parasitemia and mouse survival times were indistinguishable from − Tet controls (Figure 4A–B). In the single case where two waves of parasitemia were observed, the VSG expressed by the infecting population was lost in the second wave (Figure 4C), indicating that VSG switching remains active in the K/R mutant.
Figure 4. Motility is dispensable for infection and pathogenesis.
(A) Parasitemias of mice infected with K/R point mutants. Mice were maintained without tetracycline (solid lines, − Tet) or with tetracycline (dashed lines, + Tet) in the drinking water. Mice were infected at day 0 and parasitemia was determined beginning three days post infection (dpi). Representative data are shown for two − Tet and + Tet mice from a total of 18 −Tet and 20 +Tet infections. Typically, mice exhibited a single wave of parasitemia (triangles) but one mouse in each group showed two waves (circles). (B) Survival curves for all K/R-infected mice, maintained without tetracycline (−Tet, solid lines, n = 18) or with tetracycline (+Tet, dashed line, n = 20) in the drinking water. Any differences were not significant (p = 0.0750). One mouse in each group did not show detectable parasitemia. (C) Western blot of total protein prepared from K/R point mutants grown in culture (culture) or purified from infected mice (mice) maintained with (+) or without (−) Tet. Parasites from mice were taken at parasitemia peak 1 or peak 2 as indicated. Blots were probed with antibody against VSG221 or β-tubulin as a loading control. (D–F) Uninfected mice (uninf) or mice infected with K/R point mutants maintained without (− Tet) or with (+ Tet) tetracycline, were examined for total body weight (D) anemia (E) and spleen weight (F). Body weight and spleen weight were determined seven dpi. (Uninfected mice n = 3; mice infected with K/R mutants − Tet n = 4; + Tet n = 6.). Anemia was assessed seven dpi. (Uninfected mice n = 4; − Tet infections n = 5; + Tet infections n = 7.) Error bars show standard deviation.
Splenomegaly and anemia are hallmark clinical features of trypanosomiasis in many hosts including humans (Amole et al., 1982) and we therefore examined infected mice for these pathologies. Mice infected with K/R mutants and treated with tetracycline exhibited weight loss, anemia and splenomegaly that were indistinguishable from that observed in control (− Tet) infections (Figure 4D–F). We did not observe any discernible difference in mouse behavior between − Tet and + Tet infections. Hepatomegaly was not observed in either group of mice (not shown). Therefore, + Tet mice infected with K/R mutants established bloodstream infection and showed clinical features of trypanosomiasis that parallel that observed with − Tet control infections.
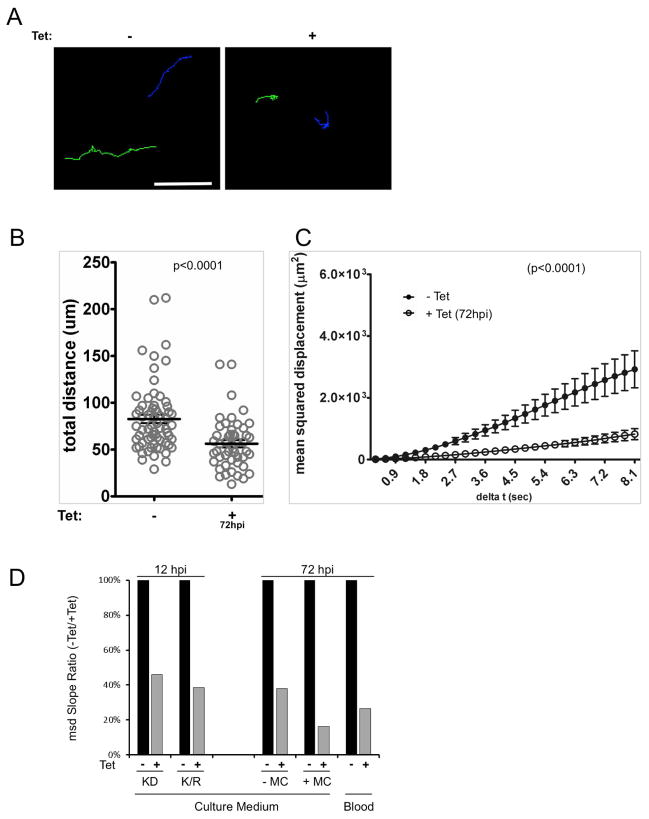
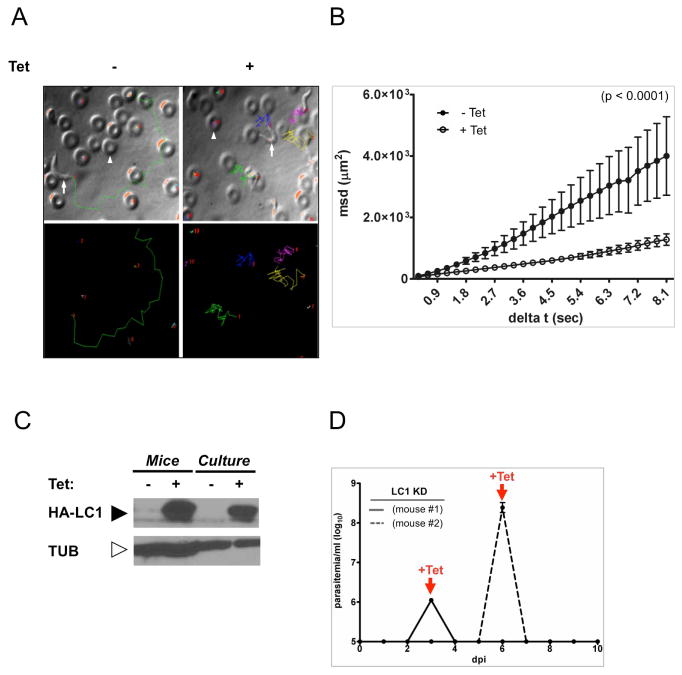
Infection of mice by + Tet K/R motility mutants was a surprising result. We considered the possibility that the mouse environment might select for revertants with wild type motility and these wild type parasites are responsible for the infection. We also considered the possibility that Tet was somehow not effective under the conditions used. If either of these situations occurred, parasites from infected +Tet mice would no longer exhibit a motility defect. We therefore examined motility and protein expression in parasites taken directly from infected mice. As shown in figure 5, K/R mutants taken directly from + Tet mice retained the motility defect (Figure 5A–B and Supplemental Videos 8–9) and expression of mutant LC1 protein (Figure 5C) that were observed in culture. As an independent test, we asked whether Tet treatment was able to clear infection by the parental LC1 knockdown line as well as Tet-inducbile trypanin knockdown parasites, both of which are non-viable under +Tet conditions (Ralston et al., 2011). In both cases, Tet treatment cleared the infection within 24 hr of treatment and no revertant cells emerged (Figure 5D and Supplemental Figure S1F). Therefore, using multiple independent tests, Tet was effective under the conditions used and mouse infection did not simply select for revertants.
