Abstract
Lymphoid tissue often forms within sites of chronic inflammation. Here we report that expression of the proinflammatory cytokine TNFa drives development of lymphoid tissue in the intestine. Formation of this ectopic lymphoid tissue was not dependent on the presence of canonical RORgt+ lymphoid tissue inducer (LTi) cells, because animals expressing increased levels of TNFα but lacking RORgt+ LTi cells (TNF/Rorc(gt)−/− mice) developed lymphoid tissue in inflamed areas. Unexpectedly, such animals developed several lymph nodes that were structurally and functionally similar to those of wild type animals. TNFα production by F4/80+ myeloid cells present within the anlagen was important for activation of stromal cells during the late stages of embryogenesis and for the activation of an organogenic program that allowed development of lymph nodes. Our results show that lymphoid tissue organogenesis can occur in the absence of LTi cells and suggest that interactions between TNFα-expressing myeloid cells and stromal cells have an important role in secondary lymphoid organ formation.
Introduction
Lymphoid organs are critical for generation of adaptive immune response. Secondary lymphoid organs (SLO) are formed at predefined areas during embryogenesis whereas tertiary lymphoid organs (TLO) are formed after birth in tissues with ongoing inflammatory processes1, 2. Both secondary and tertiary lymphoid organs have lymphocytes that are topologically segregated, and diverse sets of myeloid and stromal cells. In addition, they have specialized vasculature such as high endothelial venules (HEV), and a lymphatic network.
The two major cell types involved in lymph node organogenesis are the hematopoietic lymphoid tissue inducer (LTi) cells and non-hematopoietic lymphoid tissue stromal “organizer cells” (LTo)1. Clustering of LTi and LTo cells is an essential step in lymph node development3. Animals that are deficient in the nuclear retinoid orphan receptor (ROR)γ, encoded by the Rorc gene, or the negative regulator of basic helix-loop-helix protein signaling Id2, lack LTi cells and therefore fail to form lymph nodes and Peyer’s patches4–6. The current model for development of lymphoid organs posits that LTi cells originate in the fetal liver from common lymphoid progenitors and that they migrate to the sites where the lymph nodes are formed (lymph node anlagen)1, 7. At these sites, binding of the TNFα family ligand Receptor Activator of NF-kB (RANKL) to its receptor RANK induces the differentiation and survival of LTi cells and trigger expression of LTα1β2 on their surface3, 8–11. A key step in the development of LNs is the engagement of Lymphotoxin a1b2 (LTα1β2) expressed by LTi cells to its receptor LTβR on mesenchymal organizer cells12, 13. This interaction promotes upregulation of intracellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1) and mucosal addressin cell adhesion molecule (MAdCAM-1) on the surface of LTo cells14, 15 and the expression of the chemokines CCL19, CCL21, and CXCL137.
Animals genetically deficient in LT-alpha and LTβR do not form lymph nodes or Peyer’s patches10, 12, 16. Furthermore, genetic deletion of molecules in the LTβR signaling pathway (NF-kappa B non canonical pathway) such as NF-kappa B-inducible kinase (NIK)17 and RelB18 precludes LN formation. While the role of LTα1β2/LTβR is firmly established in the process of lymphoid organogenesis, the role of other members of the TNFα superfamily is unclear.
Female mice injected in utero with LTβR-Ig fusion protein retain cervical and mesenteric lymph nodes (mLN) but fail to form other lymph nodes19, 20. However, simultaneous treatment LTβR-Ig fusion protein and anti-TNFR1 antibody, or LTβR-Ig plus anti-TNFα antibodies, prevents development of all lymph nodes21, which suggests that TNFα has a role in mLN organogenesis. However, TNFα or TNF-R1-deficient mice have all lymph nodes, including mLN, but they fail to form B cell follicles. These results suggest that TNFα activity in lymphoid organogenesis may be secondary to other TNFα members such as LT. However, simultaneous deficiency of TNFR1 and RelA abrogates the development of all lymph nodes, despite the presence of a normal complement of LTα1β2+ LTi cells22. Thus, the role of TNFα in lymphoid organogenesis remains poorly defined.
Here we used TNFΔARE/+ mice, a well-established model of human inflammatory disease, to study the role of TNFα in lymphoid organogenesis. These animals express increased levels of TNFα under basal conditions, due to mutation in the 3′ region of the Tnfa gene that causes higher stability of its mRNA and, consequently, increased levels of TNFα protein23. Intercross of TNFΔARE/+ mice with Rorc(γt)−/− mice led to the generation of TNF/Rorc(γt)−/− mice. Surprisingly, TNF/Rorc(γt)−/− mice developed TLO and several SLO (mesenteric, axillary and cervical LN and others) despite the lack of the classical RORγt+ LTi cells. Development of lymph nodes was mechanistically linked to activation of stromal cells by TNFα produced by myeloid cells present in the anlagen, and expression of molecules involved in lymphoid organogenesis. These results establish that lymphoid organogenesis can occur in the absence of Rorc if there is increased TNFα signaling.
Results
Increased expression of TNFα promotes development of TLO in the absence of LTi cells
Two types of lymphoid aggregates can be identified in the intestine of adult mice: isolated lymphoid follicles (ILF) and tertiary lymphoid organs (TLO). ILFs are genetically programmed clusters of B cells present at the base of the villi, that require RORγt+LTi cells and LTβR signaling for their formation5, 24–26. TLO are composed by large clusters of B220+ cells that contain CD3+ lymphocytes, and are formed in response to infection or inflammation27, 28. To further define the role of LTi cells and TNFα in the formation of lymphoid aggregates in the intestine we examined the presence of these structures in the ileum of TNFΔARE/+ mice. The inflammatory infiltrates in the ileum are composed of neutrophils, macrophages, and T cells that are distributed throughout the submucosa and muscular layers and sometimes reach the serosa. Large mononuclear aggregates rich in B cells, or TLO, are also found in the terminal ileum of the TNFΔARE/+ mice29. To determine whether the formation of these aggregates is dependent on RORγt+LTi cells we crossed Rorc(γt)−/− mice with TNFΔARE/+ mice to generate TNF/Rorc(γt)−/− mice. Histological analysis of the terminal ileum of age-matched wild type (WT), Rorc(γt)−/−, TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice at 16–20 weeks of age showed that TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice, but not WT or Rorc(γt)−/− mice, had marked submucosal inflammation, vilus blunting, patchy transmural inflammation, and lymphoid aggregates (Figure 1a). The lymphoid aggregates in TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice contained large clusters of B220+ B cells and few CD3+ T cells (Figure 1b and 1c), which were absent in Rorc(γt)−/− mice. These results indicate that TLO can be formed in the ileum in the absence of RORγt+ LTi cells.
Figure 1. TLO are formed in the ileum TNF/Rorc(γt)−/− mice.
