Abstract
Sirolimus (SRL) is a promising alternative to calcineurin inhibitors, such as tacrolimus (TAC), in kidney transplant recipients (KTRs), but the immunological benefits of conversion from calcineurin inhibitors to SRL are not fully investigated. In the present study, we evaluated the effect of conversion from TAC to SRL on the T helper type 17/regulatory T (Th17/Treg) axis in three separate studies. First, the effect of SRL on the Th17/Treg axis was evaluated in vitro using peripheral blood mononuclear cells (PBMCs). Second, the effect of conversion from TAC to SRL on the Th17/Treg axis was studied in KTRs. Finally, the effect of SRL on CD8+ Treg cells was evaluated. In vitro analysis of PBMCs isolated from KTRs showed that SRL suppressed Th17 cell differentiation but TAC did not. Conversion from TAC to SRL markedly decreased the number of effector memory CD8+ T cells and significantly increased the proportion of CD4+ and CD8+ Treg cells compared with TAC in KTRs. SRL treatment induced the CD8+ Treg cells, and these cells inhibited the proliferation of allogeneic CD4+ T cells and Th17 cells. In conclusion, conversion from TAC to SRL favourably regulates Th17 and Treg cell differentiation in KTRs. These findings provide a rationale for conversion from TAC to SRL in KTRs.
Keywords: cytokines, mTOR, regulatory T cells, T cells, transplantation
Introduction
Recent advances in immunosuppression methods have led to a marked improvement in allograft survival after kidney transplantation.1,2 Calcineurin inhibitors (CNIs) such as tacrolimus (TAC) are currently most widely used and have significantly reduced the episodes of acute rejection compared with previous regimens containing azathioprine2. However, long-term treatment with CNIs is associated with complications such as nephrotoxicity, diabetes mellitus and secondary malignancies.3–5 Additionally, CNIs induce undesirable changes in the immune response after kidney transplantation, including a reduction in the proportion and function of CD4+ regulatory T cells (CD4+ Treg cells) within the peripheral blood mononuclear cell (PBMC) population,6–8 and an up-regulation of T helper type 17 (Th17) cell-associated pathways, which are involved in allograft rejection.9–17 Hence, CNI-based immunosuppression may in fact contribute to the progression of chronic allograft dysfunction in kidney transplant recipients (KTRs).
During the late 1990s, sirolimus (SRL), a mammalian target of rapamycin (mTOR) inhibitor, was introduced as an immunosuppressive agent after kidney transplantation.18–20 mTOR is involved in modulating adaptive immune response and regulating T-cell polarization towards CD4+ or CD8+ T cells.21–26 Hence, unlike CNIs, SRL may have potential immunoregulatory effects. Clinical studies show that, compared with CNIs, SRL reduces chronic allograft nephropathy27 and chronic vascular changes, and improves long-term renal function.28 However, the mechanisms underlying the immunological benefits of SRL have not been fully investigated. Therefore, the aim of the present study was to investigate the immunosuppressive effects of SRL on T-cell-mediated immune responses involving the Treg/Th17 pathway, and compare them with those of TAC. We also investigated the immunoregulatory effects of SRL in KTRs by examining CD8+ Treg cells, which are known to suppress Th17 cells.
Materials and methods
Patients and clinical information
Five KTRs (within 6 months after transplantation) who received TAC as the main immunosuppressant and showed stable allograft function were enrolled in the study. Eleven healthy volunteers were also enrolled as controls. All the subjects provided written informed consent in accordance with the Declaration of Helsinki. An additional five KTRs receiving TAC and showing stable allograft function without acute rejection episode were enrolled for the in vivo conversion study (Table 1). The conversion from TAC to SRL was performed as previously described.29 Briefly, on the day of conversion, SRL (2 mg/day) was introduced along with a simultaneous 50% reduction in the TAC dose. The target SRL trough level was 8–12 ng/ml. After achieving the target trough level, CNI was withdrawn on day 14. The immune cell subsets within the PBMC population were examined both before and 1 month after conversion. The study was approved by the Institutional Review Board of Seoul St Mary's Hospita l (KC10SISI0235).
Table 1.
Baseline clinical characteristics of patients (n = 5)
| Parameter | Value |
|---|---|
| Age (years) | 39·0 ± 6·6 |
| Male gender, n (%) | 5 (100) |
| Primary renal disease | |
| Chronic glomerulonephritis, n (%) | 3 (60) |
| Hypertension, n (%) | 2 (40) |
| Duration from kidney transplant | 4·9 ± 2·3 |
| Mean trough tacrolimus level at conversion (ng/ml) | 5·3 ± 1·9 |
KT, kidney transplantation; Tac, tacrolimus.