Figure 5. Defective motility in K/R point mutants from infected mice.
(A) Motility traces of K/R parasites taken from infected mice that were maintained without (− Tet) or with (+Tet) tetracycline in the drinking water. Each line traces the path of single cell over 8.1 seconds. Upper panels show traces overlaid on phase contrast images. Representative trypanosomes (arrows) and red blood cells (arrowheads) are indicated. Lower panels show traces. Each line color represents a different cell. (B) Mean squared displacement is plotted as a function of time interval for K/R parasites from infected mice, maintained with (+Tet, open circles, n = 39 parasites) or without (−Tet, closed symbols, n = 17) tetracycline in the drinking water. Error bars show standard error of the mean, p <0.0001. (C) Western blot of total protein from K/R parasites purified from infected mice or maintained in culture. Tetracycline was provided in the mouse drinking water or culture medium as indicated. Blots were probed with antibody against the HA epitope to detect HA-LC1, or β-tubulin as a loading control. (D) Parasitemias of two mice infected with LC1 knockdown parasites (LC1-KD). Mice were infected with LC1-KD parasites at day 0 and then treated with tetracycline in drinking water upon detection of parasites in blood (+Tet arrows). Mice cleared infection within 24 h of tetracycline treatment, demonstrating the efficacy of tetracycline treatment.
DISCUSSION
African trypanosomes are deadly pathogens and infection of the mammalian host proceeds via multiple steps. Following a tsetse bite, parasites must first establish infection in the bloodstream and then defend against a potent immune response in order to maintain the infection. Subsequently, parasites invade extravascular tissues, including the CNS, leading to host tissue damage, neurologic dysfunction and an ultimately lethal outcome (Stich et al., 2002; Rodgers 2010). Here we used trypanosome motility mutants in a mouse infection model to examine impact of parasite motility on the first of these steps, namely establishment and maintenance of a bloodstream infection. We also examined pathogenic features and progression to terminal outcome. Surprisingly, infection dynamics, including time-course, maximum parasitemia, and number of waves of parasitemia, as well as pathological features and mouse survival rates were indistinguishable between control and motility mutant parasites.
Parasite motility is generally considered to be important for infection and motility functions of the trypanosome flagellum are considered candidate targets for therapeutic intervention (Broadhead et al., 2006; Ralston and Hill 2006; Ginger et al., 2008). Our studies present the first direct tests of the role for parasite motility in mouse infection and were made possible by availability of viable bloodstream-form motility mutants (Ralston et al., 2011). Previously, T. brucei motility mutants were generated using RNAi to simply deplete endogenous flagellar proteins and this is lethal in cultured bloodstream form cells (Branche et al., 2006; Broadhead et al., 2006; Ralston and Hill 2006), thereby limiting their utility for mouse infection studies. The K/R mutant differs in that the endogenous LC1 protein is replaced with a nonfunctional mutant and this mutant is viable (Ralston et al., 2011).