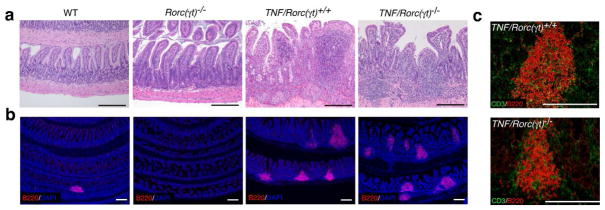
(a) Representative H&E sections of the Ileum of WT, Rorc(γt)−/−, TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice at 16 wks. Notice the presence of inflammatory infiltrates in the ileum of TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice. (b) Ileum sections of indicated mice were stained with anti-B220 antibody to visualize B cell aggregates and DAPI for nuclear staining. Small B cell clusters were found in the ileum of WT but were absent in the ileum of Rorc(γt) −/− mice. (c) Overexpression of TNF induced the formation of large B cell clusters with few T cells in the ileum of TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice. Scale bars = 250 μm, n=4/group.
TNF/Rorc(γt)−/− mice develop secondary lymphoid organs
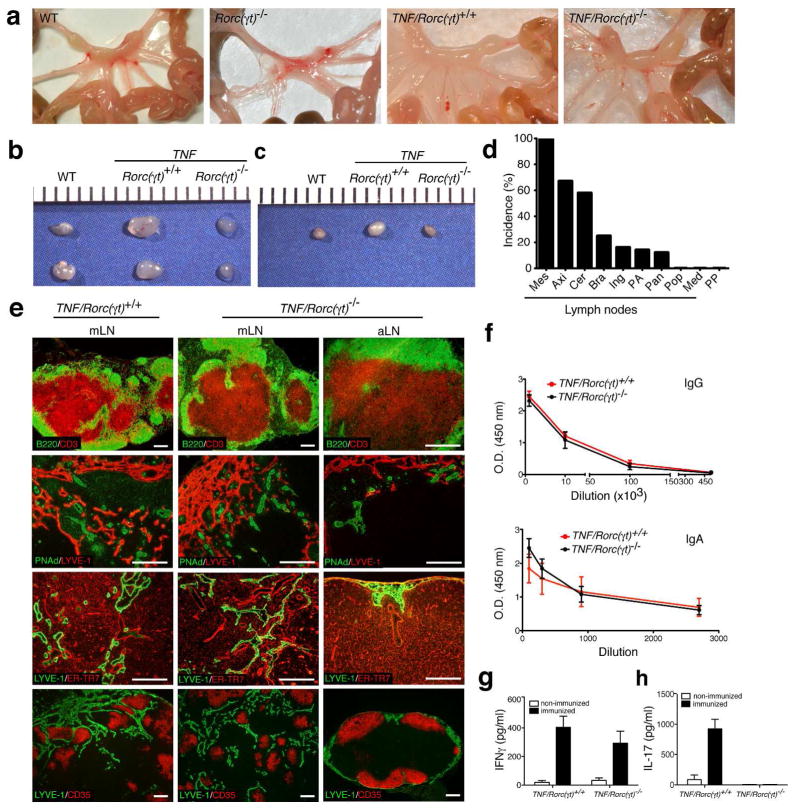
Rorc is essential for development of secondary lymphoid organs5. As expected, no lymph nodes were found in the Rorc(γt)−/− mice examined (Figure 2a). However, we were surprised to find that 100% of the TNF/Rorc(γt)−/− mice had fully developed mesenteric LN (mLN) that were grossly indistinguishable from those found in WT mice. Axillary (Figure 2b), cervical (Figure 2c), brachial, inguinal, para-aortic, and peripancreatic LN were also present at lower frequency (Figure 2d). Mediastinal and popliteal LN, as well as Peyer’s patches, were not observed in these animals.
Figure 2. Increased expression of TNF induces development SLO in the absence of RORγt+ LTi cells.
(a) Photograph of the mesentery of WT, Rorc(γt)−/−, TNF/Rorc(γt)+/+, and TNF/Rorc(γt)−/− mice. Photograph of axilary (b) and cervical (c) lymph nodes of WT, TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice. (d) Incidence of mesenteric (Mes), axillary (Axi), cervical (Cer), brachial (Bra), inguinal (Ing), para aortic (PA), peripancreatic (Pan), popliteal (Pop), mediastinal (Med) lymph nodes, and Peyer’s patches (PP) formed in TNF/Rorc(γt)−/− mice (n = 80). (e) Lymph nodes from TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice at 6 wk of age were analyzed by immunostaining. Note segregation of T and B cell areas, presence of PNAd+ HEV and lymphatic vessels, normal distribution of ER-TR7+ meshwork and CD35bright FDC in mesenteric (mLN) and axillary (aLN) lymph nodes of TNF/Rorc(γt)−/− mice. These features were similar to the ones observed in the mLn of TNF/Rorc(γt)+/+ mice (n = 5 mice/group). Scale bars = 250μm. (f) OVA-specific IgG and IgA measured in the serum of TNF/Rorc(γt)+/+ (n = 5) and TNF/Rorc(γt)−/− (n = 4) obtained after 5 rounds of immunization. (g) IFN-γ and (h) IL-17 levels in supernatants of cultured MLN cells 7 weeks after OVA immunization.
To further characterize the structure of the LNs present in TNF/Rorc(γt)−/− mice we performed immunostaining. LNs of WT and TNF/Rorc(γt)−/− mice had segregated T and B cell areas, PNAd+ high endothelial venules, an extensive lymphatic network, ER-TR7+ lymph node stroma and CD35bright follicular dendritic cells (Figure 2e). To determine if these LN were functional we immunized TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice orally with OVA and cholera toxin 7 times at 1-wk intervals and assessed OVA-specific antibody serum titers by ELISA (Figures 2f). The serum levels of OVA-specific IgA and IgG were similar between TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice, indicating that both strains responded to oral immunization with OVA. We next examined if cells from the mesenteric lymph nodes could produce cytokines after immunization. mLNs were collected and cultured with media alone or with 50μg/ml of OVA. Supernatants were harvested 72 hours later and IFNγ and IL-17 were measured by ELISA. As shown in Figure 2g, similar levels of IFNγ were produced by mesenteric LN cells of TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice. As expected, IL-17 was not detected in TNF/Rorc(γt)−/− cells since RORγt is required for IL-17 production (Figure 2h) 30. Collectively these results indicate that increased expression of TNFα can drive the formation of secondary lymphoid organs in the absence of RORγt+ LTi cells.
