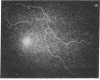
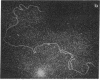
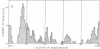
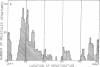
Abstract
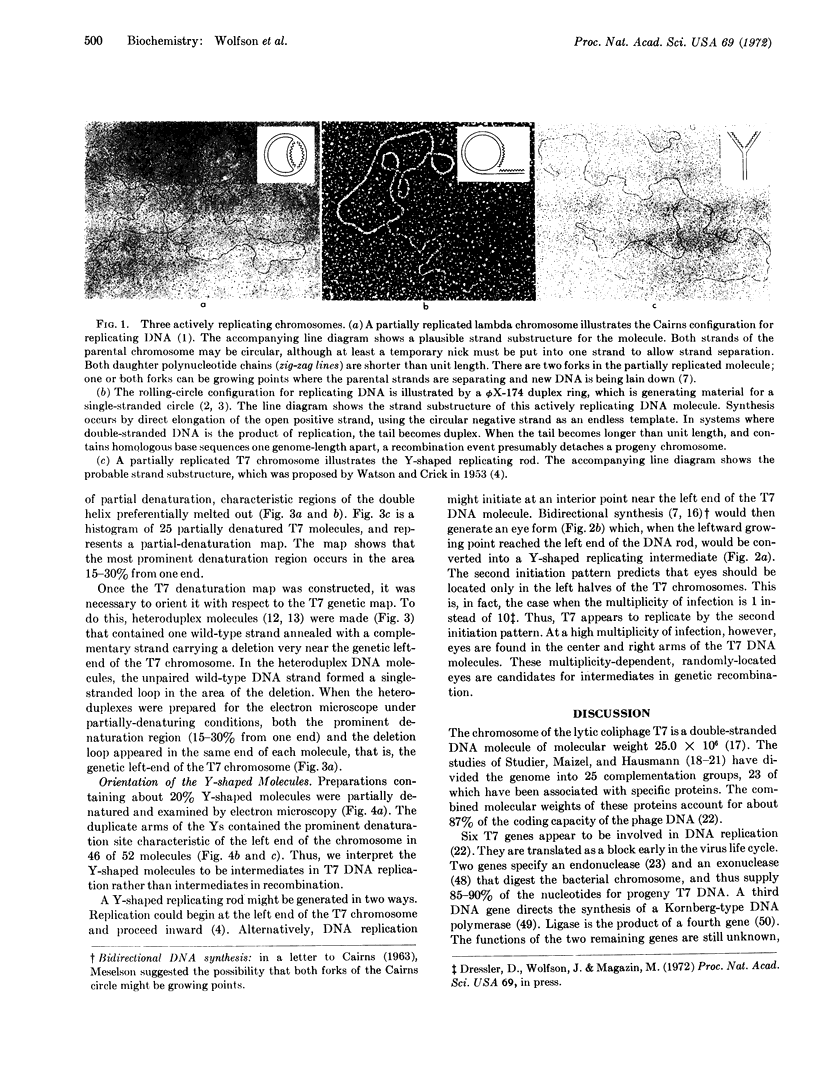
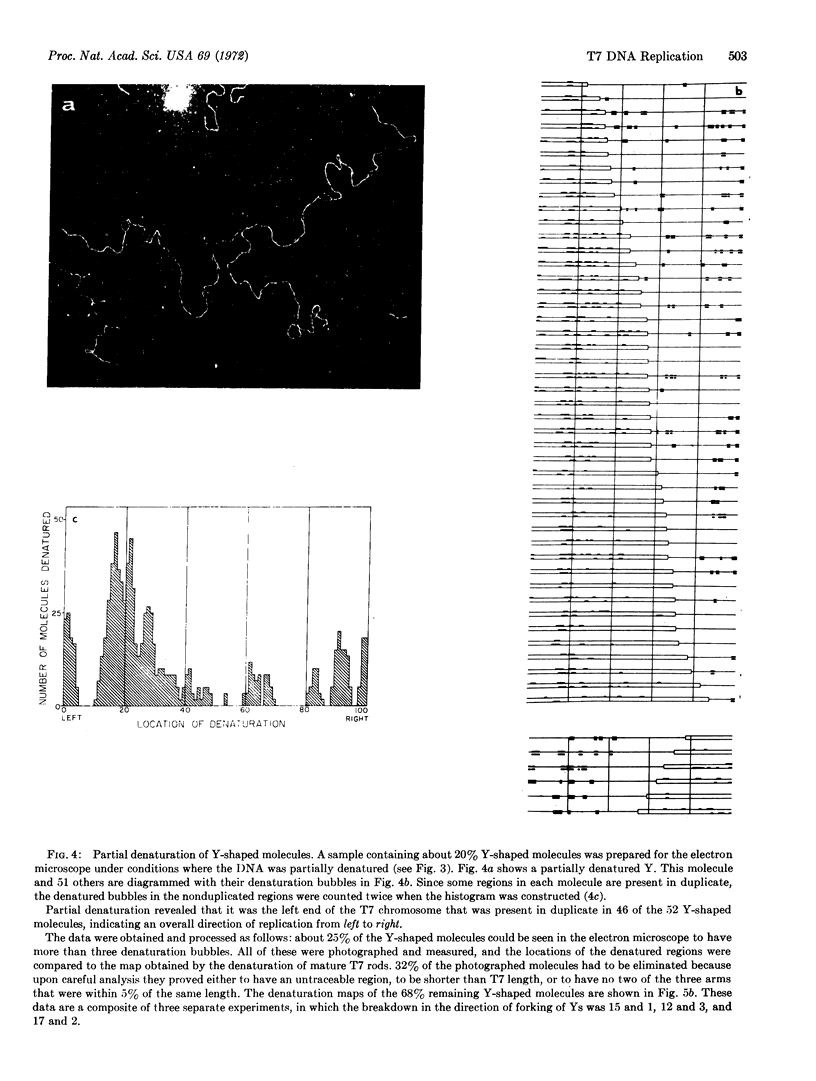
The T7 chromosome in the first round of replication is a Y-shaped DNA rod. Thus, it differs from previously observed bacterial and viral replicating chromosomes that are circular.