The induced K/R mutant retains some residual motility and we cannot rule out the possibility that this is enough to support any motility needs for bloodstream infection. However, induced K/R mutant parasites exhibit a pronounced motility defect relative to control cells under all conditions examined, i.e. culture medium alone, culture medium containing methyl cellulose to increase viscosity, whole blood and in diluted blood taken directly from infected mice. The defect is at least as severe as that seen in the parental LC1 knockdown line, which is non-viable. Moreover, induced K/R mutants do not show significant increase in velocity or propulsive motility when the viscosity of culture medium is raised. Cell velocity and propulsive motility of control trypanosomes increase when viscosity of the culture medium is raised (Figure 2) (Heddergott et al., 2012). A hallmark of T. brucei cell propulsion is a spiral motility mechanism (Rodriguez et al., 2009; Heddergott et al., 2012). Specific biophysical features of this type of motion causes microbes that move with a spiral mechanism, such as trypanosomes and spirochetes, to increase motility when viscosity is increased (Berg and Turner 1979; Heddergott et al., 2012). This result is the opposite of what happens for organisms such as E. coli that do not use a spiral mechanism (Berg and Turner 1979; Heddergott et al., 2012). Therefore, our results indicate that more than just being slow, cell movement in induced K/R mutants is fundamentally altered, such that they no longer use a spiral motility mechanism.
We considered the possibility that infection is caused by revertants, which might arise for example if cells become insensitive to tetracycline, become resistant to RNAi, or develop mechanisms to overcome LC1 functional defects. This was not the case, because K/R mutants taken directly from infected mice retain their motility defect. Likewise, revertants did not develop during infection with two independent tet-inducible RNAi lines. Moreover, mice infected with K/R mutants do not show any delay in time course of infection that would be expected if revertants had to emerge. Our findings therefore demonstrate that T. brucei can accommodate severe disruptions in motility mechanism and overall capacity for cell movement without affecting bloodstream infection. We note that infection via intraparatoneal injection used here differs from the natural route via a tsete bite, and it remains possible that motility may be required for infection through a tsete bite.
While not essential for survival in the bloodstream during acute infection, we favor the idea that parasite motility is required for subsequent steps of infection, such as persistence in the bloodstream during a chronic infection (MacGregor et al., 2011) or penetration of the CNS (Jennings et al., 1979). For example, trypanosome forward motility facilitates removal of host immunoglobulin from the cell surface in culture (Engstler et al., 2007) and this activity likely enables parasite persistence in the bloodstream over extended periods in the face of potent immune responses by the host. Persistent infection also enables CNS invasion (Jennings et al., 1982). It is generally thought that T. brucei requires more than two weeks to gain access to brain tissue (Jennings et al., 1979; Poltera et al., 1980; Gray et al., 1982), although this has been debated recently (Frevert et al., 2012). Rapid progression of infection with 427-derived parasites used in our studies, with maximum mouse survival ≤14 days, precludes reliable assessment CNS penetration, and additional work with motility mutants in chronic infection models (Jennings et al., 1979; Gray et al., 1982; MacGregor et al., 2011) will be required. T. brucei isolates that give chronic infection have historically been recalcitrant to molecular genetic manipulation, although advances in this direction have been made recently (Macgregor et al., 2013). 427-derived parasites were selected for our studies because of their experimental tractability and because they allowed the first-ever direct analysis of motility in any aspect of T. brucei infection. The demonstration here that induced K/R mutants are viable and mammalian-infectious will now enable studies of parasite motility in chronic infection models.
Another potential reason to retain motility in the bloodstream may be to keep the parasite prepared for uptake and transmission by the tsetse fly vector. T. brucei development in the tsetse requires an ordered series of parasite movements from the midgut to the salivary glands before the parasites transform into mammalian infectious trypomastigotes (Roditi and Lehane 2008; Sharma et al., 2009; Rotureau et al., 2012). Indeed, important new studies by Rotureau and colleagues (Rotureau et al., 2013) report that cell motility is required for parasite development and transmission. In this context, parasite motility might be maintained in bloodstream cells so that the parasite is ready to move through the tsetse following uptake with a blood meal.
The role of parasite motility in infection and pathogenesis of trypanosomiasis is a longstanding question that has not previously been amenable to experimental investigation. As such, development of a motility mutant infection model describe here provides an important foundation for future studies of flagellum biology in the context of host infection.
Experimental procedures
Ethics Statement
All animal experiments strictly complied with the Institutional Animal Care and Use Committee of University of California, Los Angeles (Approved protocol permit: ARC # 2001-065).