F4/80+CD11b+ cells are the source of TNFα in the mLN anlagen
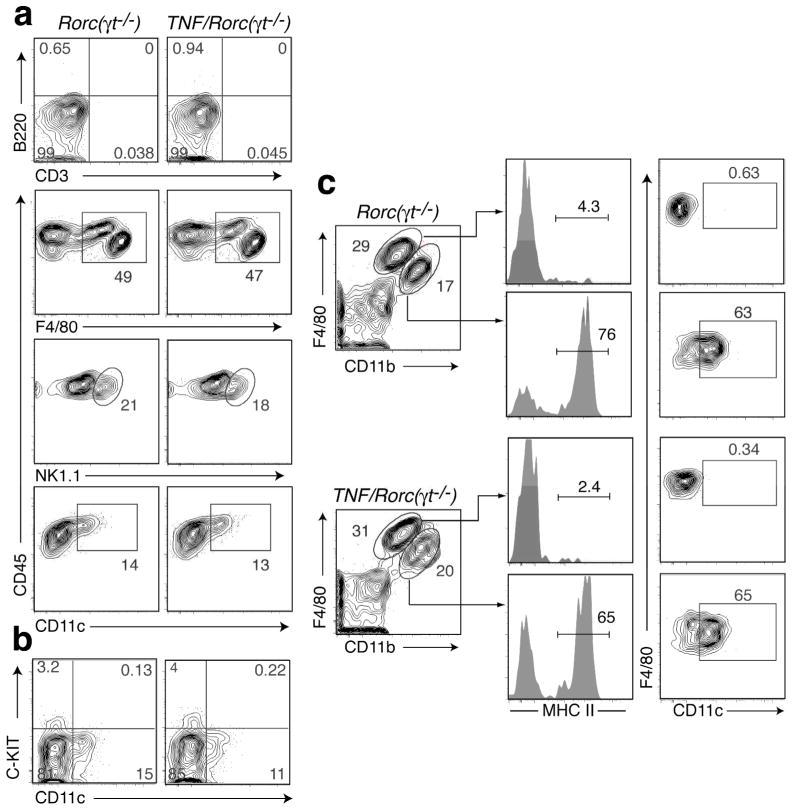
Our results suggested that a RORgt-independent cell type could play a role in the formation of SLO in TNF/Rorc(γt)−/− mice. To start addressing this hypothesis we first examined the cellular composition of the mLN of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice at P1. Very few lymphocytes were present to the mLN anlagen of TNF/Rorc(γt)−/− mice at this stage (Figure 3a). F4/80+, NK1.1+ and CD11c+ cells were the most abundant CD45+ leukocytes present in the mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice, but their relative proportions were comparable. CD11c+ cells in the mLN of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice did not express c-Kit (Figure 3b) and thus were distinct from the c-Kit+CD11c+ lymphoid tissue initiator cells shown to be important in the formation of Peyer’s patches (PP)31. Further flow cytometric analyses showed that the F4/80+ cells comprised two populations: F480hi/CD11blow/MHC IIneg/CD11c− and F4/80low/CD11bhi/MHC IIpos/CD11c+ cells (Fig. 3c). These results indicate that there were no marked differences in the type and relative number of leukocytes in the mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice at P0.5-P1
Figure 3. Phenotype of the cells present in the mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice.
Cell suspensions from the mLN region of RORγt−/− and TNF/Roc(γt)−/− mice at P0.5-P1 stage were analyzed by flow cytometry for the expression of the indicated markers. Cells were gated on PI−CD45+. Representative plots of 3 independent experiments (n=2–3/group).
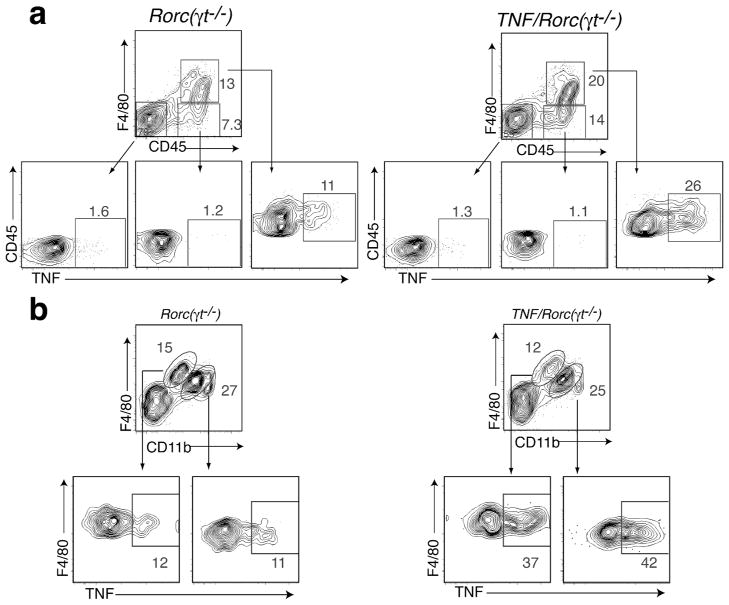
We asked next if TNFα was expressed in the mLN during development. TNFα expression was detected in the mLN anlagen of WT mice at steady state during embryogenesis (Supplemental Figure 1b). Next we compared the levels of TNFα mRNA in mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice, and found it to be upregulated in the latter at all embryonic and postnatal stages examined (Supplemental Figure 1a). We then used flow cytometry to determine the cellular source of TNFα in the mLN anlagen. In WT mice, TNFα was detected in F4/80+ myeloid cells as early as E15.5 in WT mLN while TNFR1 was expressed in both myeloid and CD45− stromal cells (Supplemental Figure 1c). Analysis of the mLN anlagen of P0.5-P1 Rorc(γt)−/− and TNF/Rorc(γt)−/− mice showed that TNFα was mainly produced by CD45+F4/80+ cells and not by other CD45+ or stromal (CD45−) cells (Figure 4a). A two fold increase in the production of TNFα by F4/80+ cells was observed in the mLN anlagen of TNF/Rorc(γt)−/− mice. Further flow cytometric analyses showed that TNFα was expressed by both F4/80hi/CD11blow and F4/80low/CD11bhi cells (Figure 4b). Together these results indicate that: 1) TNFα is physiologically expressed by F4/80+ cells in the mLN anlagen of WT mice during embryogenesis, 2) that TNFα is expressed by F4/80+ cells in both Rorc(γt)−/− mice TNF/Rorc(γt)−/− mice, and, 3) that TNFα expression is increased during embryogenesis and early postnatal life in the mLN of TNF/Rorc(γt)−/− mice compared to Rorc(γt)−/− mice.
Figure 4. F4/80+/CD11b+ cells produce increased levels of TNF in the mLN of TNF/RORγt−/− mice.
(a) Flow cytometric analysis of TNF production by CD45−, CD45+F4/80− and CD45+F4/80+ cells isolated from the mLN region of RORγt−/− and TNF/Roc(γt)−/− mice at P0.5-P1 stage. (b) Analysis of TNF production by CD45+F4/80hiCD11blow and CD45+F4/80lowCD11bhigh cells. Representative plot of two independent experiments, (n=4–5 animals/group).