Isolation and purification of CD4+ and CD8+ T cells from the PBMCs
Peripheral blood mononuclear cells were isolated from heparinized blood samples by Ficoll–Hypaque (GE Healthcare, Pittsburgh, PA) density-gradient centrifugation. The isolated cells were cultured as previously described.30 All five KTRs and the healthy individuals were Korean, aged 25–40 years, non-smokers, and showed no evidence of recent infection. In addition, the effects of SRL were examined ex vivo in five patients who had previously undergone kidney transplantation at Seoul St Mary's Hospital and had consented to participate in a clinical study to examine the effects of conversion from Tac (Prograf, Astellas Pharma, Tokyo, Japan) to SRL (Rapamune, Wyeth Pharma, Madison, NJ). Informed consent was obtained from all the patients, and the current study to examine the effects of conversion from TAC to SRL was approved by the Institutional Review Board (KC11OISI0917) of Seoul St Mary's Hospital. All the clinical investigations were conducted according to the principles set forth in the Declaration of Helsinki. CD4+ T cells were isolated from the PBMCs of healthy individuals using monoclonal anti-human CD4 antibody conjugated to microbeads (MicroBeads; Miltenyi Biotech, Bergisch Gladbach, Germany). To induce CD8+ Treg cells, PBMCs (1 × 106/ml) were cultured in 24-well plates in RPMI-1640 medium supplemented with penicillin/streptomycin/glutamine, 10% fetal calf serum, 5 ng/ml recombinant interleukin-15 (IL-15) 0·1 ng/ml anti-CD3 and 50 ng/ml SRL. After 6 days, CD8+ T cells were obtained by sorting CD8+ CCR7+ T cells using phycoerythrin (PE) -conjugated CCR7 (BD Biosciences, San Jose, CA), allophycocyanin (APC) -conjugated CD8 (BD Biosciences, San Jose, CA), and a FACSAria III cell sorter (BD Biosciences). The purity of the cell population was consistently > 90%.
Effects of TAC or SRL on Th0 and Th17 cells in vitro
Peripheral blood mononuclear cells (5 × 105) were isolated from five KTRs and four healthy individuals and incubated for 48 hr with anti-CD3 (1 µg/ml) and anti-CD28 (1 μg/ml) monoclonal antibodies (mAbs) to induce Th0 cells, or with anti-CD3 (1 μg/ml), anti-CD28 (1 μg/ml), IL-1β (20 ng/ml), IL-6 (20 ng/ml), and IL-23 (20 ng/ml) to induce Th17 cells. To examine the immunosuppressive effects of TAC and SRL, PBMCs isolated from healthy individuals and KTRs were pre-incubated for 1 hr with TAC or SRL, and then stimulated as described above to induce Th0 or Th17 cells. Interferon-γ (IFN-γ) -neutralizing antibody (2 μg/ml) (25723; R&D Systems, Inc. Minneapolis, MN) and IL-4-neutralizing antibody (2 μg/ml) (34019; R&D Systems, Inc.) were also added to the appropriate samples.
Flow cytometry analysis
In all cases, flow cytometry analysis was performed within a few hours after collection of peripheral blood. The cells were surface-stained with different combinations of the following mAbs: CD4-PE/Cy7 (RPA-T4, IgG1; BioLegend, San Diego, CA), CD8-APC (RPA-T8, IgG1,κ; Pharmingen, San Diego, CA), CD45RA-FITC (HI100, IgG2b, κ; Pharmingen), and CD25-APC (M-A251, IgG1, κ; Pharmingen). An anti-CCR7 antibody was used to detect chemokine receptors (3D12, IgG2a, κ; Pharmingen). For intracellular staining, the cells were washed, fixed, permeabilized and incubated with mAbs against IL-17 (PE, eBio64dec17, IgG1, κ; eBioscience, San Diego, CA), IFN-γ (FITC, 4S.B3, IgG1, κ; eBioscience; and PE, B27, IgG1, κ; Pharmingen), IL-4 (APC, MP4-25D2, IgG1, κ; eBioscience), IL-17 (FITC, eBio64DEC17, IgG1, κ; eBioscience), and Foxp3 (FITC, PCH101, IgG2a, κ; eBioscience). Appropriate isotype controls were used for gating purposes. Cells were analysed using a FACS Calibur flow cytometer (BD Biosciences). The data were analysed using the flowjo software (Tree Star, Ashland, OR).
Real-time RT-PCR
Messenger RNA was extracted in PBMCs from five KTRs using the TRIzol Reagent (Molecular Research Center, Inc., Cincinnati, OH), according to the manufacturer's instructions. Complemetary DNA was synthesized in a PerkinElmer Cetus DNA thermal cycler (PerkinElmer, Inc, Waltham, MA) using the SuperScript Reverse Transcription system (Takara, Shiga, Japan).
Real-time PCR
A LightCycler 2.0 instrument (Roche Diagnostics; software version 4.0, Indianapolis, IN) was used for PCR amplification. All the PCRs were performed using LightCycler FastStart DNA Master SYBR Green I (Takara), according to the manufacturer's instructions. The following primers were used for each molecule: for IL-17, 5′-CAA CCG ATC CAC CTC ACC TT-3′(sense) and 5′-GGC ACT TTG CCT CCC AGA T-3′(antisense); for Foxp3, 5′-CAC TGC CCC TAG TCA TGG T-3′(sense) and 5′-GGA GGA GTG CCT GTA AGT GG-3′(antisense); and for β-actin, 5′-GGA CTT CGA GCA AGA GAT GG-3′(sense) and 5′-TGT GTT GGG GTA CAG GTC TTTG-3′(antisense). Housekeeping genes (β-actin) were amplified for normalization. Heat-map images were analysed using the template designated by the manufacturer.