Trypanosome culture
Cultivation, transfection and RNAi induction of trypanosomes in culture were done as described previously (Ralston et al., 2006). The T. brucei bloodstream trypanin knockdown, LC1 knockdown, LC1-K203A/R210A (K/R) motility mutant and bloodstream single marker (BSSM), cells were described previously (Wirtz et al., 1999; Ralston et al., 2011). K/R-mCherry cells were generated by stable transfection of K/R cells with the pNKmCherry plasmid (described below). To generate pNKmCherry, the mCherry gene (accession number AY678264) was PCR amplified from pmCherry plasmid (Hajagos et al., 2012) and cloned into the expression vector pHD496-H (Hutchings et al., 2002) using the HindIII and BamHI cloning sites. pHD496-H contains a ribosomal RNA promoter to drive constitutive expression of the mCherry gene and a hygromycin-resistance cassette to enable selection (Biebinger and Clayton 1996; Hutchings et al., 2002). Primers used to PCR amplify mCherry are as follow: 5′ ATGGTGAGCAAGGGCGAGG 3′ (forward primer), 5′ TTACTTGTACAGCTCGTCCATGC 3′ (reverse primer). All DNA sequences were verified by direct sequencing.
Mouse infections
Mouse infections were done essentially as described (Dubois et al., 2005). BALB/c female mice (The Jackson Laboratory, Bar Harbor, ME), 6 to 10 weeks old, were injected intraperitoneally with 100 parasites in 0.2 ml cold phosphate buffered saline (PBS) (pH 7.4) supplemented with 1% glucose. To maintain cell viability in PBS, parasites were kept on ice prior to injection into mice. For + Tet infections, parasites were induced with 1 μg/ml tetracycline in culture three days prior to injection into mice and mice received 1 mg/ml doxycycline, a tetracycline version, in their drinking water 5 to 7 days prior to infection and throughout the course of infection. Parasitemia was monitored daily beginning 3 days post infection using an improved Neubaeur hemocytometer (Herbert and Lumsden 1976). After euthanasia, mice were weighed and tissues were collected for weighing. For trypanin knockdown and LC1 knockdown infections, parasites were maintained in culture without tetracycline and mice received 1 mg/ml doxycycline in drinking water only when animals showed the first wave of parasitemia.
Parasite motility assays
Knockdown, K/R and K/R-mCherry parasites were grown without or with tetracycline to a density of 1.5–2.0 × 106 cells/ml and cell motility analysis was done at the hours post induction (hpi) described in each figure. For methyl cellulose experiments, cells were maintained with or without addition of 0.4% (v/v) to approximate the viscosity of blood (Heddergott et al., 2012). For mCherry K/R parasites, cells (50 microliters) were added to freshly drawn, undiluted mouse blood (500 microliters). All samples were analyzed in a pre-warmed motility chambers within 5 minutes after collection. Motility traces were done as described (Ralston et al., 2011). Briefly, parasites were examined in motility chambers using dark field optics on a Zeiss Axioskop II compound microscope with a 10x objective (Figures 1 and 2) or using fluorescence optics on a Zeiss Axiovert 200 M inverted microscope with a 20x objective (Figure 3). Videos were captured using a COHU analog video camera. Analog format movies were converted to digital format with an ADVC-300 digital video converter (Canopus, Co., Ltd.). Movies were recorded at 30 frames per second (fps) and converted to AVI format and then to stacks of TIFF images using Adobe Premiere Elements software (Adobe Systems). TIFF image stacks were analyzed using Metamorph software (Molecular Devices) to trace parasite movement over the indicated time period. Trace data were used to calculate the total distance travelled, as well as the mean squared displacement (MSD) of individual cells in the x and y dimensions. MSD was calculated according to the formula MSD = <ri(t)2> = <(pi(t) − pi(0))2>, where ri is the distance travelled by the parasite over time interval t and pi is the position of the parasite at any given time. For figures 1 and 2, the timescale of t ranged from 1 to 25 seconds in increments of 1 second. For figures 3 and 5, the timescale of t ranged from 1 to 8 seconds in increments of 0.3 second. MSD is calculated for each instance i of a given time interval. Several cell MSDs were then averaged to obtain an ensemble average.
For motility experiments on parasites from mouse infections (Figure 5), blood samples were collected from infected mice 7–13 days post infection (dpi). Samples were kept at 37°C and analyzed in a pre-warmed motility chamber within 5 to 10 minutes after collection. To record motility traces, blood samples were diluted 1:100 in warm HMI-9 culture medium to enable tracing of individual parasites among red blood cells and videos were recorded and processed as described above, using differential interference contrast (DIC) optics on a Zeiss Axiovert 200 M inverted microscope with a 100x oil-immersion objective. For high-resolution videos of individual cells (Supplemental Videos 1–9), videos were captured in motility chambers using DIC optics (Supplemental Videos 1–6, 8–9) or using fluorescence optics (Supplemental Videos 7) on a Zeiss Axiovert 200 M inverted microscope with a 100x oil-immersion (Supplemental Videos 1–6, 8–9) or 20x (Supplemental Videos 7) objective.