TNFα does not bypass the requirement of ID2 for lymphoid organogenesis
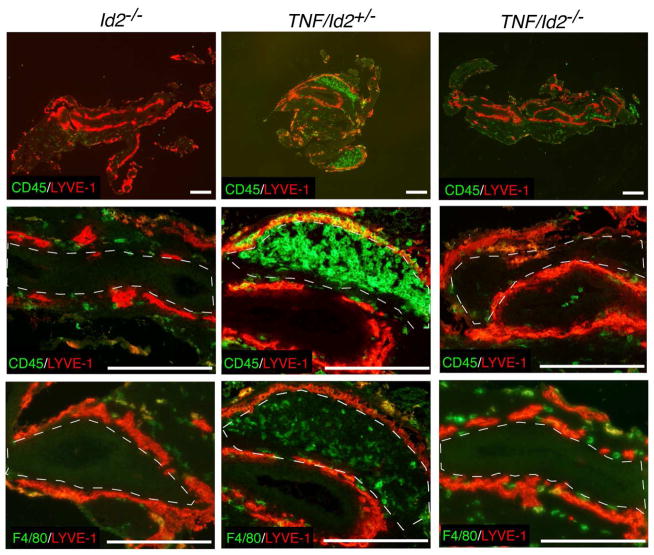
Id2-deficient mice lack LTi cells6, NK cells6 and fetal CD11b+ myeloid cells5 in the lymph node anlagen, and do not develop SLO. We had shown above that TNFα overexpression bypasses the requirement for Rorc(γt)+ cells in SLO formation, thus we investigated next if TNFα would bypass the requirement for Id2 in lymph node organogenesis. To do so, we intercrossed TNFΔARE/+ mice with Id2−/− mice to generate TNF/Id2−/− mice (Figure 5). None of the TNF/Id2−/− mice examined at birth (n=7) had mLN. We also examined the presence of F4/80+ myeloid cells, and found them to be present in the mLN anlagen of WT and TNF/Id2+/− mice but absent in TNF/Id2−/− mice (Figure 5, dashed lines), and in Id2−/− mice, in agreement with previous reports5. Myeloid cell migration to the mLN anlagen of TNF/Id2−/− mice was impaired and, strikingly, no LNs were formed in these mice. These results indicate that TNFα does not bypass the requirement for Id2 in lymphoid organogenesis and suggest that TNFα-producing F4/80+CD11b+ cells or NK cells are important for development of lymph nodes in TNF/Rorc(γt)−/− mice.
Figure 5. TNF/Id2−/− mice lack F4/80+ cells in the mLN anlagen and do not develop SLO.
mLN region of TNF/Id2+/− and TNF/Id2−/− at P0 stained with CD45, F4/80, LYVE-1 antibodies. Notice the absence of F4/80+ cells in the MLN region of Id2−/− and TNF/Id2−/− mice (dashed lines). Representative staining (n = 3/group). Scale bars; 250μm.
NK cells are not critical for development of mLN in TNF/Rorc(γt)−/− mice
Because Id2-deficient mice have defective NK cell development6 it remained possible that NK cells played a role in the formation of SLO. To test this hypothesis, we first examined if NK cells were present in the anlagen. As shown in Figure 3, NK cells were present in the mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− at P1. To determine if they played a role in SLO development we depleted them from TNF/Rorc(γt)−/− mice. To do so, we injected pregnant mothers at E15 and E18 with 200μg of isotype control or with the anti-NK monoclonal antibody PK136, which depletes NK cells in vivo. TNF/Rorc(γt)−/− offspring received additional injection of 100μg of control or PK136 on days 0, 3, 6 and 9. On day 15 the mLN were collected and the number of NK cells and the formation of mLN were examined (Supplementary Figure 2a and 2b). Treatment of TNF/Rorc(γt)−/− mice with anti-PK136 caused a complete reduction in the number of NK cells in the mLN (Supplementary Fig. 2a), but did not prevent normal development of mLN (Supplementary Fig. 2b). These results indicate that NK cells do not contribute significantly to SLO formation in TNF/Rorc(γt)−/− mice and suggest that the F4/80+ cells are the important cells in the process, as they are the sole source of TNFα in the TNF/Rorc(γt)−/− lymph node anlagen.
TNFα triggers expression of several genes involved in lymphoid organogenesis
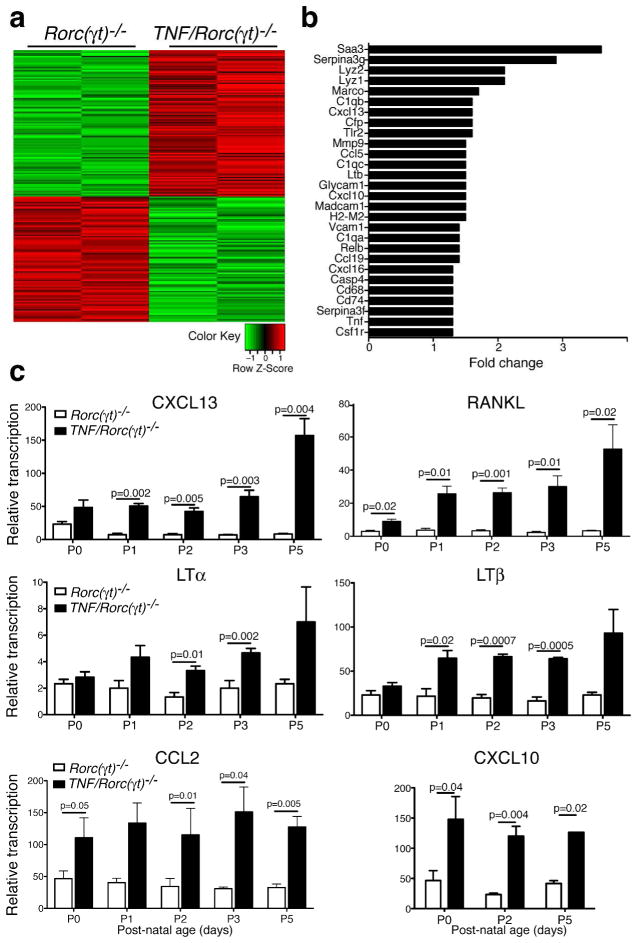
To determine how TNFα expression by myeloid cells could contribute to lymph node organogenesis, we compared the transcriptomes of the mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice at postnatal day 1 (P1), using the Ilumina gene arrays (Figure 6a and 6b). Consistent with a TNFα-driven signature, the highest expressed genes in the mLN of TNF/Rorc(γt)−/− mice were acute-phase response genes (Saa3 and Serpina-3g). Expression of several genes involved in lymphoid organogenesis such as Cxcl13, Lymphotoxin beta (LTb), Relb, Ccl19 and Madcam-1, was increased in the mLN of TNF/Rorc(γt)−/− mice. Expression of macrophage related genes (Lyz2, Lyz1, csfr1, Mmp9), MHC molecules (H2-M2) and chemokines (Ccl5, Cxcl10 and Cxcl16) were also increased in the mLN of TNF/Rorc(γt)−/− mice. To validate and extend these findings we performed qPCR analysis (Figure 6c). Expression of Cxcl13, RANKL, Ltb, was confirmed to be upregulated in the mLN of TNF/Rorc(γt)−/− animals at different stages of postnatal development. Interestingly, transcripts for LTa were significantly upregulated in the mLN of TNF/Rorc(γt)−/− mice after P1, but not at earlier time points. These results indicate that increased expression of TNFα promotes expression of genes involved in lymphoid tissue organogenesis during embryogenesis.
Figure 6. Increased expression of genes involved in lymph node organogenesis in the mLN of TNF/Rorc(γt)−/−mice.
(a–b) Transcriptional profiles of mLN anlagen of Rorc(γt)−/− and TNF/Rorc(γt)−/−mice at P1. Samples (each column corresponds to a pool of 2–3 anlagen) were compared using MouseRef-8 v2.0 Expression BeadChip. Quantile-normalized expression values were filtered for p < 0.01 and log fold change (logFC) > 1.25 (= fold 2.38). (a) Heatmap analysis sorted by logFC of the 193 resulting probe sets were Z-score normalized and subjected to hierarchical clustering; increased (red) decreased (green) expression in TNF/Rorc(γt)−/− compared to Rorc(γt)−/− mice. (b) Fold-change of selected up-regulated genes. (c) qPCR analysis of selected genes in the MLN region of Rorc(γt)−/− and TNF/Rorc(γt)−/−mice at different stages (n=3/group).