Keywords: gradient centrifugation, electron microscopy, E. coli, DNA partial denaturation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Effects of 9-aminoacridine on bacteriophage T4 deoxyribonucleic acid synthesis. J Mol Biol. 1970 Jun 14;50(2):263–277. doi: 10.1016/0022-2836(70)90191-9. [DOI] [PubMed] [Google Scholar]
- Bode H. R., Morowitz H. J. Size and structure of the Mycoplasma hominis H39 chromosome. J Mol Biol. 1967 Jan 28;23(2):191–199. doi: 10.1016/s0022-2836(67)80026-3. [DOI] [PubMed] [Google Scholar]
- Botstein D., Matz M. J. A recombination function essential to the growth of bacteriophage P22. J Mol Biol. 1970 Dec 28;54(3):417–440. doi: 10.1016/0022-2836(70)90119-1. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Davidson N. Electron-microscopic visualization of deletion mutations. Proc Natl Acad Sci U S A. 1968 May;60(1):243–250. doi: 10.1073/pnas.60.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Dressler D. The rolling circle for phiX DNA replication. II. Synthesis of single-stranded circles. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1934–1942. doi: 10.1073/pnas.67.4.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Wolfson J. The rolling circle for phi X DNA replication. 3. Synthesis of supercoiled duplex rings. Proc Natl Acad Sci U S A. 1970 Sep;67(1):456–463. doi: 10.1073/pnas.67.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Bacteriophage T3- and T7-directed deoxyribonucleases. J Virol. 1968 Mar;2(3):265–266. doi: 10.1128/jvi.2.3.265-266.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Ihler G. M., Thomas C. A., Jr Equal incorporation of both parental bacteriophage T7 deoxyribonucleic acid strands into intracellular concatemeric deoxyribonucleic acid. J Virol. 1970 Dec;6(6):877–880. doi: 10.1128/jvi.6.6.877-880.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Replicating DNA: structure of colicin factor E1. Science. 1970 Aug 7;169(3945):590–592. doi: 10.1126/science.169.3945.590. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. DNA of vegetative bacteriophage lambda. VI. Electron microscopic studies of replicating lambda DNA. Proc Natl Acad Sci U S A. 1971 Jan;68(1):112–115. doi: 10.1073/pnas.68.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R. H., Wolstenholme D. R., Gross N. J. Replicating molecules of circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1466–1472. doi: 10.1073/pnas.60.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davidson N. Physicochemical studies on the minicircular DNA in Escherichia coli 15. Biochim Biophys Acta. 1970 Apr 15;204(2):285–295. doi: 10.1016/0005-2787(70)90146-2. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Masamune Y., Frenkel G. D., Richardson C. C. A mutant of bacteriophage T7 deficient in polynucleotide ligase. J Biol Chem. 1971 Nov 25;246(22):6874–6879. [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. I., Fuke M. Replication of bacteriophage DNA, II. Structure of replicating DNA of phage lambda. Proc Natl Acad Sci U S A. 1968 Jul;60(3):861–865. doi: 10.1073/pnas.60.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Ihler G. Strand selection during bacterial mating. Cold Spring Harb Symp Quant Biol. 1968;33:647–650. doi: 10.1101/sqb.1968.033.01.073. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Smith M. G., Skalka A. Some properties of DNA from phage-infected bacteria. J Gen Physiol. 1966 Jul;49(6):127–142. doi: 10.1085/jgp.49.6.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Hausmann R. Integration of two sets of T7 mutants. Virology. 1969 Nov;39(3):587–588. doi: 10.1016/0042-6822(69)90106-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. The structure of DNA. Cold Spring Harb Symp Quant Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- Werner R. Distribution of growing points in DNa of bacteriophage T4. J Mol Biol. 1968 May 14;33(3):679–692. doi: 10.1016/0022-2836(68)90313-6. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]