Hi-speed videos
The experimental setup used to record high-speed trypanosome motility consists of an inverted DMI6000 B microscope (Leica Microsystems, Wetzlar, Germany) equipped with a 100X objective (Leica HCX PL APO 100x/1.40-0.70 oil) and Differential interference Contrast (DiC) optics, a halogen-based white trans-illumination system and a PC-controlled Photron FocusScope SV200-i high-speed CMOS camera (Photron USA Inc.) mounted in the trinocular port. The images provided by the high-speed camera are directly observed in the PC monitor. System control is achieved through the Photron FASTCAM Viewer software (PFV Version 3). Images were captured at 1,000 frames per sec (fps) with 512 × 512 pixel image resolution.
Western blot analysis of parasites from mice
Western blots were done as described previously (Ralston et al., 2011). To obtain parasites from mice, mice were euthanized and blood was collected in heparinized tubes 7–13 days post infection. Parasites were separated from whole blood using DE 52 anion exchange columns, as described (Lanham and Godfrey 1970). Purified parasites were examined with a microscope to assess purity and parasite yield was determined by counting in a hemocytometer. Purified parasites were washed three times in PBS and then boiled in Laemmli sample buffer for Western blot analysis. Blots were probed with anti-HA monoclonal antibody (Covance) at 1:5000 diliution, anti-β-tubulin monoclonal antibody (Developmental Studies Hybridoma Bank, University of Iowa) at 1:5000 diliution, or anti-variable surface glycoprotein VSG 221 (McDowell et al., 1998) at 1:100,000 dilution
Statistical analysis
Statistical analysis and graphical output was done with GraphPad Prism 5. The significance of survival was determined by Log-rank (Mantel-Cox) Test. We used the Student unpaired two-tailed t test to determine significant differences in motility. The significance of total body weight, spleen weight, liver weight, and number of red blood cells was calculated by one-way ANOVA with Bonferroni’s post-test for multiple comparisons. Significance (p value) is reported in each figure.
Supplementary Material
(A) Cartoon of a T. brucei cell. (B) A schematic diagram of an axoneme cross section as would be observed looking posterior to anterior near the boxed region in (A). (C) Simplified diagram of outer arm dynein, showing the two heavy chains (α and β) and the location of LC1. (D) LC1 schematic showing residues mutated in the K/R mutant used in this study. Panel A adapted from (Hill et al., 2000) with permission. (E) Mean squared displacement of K/R parasites in media without or with methyl cellulose. Same graph as shown in Figure 2C, but including error bars that show standard error of the mean (SEM). See figure 2C legend for details. (F) Tetracycline treatment clears infection by trypanin knockdown parasites. Parasitemias of two mice infected with trypanin knockdown parasites (TPN-KD). Mice were infected with TPN-KD parasites at day 0 and then treated with tetracycline in drinking water upon detection of parasites in blood (+Tet arrows). Mice cleared infection within 24 h of tetracycline treatment, demonstrating the efficacy of tetracycline treatment.
Representative live video shows propulsive motility of LC1 knockdown parasites taken from −Tet cultures. Parasites move rapidly and translocate with tip of flagellum leading. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of LC1 knockdown parasites grown in +Tet cultures for 12 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows cell division failure of LC1 knockdown parasites grown in +Tet cultures for 26 hours. Cells accumulate as an amorphous mass, but the flagella continue to beat vigorously. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of K/R parasites grown in +Tet cultures for 12 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of K/R parasites grown in +Tet cultures for 72 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
The first clip (from 0 to 8 seconds) shows representative flagellar beating in control cells (LC1 grown in the absence of Tet). Tip-to-base flagellar beats are evident. The second clip (from 10 to 18 seconds) shows representative flagellar beating in LC1 knockdown parasites grown in +Tet cultures for 12 hours. The third clip (from 20 to 28 seconds) shows representative flagellar beating in K/R parasites grown in +Tet cultures for 12 hours. The fouth clip (from 31 to 39 seconds) shows representative flagellar beating in K/R parasites grown in +Tet cultures for 72 hours. For all videos, frame rate for capture is 1000 frames/sec and playback is 60 frames/sec.
The first clip (from 0 to 5 seconds) shows representative motility of mCherry K/R trypanosomes from −Tet cultures, added to mouse blood. Parasites move rapidly and translocate with tip of flagellum leading. Frame rate for capture and playback is 30 frames/sec. The second clip (from 7 to 12 seconds) shows representative motility of mCherry K/R trypanosomes from +Tet cultures, added to mouse blood. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec. For both videos, cultured trypanosomes were added directly to whole blood.