TNFα induces stromal cell maturation
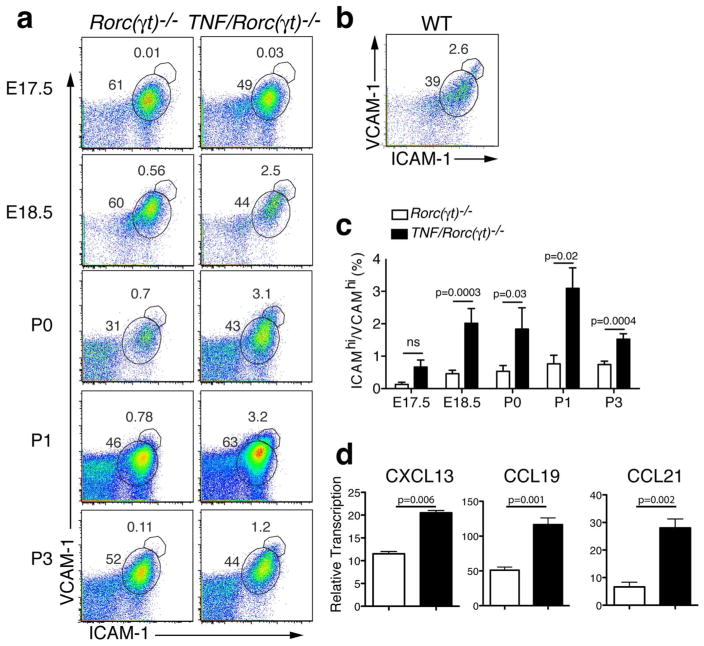
Maturation of mesenchymal stromal cells into organizer cell is a key step in lymphoid organogenesis1. The existing evidence suggests that activation of the stromal cells is mediated by interaction of LTα1β2 present on the RORγt+ LTi cells with LTβR expressed on stromal cells. This interaction leads to upregulation of ICAM-1, VCAM-1 and MAdCAM-1 expression on the surface of the stromal cells14, 32. To examine whether stromal maturation to “organizer” cells could occur in the absence of LTi cells, we analyzed the presence of ICAM-1hiVCAM-1hi cells in the mLN region of Rorc(γt)−/− and TNF/Rorc(γt)−/− (Figure 7a) and WT mice (Figure 7b) mice by flow cytometry. Cells were gated in the CD45− stromal cell population. ICAM-1hiVCAM-1hi cells were present at a significantly higher frequency in the mLN of TNF/Rorc(γt)−/− mice at E18.5 onwards when compared with the same region of Rorc(γt)−/− mice33 (Figure 7a and 7c). One day after birth the frequency of ICAM-1hiVCAM-1hi cells in mLN stroma of TNF/Rorc(γt)−/− was higher than that of Rorc(γt)−/− mice, but comparable to that of WT mice (Figure 7b). Another parameter of stromal cell activation is the production of chemokines. To examine if the stromal cells from TNF/Rorc(γt)−/− mice produced chemokines, we sorted CD45− cells from the mLN anlagen and performed QPCR analyses. Sorted stromal cells (CD45-) from the mLN anlagen of TNF/Rorc(γt)−/− mice at P1 expressed increased levels of Cxcl13, Ccl19 and Ccl21 mRNA when compared to sorted stromal cells of of Rorc(γt)−/− mice (Figure 7d). Taken together these results indicate that increased levels of TNFα are sufficient to induce lymph node stromal cell maturation in the absence of LTi cells.
Figure 7. TNF induces stromal cell activation.
(a) FACS analysis of single-cell suspensions from the mLN region of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice at E17.5, P0, P1, and P3 showing the increased expression of ICAM-1/VCAM-1 in the CD45 negative stromal cell population. (b) Expression of ICAM-1/VCAM-1 in stromal cell population in the mLN of WT and TNFΔARE/+ mice at P1. (c) Relative number of ICAM-1hiVCAM-1hi cells in the CD45 negative stromal cells in the mLN region of Rorc(γt)−/− and TNF/Rorc(γt)−/− at E17.5, E18.5, and P0 (n = 4 mice/group), P1 (n = 7 mice/group), and P3 (n= 6 mice/group). (d) Expression of CXCL13, CXCL19 and CCL21 in the stromal (CD45 negative) cell population sorted from the mLN region of Rorc(γt)−/− and TNF/Rorc(γt)−/− mice at P0.5-P1 (n = 9–10 anlagen/group).
TNFα overexpression does not bypass the requirement for LTβR signaling in lymphoid organogenesis
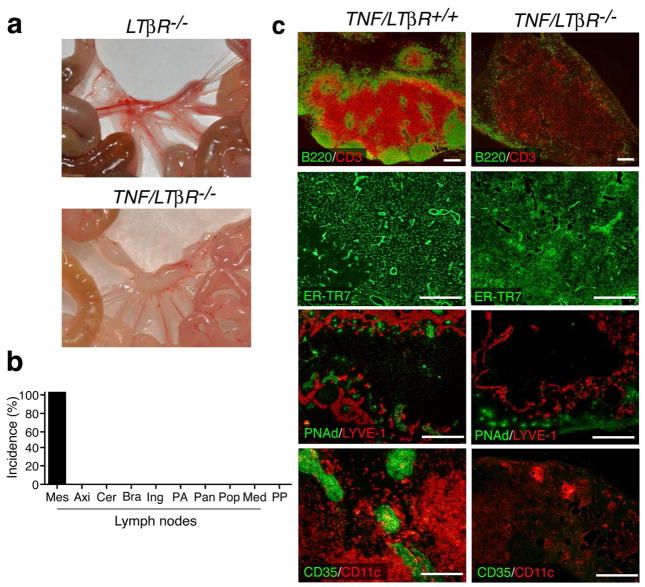
The high expression levels of LTβR ligands in the mLN of TNF/Rorc(γt)−/− mice after birth suggested a role for Lymphotoxin-LTβR signaling in the development of LN in this model. To determine if this was indeed the case, we crossed TNFΔARE/+ mice with LTβR-deficient animals (referred to as TNF/LTβR−/− mice) (Figure 8). With exception of mLN, no lymph nodes, Peyer’s Patches and TLO were found in any of the TNF/LTβR−/− mice animals examined (age 3–36 wk, n = 19) (Figures 8a, 8b and Supplementary Figure 3a). The mLN of TNF/LTβR−/− mice were markedly abnormal as shown by the absence of B cell follicles and T cell areas and the impaired recruitment of lymphocytes to these organs (Figure 8c). In addition, HEVs appear to be absent and the number of lymphatic vessels is also reduced. Finally, they lacked CD35bright FDC, and had an aberrant ER-TR7+ stroma.
Figure 8. TNF-driven formation of most SLO is dependent on LTβR signaling.
(a) Mesentery of LTβR−/− and TNF/LTβR−/− mice. (b) With the exception of mLN, SLO were absent in TNF/LTβR−/− mice (n = 16). (c) Abnormal organogenesis of mLN in TNF/LTβR−/− mice. mLN of TNF/LTβR+/+ and TNF/LTβR−/− mice were stained with the indicated antibodies. Notice the lack of distinct T and B cell areas, absence of of PNAd+ HEVs and reduced lymphatic vasculature, lack of CD35+ FDC, and disorganized ER-TR7 stroma in the MLN of TNF/LTβR−/− mice. Representative staining (n = 5/group). Scale bars = 250μm.
LTβR-deficient mice have severe splenic defects that include loss of T/B cell segregation and an abnormal stroma12. Interestingly, the spleen of TNF/LTβR−/− mice displayed normal T/B cell distribution, had MAdCAM-1+ cells and a normal ER-TR7+ cell network (Supplementary Figure 3b). Thus, TNFα overexpression can compensate for the absence of LTβR signaling and promote development of a normal spleen. Together the results indicate that TNFα overexpression corrects the splenic defects, but not the lack of SLO associated with abrogation of LTβR signaling.
Influx of hematopoietic cells into the anlagen of TNF/Rorc(γt)−/− mice
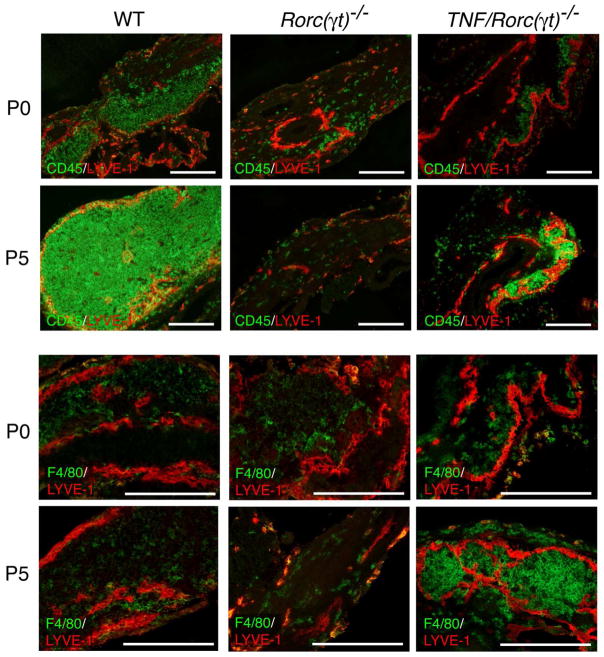
To gain further insights into the mechanisms of SLO formation, we compared the kinetics of hematopoietic cell recruitment to the mLN anlagen of WT, Rorc(γt)−/− and TNF/Rorc(γt)−/− mice. A significant number of CD45+ cells were present in mLN of WT mice at P0 while very few cells were detected in the mesenteric area of both Rorc(γt)−/− and TNF/Rorc(γt)−/− mice (Figure 9). In contrast, by P5 the mLN anlagen of TNF/Rorc(γt)−/− mice appeared to be populated by CD45+ cells while almost no bone marrow derived cells were present in a similar area in Rorc(γt)−/− mice. Consistent with our flow cytometric data, F4/80+ cells were present within the anlagen of WT and TNF/Rorc(γt)−/− mLNs, and their frequency was proportionally increased in the latter strain.
Figure 9. Hematopoietic cell influx into the mLN anlagen.
Sections of the mLN region of WT, Rorc(γt)−/− and TNF/Rorc(γt)−/− (P0 and P5) mice were stained with anti-CD45, -F4/80 and -LYVE-1 antibodies. Representative staining (n = 3/group). Scale bars = 250μm.
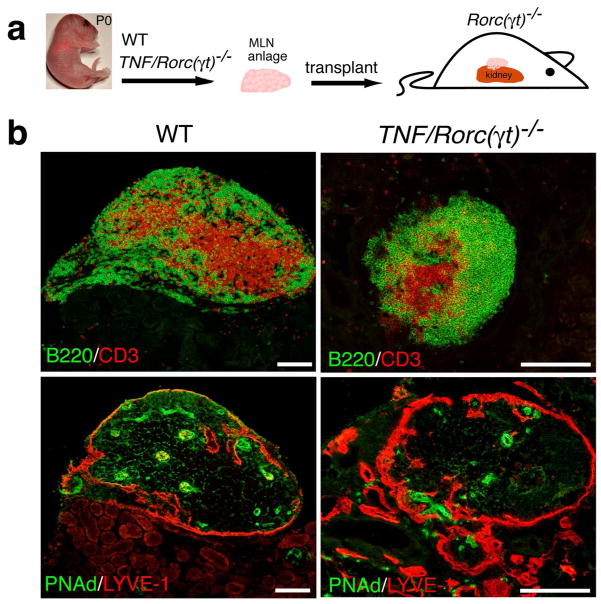
Grafting of TNF/Rorc(γt)−/− anlagen under the kidney capsule induces lymphoid neogenesis
Grafting of re-aggregates of embryonic and neonatal RORγt+LTi cells and LTo cells under the kidney capsule of adult mice promotes the formation of structures that resemble lymph nodes that recruit and organize host T and B cells34. At P0 the mLNs of WT mice contain mostly LTi cells and myeloid cells and are not organized. Kidney grafts of wild type mLNs result in organized tissues populated by host lymphocytes only 2–3 weeks after grafting. To test whether the anlagen of TNF/Rorc(γt)−/− mice could promote development of LN-like structures in adult mice, we grafted them under the kidney capsule of Rorc(γt)−/− recipient mice (Figure 10). Three weeks later the kidneys were removed and processed for histological analysis. All (100%) animals transplanted with WT mLN anlagen (n = 3) and 71% of those transplanted with TNF/Rorc(γt)−/− anlagen (n = 14) developed lymphoid aggregates under the kidney capsule. These aggregates, contained segregated T and B cell areas, HEV and lymphatic vessels, and were similar to host LN. We conclude that mLN anlagen of TNF/Rorc(γt)−/− mice can promote the formation of lymphoid organs at a non-predestined site in adult mice in the absence of RORγt+LTi cells, and that this response is not dependent on systemically increased levels of TNFα.
Figure 10. mLN anlagen from WT and TNF/Rorc(γt)−/− mice promote development of LN-like structures when grafted under the kidney capsule.
(a) Schematic representation of the transplantation experiment. (b) mLN anlagen isolated from WT (n = 3) and TNF/Rorc(γt)−/− (n = 14) newborn mice were grafted under the kidney capsule of Rorc(γt)−/− mice. Notice normal segregation of T and B cells and development of PNAd+ HEV from WT and TNF/Rorc(γt)−/− grafts. Scale bars = 250μm.
Discussion
RORγt is a transcription factor encoded by the Rorc gene whose expression is critical for development of embryonic LTi cells and other types of group 3 innate lymphoid cells35. Our results show that lymphoid organogenesis can occur in the absence of Rorc, provided that there is increased expression of TNF. Formation of most lymph nodes under these circumstances is dependent on LTβR signaling.