Representative live video shows propulsive motility of parasites taken from mice infected with K/R trypanosomes and maintained without tetracycline. Parasites move rapidly and translocate with tip of flagellum leading. Samples were taken seven days post-infection. Red blood cells are readily distinguishable from parasites as biconcave disks. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of parasites taken from mice infected with K/R trypanosomes and maintained with tetracycline in the drinking water. Flagellum beating is evident, but parasite propulsive motility is blocked. Samples were taken seven days post-infection. Red blood cells are readily distinguishable from parasites as biconcave disks. Frame rate for capture and playback is 30 frames/sec.
Summary.
Trypanosoma brucei is a deadly human pathogen that causes significant human mortality and limits economic development in some of the most impoverished regions of the world. Trypanosome motility is considered to be important for infection and pathogenesis. However, until the present work, it was not possible to test this hypothesis because all motility mutants generated previously were not viable. We recently overcame this barrier by identifying a viable motility mutant and in the present study, we have used this mutant to provide the first analysis of the impact of trypanosome motility on infection and pathogenesis. We found that normal motility is dispensable for trypanosome bloodstream infection and pathogenesis in mice. These studies represent an important contribution to understanding pathogenesis mechanisms and host-parasite interactions, and inform efforts to exploit the flagellum as a drug target.
Acknowledgments
Funding for this work was supplied by grants to K.L.H. from the Burroughs Wellcome Fund and NIH-NIAID (AI052348). Neville K. Kisalu is a recipient of the Shapiro Fellowship and the UCLA Dissertation Year Fellowship. Gerasimos Langousis is recipient of the Warsaw fellowship. We are grateful to John Mansfield and Karen Demick (University of Wisconsin) for guidance with mouse infections. We thank Jose Rodriguez (UCLA) for technical support with motility trace analysis. We thank Josh Beck (UCLA) for kindly providing us with the mCherry DNA. We are also appreciative of Shimon Weiss (UCLA) and members of our laboratory for pertinent comments on the work and critical reading of the manuscript. High-speed video microscopy was performed at the California NanoSystems Institute Advanced Light Microscopy/Spectroscopy and the Macro-Scale Imaging Shared Facilities at UCLA.
Footnotes
Authors have no conflict of interest.
Author contributions: Conceived the study: N.K.K, K.S.R, K.L.H. Performed the experiments: N.K.K, G.L, L.A.B. Analyzed the data: N.K.K, G.L, K.S.R, L.A.B, K.L.H. Wrote the paper: N.K.K and K.L.H.
References
- Amole BO, Clarkson AB, Jr, Shear HL. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1982;36(3):1060–1068. doi: 10.1128/iai.36.3.1060-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JD, McCulloch R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol. 2001;49:1–70. doi: 10.1016/s0065-308x(01)49037-3. [DOI] [PubMed] [Google Scholar]
- Berg HC, Turner L. Movement of microorganisms in viscous environments. Nature. 1979;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Biebinger S, Clayton C. A plasmid shuttle vector bearing an rRNA promoter is extrachromosomally maintained in Crithidia fasciculata. Exp Parasitol. 1996;83(2):252–258. doi: 10.1006/expr.1996.0072. [DOI] [PubMed] [Google Scholar]
- Borst P. Antigenic variation and allelic exclusion. Cell. 2002;109(1):5–8. doi: 10.1016/s0092-8674(02)00711-0. [DOI] [PubMed] [Google Scholar]
- Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119(Pt 16):3443–3455. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440(7081):224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- Dubois ME, Demick KP, Mansfield JM. Trypanosomes expressing a mosaic variant surface glycoprotein coat escape early detection by the immune system. Infect Immun. 2005;73(5):2690–2697. doi: 10.1128/IAI.73.5.2690-2697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell. 2010;9(6):934–942. doi: 10.1128/EC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131(3):505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Frevert U, Movila A, Nikolskaia OV, Raper J, Mackey ZB, Abdulla M, et al. Early invasion of brain parenchyma by african trypanosomes. PLoS One. 2012;7(8):e43913. doi: 10.1371/journal.pone.0043913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginger ML, Portman N, McKean PG. Swimming with protists: perception, motility and flagellum assembly. Nat Rev Microbiol. 2008;6(11):838–850. doi: 10.1038/nrmicro2009. [DOI] [PubMed] [Google Scholar]
- Gray GD, Jennings FW, Hajduk SL. Relapse of monomorphic and pleomorphic Trypanosoma brucei infections in the mouse after chemotherapy. Z Parasitenkd. 1982;67(2):137–145. doi: 10.1007/BF00928109. [DOI] [PubMed] [Google Scholar]
- Gruby M. Analysis and observation of a novel hematozoan species, Trypanosoma sanguinis. C R Hebd Seqnces Acad Sci. 1843;17:1134–1136. [Google Scholar]
- Hajagos BE, Turetzky JM, Peng ED, Cheng SJ, Ryan CM, Souda P, et al. Molecular dissection of novel trafficking and processing of the Toxoplasma gondii rhoptry metalloprotease toxolysin-1. Traffic. 2012;13(2):292–304. doi: 10.1111/j.1600-0854.2011.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddergott N, Kruger T, Babu SB, Wei A, Stellamanns E, Uppaluri S, et al. Trypanosome motion represents an adaptation to the crowded environment of the vertebrate bloodstream. PLoS Pathog. 2012;8(11):e1003023. doi: 10.1371/journal.ppat.1003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert WJ, Lumsden WH. Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitemia. Exp Parasitol. 1976;40(3):427–431. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- Hill KL, Hutchings NR, Grandgenett PM, Donelson JE. T lymphocyte-triggering factor of african trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275(50):39369–39378. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
- Hutchings NR, Donelson JE, Hill KL. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J Cell Biol. 2002;156(5):867–877. doi: 10.1083/jcb.200201036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings FW, Gray GD, Whitelaw DD. Chemotherapy of Trypanosoma brucei in mice with diminazene aceturate. Variation in the aparasitaemic period over 5 years. Z Parasitenkd. 1982;67(3):337–340. doi: 10.1007/BF00927669. [DOI] [PubMed] [Google Scholar]
- Jennings FW, Whitelaw DD, Holmes PH, Chizyuka HG, Urquhart GM. The brain as a source of relapsing Trypanosoma brucei infection in mice after chemotherapy. Int J Parasitol. 1979;9(4):381–384. doi: 10.1016/0020-7519(79)90089-4. [DOI] [PubMed] [Google Scholar]
- Lanham SM, Godfrey DG. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Macgregor P, Rojas F, Dean S, Matthews KR. Stable transformation of pleomorphic bloodstream form Trypanosoma brucei. Mol Biochem Parasitol. 2013;190(2):60–62. doi: 10.1016/j.molbiopara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor P, Savill NJ, Hall D, Matthews KR. Transmission stages dominate trypanosome within-host dynamics during chronic infections. Cell Host Microbe. 2011;9(4):310–318. doi: 10.1016/j.chom.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Ransom DM, Bangs JD. Glycosylphosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem J. 1998;335 (Pt 3):681–689. doi: 10.1042/bj3350681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E. Regulation of antigen gene expression in Trypanosoma brucei. Trends Parasitol. 2005;21(11):517–520. doi: 10.1016/j.pt.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Poltera AA, Hochmann A, Rudin W, Lambert PH. Trypanosoma brucei brucei: a model for cerebral trypanosomiasis in mice--an immunological, histological and electronmicroscopic study. Clin Exp Immunol. 1980;40(3):496–507. [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Hill KL. Trypanin, a component of the flagellar Dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog. 2006;2(9):e101. doi: 10.1371/journal.ppat.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Hill KL. The flagellum of Trypanosoma brucei: new tricks from an old dog. Int J Parasitol. 2008;38(8–9):869–884. doi: 10.1016/j.ijpara.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Kabututu ZP, Melehani JH, Oberholzer M, Hill KL. The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol. 2009;63:335–362. doi: 10.1146/annurev.micro.091208.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Kisalu NK, Hill KL. Structure-function analysis of dynein light chain 1 identifies viable motility mutants in bloodstream-form Trypanosoma brucei. Eukaryot Cell. 2011;10(7):884–894. doi: 10.1128/EC.00298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Lerner AG, Diener DR, Hill KL. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell. 2006;5(4):696–711. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. Trypanosomiasis and the brain. Parasitology. 2010;137(14):1995–2006. doi: 10.1017/S0031182009991806. [DOI] [PubMed] [Google Scholar]
- Roditi I, Lehane MJ. Interactions between trypanosomes and tsetse flies. Curr Opin Microbiol. 2008;11(4):345–351. doi: 10.1016/j.mib.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Lopez MA, Thayer MC, Zhao Y, Oberholzer M, Chang DD, et al. Propulsion of African trypanosomes is driven by bihelical waves with alternating chirality separated by kinks. Proc Natl Acad Sci U S A. 2009;106(46):19322–19327. doi: 10.1073/pnas.0907001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotureau B, Ooi CP, Huet D, Perrot S, Bastin P. Forward motility is essential for trypanosome infection in the tsetse fly. Cell Microbiol. 2013 doi: 10.1111/cmi.12230. [DOI] [PubMed] [Google Scholar]
- Rotureau B, Subota I, Buisson J, Bastin P. A new asymmetric division contributes to the continuous production of infective trypanosomes in the tsetse fly. Development. 2012;139(10):1842–1850. doi: 10.1242/dev.072611. [DOI] [PubMed] [Google Scholar]