A body of work supports the notion that SLO and TLO development share common mechanisms. However, evidence first derived from analysis of CCL21-driven transgenic models36 and other models37, suggested that their development differs, as canonical LTi cells, critical for SLO development, were shown not to be absolutely required for TLO development. Here we show that the generation of TLO in the ileum of TNFΔARE/+ animals is also independent on RORγt+ LTi cells. Our results complement those of Eberl and colleagues that showed that Rorc(γt)-deficient mice that received an inflammatory insult such as DSS-induced colitis, develop TLO in the colon27. Together these studies demonstrate that inflammatory stimuli promote development of TLO in different areas of the intestine (ileum and colon) in the absence of RORγt+ LTi cells, and implicate TNFα as an important factor in their generation, since its expression is elevated in the ileum of TNFΔARE/+ mice and in the colon of DSS-treated animals29, 38. Importantly, the development of TLO in the intestine of TNF/Rorc(γt)−/− mice also demonstrates that the formation of these organs is independent of Th17+ cells and other Rorc(γt)-dependent members of the growing family of innate lymphoid cells.
While recent experimental evidence supports the concept that TLO formation can occur in the absence of canonical LTi cells, the bulk of the literature suggests that they are critical for development of SLO. The current model for SLO formation suggests that Id2+RORγt+CD3−CD4+IL-7Rα+ LTα1b2+RANK+RANKL+ LTi cells are key drivers of lymphoid organogenesis based on the fact that Id2-, Rorc-, IL-7Ra- and LTa-deficient mice lack SLO 1, 39. Exceptions to this rule include nasal associated lymphoid tissue (NALT), whose formation takes place after birth and is not dependent on LTα and Ror(c)40 and milky spots of the omentum and fat associated lymphoid clusters is also independent of LTi cells41. Here we show that many SLO can form in the in absence of RORγt+LTi cells, provided that the basal levels of TNFα are increased. Mesenteric, axillary and cervical lymph nodes were found in 60–100% of TNF/Rorc(γt)−/− mice. Other lymph nodes such as brachial, inguinal, para aortic and peripancreatic were found in more than 10% of the mice, whereas popliteal and mediastinal lymph nodes were not detected. The lymph nodes detected in TNF/Rorc(γt)−/− mice were positioned in the same region as WT nodes, had similar architecture and cellularity, and could mount an efficient immune response after immunization.
How could TNFα promote organogenesis in the absence of LTi cells? Here we show that TNFα is produced at higher levels during embryogenesis in the TNF/Rorc(γt)−/− than in Rorc(γt)−/− mice, and that the TNFR1 receptor, is expressed by stromal cells. Stromal cell activation by LTi cells is critical for the generation of lymph nodes. LTα1β2 - expressed by LTi cells binds to LTβR expressed by stromal cells, which activates both canonical and non-canonical NF-κB signaling pathways to promote the latter cells to become mature stromal “organizer” cells that express increased levels of ICAM-1, VCAM-1 and MAdCAM-1 and the B and T cell chemoattractants CXCL13, CCL19 and CCL211, 39. We suggest that increased levels of TNFα functionally compensated for the lack of LTβR signaling during embryogenesis and contributed to the maintenance of a functional anlagen. This hypothesis is supported by our observations that stromal cells in the anlagen of TNF/Rorc(γt)−/−, but not in the Rorc(γt)−/− mice, are activated. They express higher levels of ICAM-1 and VCAM-1 during embryogenesis and immediately after birth. Furthermore, cells in the TNF/Rorc(γt)−/− anlagen expressed increased levels of the TNFα-inducible chemokines CCL2 and CXCL10. These chemokines, acting in concert with TNFα, could promote recruitment of additional hematopoietic cells. At birth, influx of hematopoietic cells could further contribute to organogenesis. Interestingly, we noted that the expression of LT ligands increased after birth. This could reflect either increased expression of LT ligands by resident non-LTi cells or reflect increased influx of hematopoietic cells that express LT ligands. We favor the second hypothesis because we have observed increased influx of hematopoietic cells in the TNF/Rorc(γt)−/− anlagen during the first 5 days of life. The increased expression of LT ligands is absolutely critical for normal lymph node development as shown by the analysis of the TNF/LTβR mutants. In the newborn TNF/Rorc(γt)−/− anlagen, LT ligands could potentially synergize with TNFα to activate the transcription of several molecules related to lymph node organogenesis, macrophage function, and inflammation. A recent report has shown the synergistic effect of TNFα signaling together with the alternative NF-kB pathway to drive high expression levels of Ccl21, Cxcl13, Vcam-1, Icam-1 and Madcam-1 in spleens42. Of note, we have detected expression of Cxcl13, Ccl19 and Ccl21, by stromal cells located in the mLN of TNF/Rorc(γt)−/− mice at P1. These chemokines induce lymphoid organogenesis when expressed in vivo43–45, and their expression by stromal cells in the anlagen could account for the formation of LN in TNF/Rorc(γt)−/−mice.
Myeloid cells are essential for the maintenance46, organization47, and vascularization48 of TLO. Results presented here suggest that they may also contribute to development of SLO, working in part as LTi-like cells. We show here that TNFα is expressed by myeloid cells present in the anlagen of WT mLN as early as E15.5. Clusters of fetal CD11b+ cells and LTi cells are observed at early stages of WT LN development5. The generation of the CD11b+ cells does not require RORγt because these myeloid cells are still present in the LN anlagen of Rorc(γt)−/− mice, as shown here and in 5. The increased number of myeloid cells that express stable TNFa mRNA contribute to high levels of this protein in the lymph node anlagen in TNF/Rorc(γt)−/− mice. Consistent with a role for myeloid cells contributing to lymph node development in the TNF/Rorc(γt)−/− mouse model, is the observation that absence of these cells (and thus the source of TNFα necessary to activate the stromal cells) in lymph node anlagen of TNF/Id2−/− mice resulted in the failure to rescue the formation of these organs.
Our results indicate that the development of lymph nodes in TNF/Rorc(γt)−/− mice appears to be delayed when compared to their WT counterparts. In addition, it is not clear why Peyer’s patches and some lymph nodes do not develop in this mouse model. The development of these structures may be dependent on the local numbers or phenotype of myeloid cells in those locations. Additional research will be necessary to uncover the origin of these myeloid cells and the factors mediating their influx into different lymph node anlagen.
TNFα receptor ligation activates the NF-κB classical pathway, which involves the IκB kinase and results in the activation of RelA. LTβR ligation activates both the NF-κB classical and alternative pathways18. The alternative NF-κB pathway is mediated by the NF-κB-inducing kinase (NIK) and results in the activation of NF-kB2/Relb. Because animals genetically deficient in LtbR, Nik, and RelB do not form lymph nodes12, 17, 18 it was concluded that the alternative pathway is critically important for the generation of SLO18. However, simultaneous deletion of TNFR1 and RelA precludes formation of all LN and PP in double knockout mice due to a stromal cell defect, even in the presence of LTi cells expressing normal levels of Lymphotoxin22, which suggests that the canonical NF-kB pathway is physiologically important for normal development. It is clear however, that LTβR signaling has a profound effect in the generation of most LN and intestinal TLO, a role that cannot be bypassed even in the presence of increased levels of TNFα. While increased TNFα cannot compensate for the lack of LTβR in terms of TLO and SLO development, it can partially compensate for lack of LTβR-signaling in the development of mLN. Finally, as shown here, increased TNFα expression can correct the splenic defects associated with lack of LTβR. These results are in agreement with studies that show that TNFα overexpression can correct splenic defects associated with LTα-deficiency49, 50. Taken together, the studies highlight a significant cross-talk between these receptor systems for the development and function of lymphoid structures.
In summary: Our results support a model of LN development in TNF/Rorc(γt)−/− mice where increased expression of TNFα by F4/80+CD11b+ cells is sufficient to promote the homeostasis of lymph node stromal cells up to early postnatal life. After birth the recruitment of lymphoid cells and myeloid cells to the anlagen initiates a series of cross-talk interactions with stromal cells through LTα-LTβR signaling that induces the expression of chemokines and cell adhesion molecules to organize specific lymphoid areas and attract further cells to form the proper lymph node structure containing HEVs and lymphatic vasculature. Failure of signaling through LTβR in early postnatal life results in the collapse of the anlagen of most lymph nodes with the exception of mesenteric LN that present with a disrupted architecture as shown in TNF/LTβR−/− mice. Thus, our results show that lymphoid tissue organogenesis can occur in the absence of Rorγt+ LTi cells and suggest that interactions between TNFα-expressing myeloid cells and stromal cells have an important role in this process.
Materials and Methods
Mice
TNFΔARE/+ and LTβR−/− mice have been described12, 23. Id2−/− mice were a generous gift from Dr. Y. Yokota (University of Fukui, Japan)6. C57BL6/J and Rorc(γt)−/− mice were obtained from the Jackson Laboratories and bred in our facility. All mice were housed under specific-pathogen-free conditions in individually ventilated cages at the Mount Sinai School of Medicine Animal Facility. All experiments were performed following institutional guidelines. For timed pregnancies, the day of vaginal plug was considered as E0.5.
Immunostaining
Sections of frozen tissues were subjected to immunofluorescent staining as described36 (for details see Supplemental Procedures).
Cell isolation and flow cytometry
The area of the mesentery corresponding to where mLN are found in WT mice was microdissected from Rorc(γt)−/− and TNF/Rorc(γt)−/− animals and analyzed by flow cytometry (for details see Supplemental Procedures).
In vivo immunization
TNF/Rorc(γt)+/+ and TNF/Rorc(γt)−/− mice at 6–8 wks were immunized with ovalbumine (OVA, grade V; Sigma-Aldrich) by intragastric gavage of 100μg of OVA + 20μg cholera toxin (List Biological Laboratories) on seven occasions at 7 day intervals. One week after the last immunization, mice were killed and the mLN was collected for cytokine analysis (for details, please see Supplemental Procedures)
Analysis of mRNA expression
Total RNA was extracted from mesenteric region using the RNeasy mini Kit (Qiagen) as described36 (for details see Supplemental Procedures).
Microarray analysis
Microarrays were done with the Illumina TotalPrepTM RNA Amplification Kit (for details please see Supplemental Procedures).
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software). Differences among means were evaluated by a 2-tailed t test. P < 0.05 was considered significant. All results shown represent mean ± SEM
Transplantation of mesenteric lymph node anlagen
Rorc(γt)−/− mice (6–8 weeks) were anesthetized with ketamine/xylazine solution. A small incision in the skin and peritoneum was made in order to expose the kidney. A slight pressure to both sides of the incision was applied in order to exteriorize the kidney. A small nick in the kidney capsule was created using a 25 gauge needle, and the mesenteric lymph node anlagen was placed into the kidney capsule pocket created in the nick area. The peritoneum and skin were stitched using 5-0 silk sutures w/ a C-6 19mm needle. Formation of lymph nodes under the kidney capsule was assessed by histology 3 weeks after transplantation.
Supplementary Material
Acknowledgments
This work was supported by NIH grant 2 P01 DK072201-06A1 (to SAL). Work in JC laboratory was supported by the EU FP7 integrated project INFLACARE, a BBSRC project grant and by the College of Medical and Dental Sciences-University of Birmingham, UK.
Footnotes
Competing financial interests
The authors declare no financial interests
References
- 1.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192(10):1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 5.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 6.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397(6721):702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 7.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 8.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 9.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264(5159):703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 11.Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, et al. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182(9):5439–5445. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9(1):59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 13.Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, et al. A lymphotoxin-beta-specific receptor. Science. 1994;264(5159):707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 14.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173(5):2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 15.Okuda M, Togawa A, Wada H, Nishikawa S. Distinct activities of stromal cells involved in the organogenesis of lymph nodes and Peyer’s patches. J Immunol. 2007;179(2):804–811. doi: 10.4049/jimmunol.179.2.804. [DOI] [PubMed] [Google Scholar]
- 16.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155(4):1685–1693. [PubMed] [Google Scholar]
- 17.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24(2):429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 18.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 19.Rennert PD, Browning JL, Hochman PS. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int Immunol. 1997;9(11):1627–1639. doi: 10.1093/intimm/9.11.1627. [DOI] [PubMed] [Google Scholar]
- 20.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184(5):1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9(1):71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 22.Alcamo E, Hacohen N, Schulte LC, Rennert PD, Hynes RO, Baltimore D. Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs. J Exp Med. 2002;195(2):233–244. doi: 10.1084/jem.20011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro T, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196(12):1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305(5681):248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29(2):261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170(11):5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 27.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208(1):125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci U S A. 1992;89(21):10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viejo-Borbolla A, Martin AP, Muniz LR, Shang L, Marchesi F, Thirunarayanan N, et al. Attenuation of TNF-driven murine ileitis by intestinal expression of the viral immunomodulator CrmD. Mucosal Immunol. 2010;3(6):633–644. doi: 10.1038/mi.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature. 2007;446(7135):547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 32.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193(5):621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, et al. Ontogeny of stromal organizer cells during lymph node development. J Immunol. 2010;184(8):4521–4530. doi: 10.4049/jimmunol.0903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, et al. Lymphotoxin adependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. 2007;110(6):1950–1959. doi: 10.1182/blood-2007-01-070003. [DOI] [PubMed] [Google Scholar]
- 35.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 36.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116(10):2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107(2):353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10(9):664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 40.Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-alpha (LT alpha) and retinoic acid receptor-related orphan receptor-gamma, but the organization of NALT is LT alpha dependent. J Immunol. 2002;168(3):986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 41.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30(5):731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovas A, Weidemann A, Albrecht D, Wiechert L, Weih D, Weih F. p100 Deficiency is insufficient for full activation of the alternative NF-kappaB pathway: TNF cooperates with p52-RelB in target gene transcription. PLoS One. 2012;7(8):e42741. doi: 10.1371/journal.pone.0042741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12(5):471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 44.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol. 2006;177(10):7296–7302. doi: 10.4049/jimmunol.177.10.7296. [DOI] [PubMed] [Google Scholar]
- 45.Marchesi F, Martin AP, Thirunarayanan N, Devany E, Mayer L, Grisotto MG, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2(6):486–494. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 46.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206(12):2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206(11):2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muniz LR, Pacer ME, Lira SA, Furtado GC. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol. 2011;187(2):828–834. doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexopoulou L, Pasparakis M, Kollias G. Complementation of lymphotoxin alpha knockout mice with tumor necrosis factor-expressing transgenes rectifies defective splenic structure and function. J Exp Med. 1998;188(4):745–754. doi: 10.1084/jem.188.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Victoratos P, Lagnel J, Tzima S, Alimzhanov MB, Rajewsky K, Pasparakis M, et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24(1):65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.