- Sharma R, Gluenz E, Peacock L, Gibson W, Gull K, Carrington M. The heart of darkness: growth and form of Trypanosoma brucei in the tsetse fly. Trends Parasitol. 2009;25(11):517–524. doi: 10.1016/j.pt.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich A, Abel PM, Krishna S. Human African trypanosomiasis. BMJ. 2002;325(7357):203–206. doi: 10.1136/bmj.325.7357.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppaluri S, Nagler J, Stellamanns E, Heddergott N, Herminghaus S, Engstler M, Pfohl T. Impact of microscopic motility on the swimming behavior of parasites: straighter trypanosomes are more directional. PLoS Comput Biol. 2011;7(6):e1002058. doi: 10.1371/journal.pcbi.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PJ. Organization of function in trypanosome flagella. Nature. 1961;189:1017–1018. doi: 10.1038/1891017a0. [DOI] [PubMed] [Google Scholar]
- Weisse S, Heddergott N, Heydt M, Pflasterer D, Maier T, Haraszti T, et al. A quantitative 3D motility analysis of Trypanosoma brucei by use of digital in-line holographic microscopy. PLoS One. 2012;7(5):e37296. doi: 10.1371/journal.pone.0037296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99(1):89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Cartoon of a T. brucei cell. (B) A schematic diagram of an axoneme cross section as would be observed looking posterior to anterior near the boxed region in (A). (C) Simplified diagram of outer arm dynein, showing the two heavy chains (α and β) and the location of LC1. (D) LC1 schematic showing residues mutated in the K/R mutant used in this study. Panel A adapted from (Hill et al., 2000) with permission. (E) Mean squared displacement of K/R parasites in media without or with methyl cellulose. Same graph as shown in Figure 2C, but including error bars that show standard error of the mean (SEM). See figure 2C legend for details. (F) Tetracycline treatment clears infection by trypanin knockdown parasites. Parasitemias of two mice infected with trypanin knockdown parasites (TPN-KD). Mice were infected with TPN-KD parasites at day 0 and then treated with tetracycline in drinking water upon detection of parasites in blood (+Tet arrows). Mice cleared infection within 24 h of tetracycline treatment, demonstrating the efficacy of tetracycline treatment.
Representative live video shows propulsive motility of LC1 knockdown parasites taken from −Tet cultures. Parasites move rapidly and translocate with tip of flagellum leading. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of LC1 knockdown parasites grown in +Tet cultures for 12 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows cell division failure of LC1 knockdown parasites grown in +Tet cultures for 26 hours. Cells accumulate as an amorphous mass, but the flagella continue to beat vigorously. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of K/R parasites grown in +Tet cultures for 12 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of K/R parasites grown in +Tet cultures for 72 hours. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec.
The first clip (from 0 to 8 seconds) shows representative flagellar beating in control cells (LC1 grown in the absence of Tet). Tip-to-base flagellar beats are evident. The second clip (from 10 to 18 seconds) shows representative flagellar beating in LC1 knockdown parasites grown in +Tet cultures for 12 hours. The third clip (from 20 to 28 seconds) shows representative flagellar beating in K/R parasites grown in +Tet cultures for 12 hours. The fouth clip (from 31 to 39 seconds) shows representative flagellar beating in K/R parasites grown in +Tet cultures for 72 hours. For all videos, frame rate for capture is 1000 frames/sec and playback is 60 frames/sec.
The first clip (from 0 to 5 seconds) shows representative motility of mCherry K/R trypanosomes from −Tet cultures, added to mouse blood. Parasites move rapidly and translocate with tip of flagellum leading. Frame rate for capture and playback is 30 frames/sec. The second clip (from 7 to 12 seconds) shows representative motility of mCherry K/R trypanosomes from +Tet cultures, added to mouse blood. Flagellum beating is evident, but parasite propulsive motility is disrupted. Frame rate for capture and playback is 30 frames/sec. For both videos, cultured trypanosomes were added directly to whole blood.
Representative live video shows propulsive motility of parasites taken from mice infected with K/R trypanosomes and maintained without tetracycline. Parasites move rapidly and translocate with tip of flagellum leading. Samples were taken seven days post-infection. Red blood cells are readily distinguishable from parasites as biconcave disks. Frame rate for capture and playback is 30 frames/sec.
Representative live video shows defective motility of parasites taken from mice infected with K/R trypanosomes and maintained with tetracycline in the drinking water. Flagellum beating is evident, but parasite propulsive motility is blocked. Samples were taken seven days post-infection. Red blood cells are readily distinguishable from parasites as biconcave disks. Frame rate for capture and playback is 30 frames/sec.