ELISA
Levels of IL-17 and IL-22 in the culture supernatants from five KTRs were measured using sandwich ELISA (R&D Systems) according to the manufacturer's instructions. Absorbance at 405 nm was measured using an ELISA microplate reader (Molecular Devices, Sunnyvale, CA).
Western blot analysis
CD4+ Tcells (1 × 107) were isolated from three healthy individuals. To examine the immunosuppressive effects of TAC and SRL, CD4+ T cells were pre-incubated for 1 hr in the presence of TAC or SRL and then stimulated as described above for another 1 hr. The membrane was then incubated overnight at 4° with primary antibodies against the following: phosphorylated mTOR, mTOR, phosphorylated signal transducer and activator of transcription 3 (STAT3; 705), STAT3, phosphorylated Akt, Akt (all antibodies were from Cell Signaling Technology Inc., Danvers, MA), and β-actin (Sigma, St Louis, MO). After washing in TTBS (0.1% Tween 20, Tris-buffered saline), the reactive bands were visualized using an enhanced chemiluminescence (ECL) detection kit and Hyperfilm-ECL reagents (Amersham Pharmacia, Piscataway, NJ).
Suppression assay
Peripheral blood mononuclear cells were collected from healthy donors (n = 4). The cells were then stimulated with anti-CD3 (1 μg/ml) and T-cell-depleted, irradiated antigen-presenting cells in the presence or absence of CD8+ Treg (CD8+ CCR7+) cells isolated using a cell sorter (Beckman MoFlo, Brea, CA) followed by differentiation in response to a plate-bound anti-CD3 antibody (1 μg/ml) and recombinant human (rh) IL-15 (50 ng/ml) in the presence of SRL. The purity of all T-cell subsets was > 95% as determined by FACS analysis (data not shown). Isolated effector cells were > 95% pure. We used effector T and CD8+ Treg cells from the same donor. For the Treg suppression assay, CD4+ effector T cells (1 × 105) were co-cultured with T-cell-depleted, irradiated antigen-presenting cells (1 × 105), an anti-CD3 antibody (1 μg/ml), and the CD8+ CCR7+ Treg cells (5 × 104) for 3 days. The proliferation of CD4+ T cells was examined by adding [3H]thymidine (1 μCi/well; GE Healthcare) to the culture incubated for 8 hr. The level of [3H]thymidine incorporation was measured using a liquid β-scintillation counter (Beckman).
Statistical analysis
Statistical analysis was performed using the spss software (version 16.0; SPSS Inc., Chicago, IL). Data before transplantation and at 1 and 3 months after transplantation were compared using a paired t-test or one-way analysis of variance. Chi-square frequency analysis was used to examine categorical variables. A non-parametric, Wilcoxon signed-rank test was used for T-cell suppression, cytokine production and gene expression comparisons between control and treatments. Analysis of pre-conversion versus post-conversion measurements of immune assays and clinical outcomes was performed using Wilcoxon's signed-rank test. Chi-squared/Fisher's exact test was used for categorical measures. The results were expressed as mean ± standard deviation (SD). A P value of < 0·05 was considered significant.
Results
SRL, but not TAC, suppresses Th1, Th2 and Th17 cells isolated from the PBMCs of healthy donors and cultured under Th0-polarizing conditions
Peripheral blood mononuclear cells were isolated from healthy individuals and cultured in the presence or absence of SRL under Th0-polarizing conditions. Pre-incubation with SRL (10 ng/ml) resulted in a significant reduction in the percentage of CD4+ IFN-γ+ [8·1 ± 0·9% (+SRL) versus 28·7 ± 0·3% (Th0-polarizing conditions alone)], CD4+ IL-4+ [0·06 ± 0·01% (+SRL) versus 1·4 ± 0·5% (Th0-polarizing conditions alone)], and CD4+ IL-17+ [0·4 ± 0·06% (+SRL) versus 1·1 ± 0·1% (Th0-polarizing conditions alone)] cells; however, SRL had no significant effect on the proportion of CD4+ Treg cells [23·9 ± 12·9% (+SRL) versus 36·2 ± 5·8% (Th0-polarizing conditions alone)] (Fig. 1a). Under the same conditions, TAC (10 ng/ml) caused a significant reduction in the percentage of CD4+ IFN-γ+ [2·5 ± 2·8% (+TAC) versus 28·7 ± 0·3% (Th0-polarizing conditions alone)], CD4+ IL-4+ [0·01 ±0·004% (+TAC) versus 1·4 ± 0·5% (Th0-polarizing conditions alone)], and CD4+ Foxp3+ [15·2 ± 10·0% (+TAC) versus 36·2 ± 5·8% (Th0-polarizing conditions alone)], whereas the percentage of Th17 cells was unaffected [1·3 ± 0·05% (+TAC) versus 1·1 ± 0·1% (Th0-polarizing conditions alone)] (Fig. 1a). TAC did not suppress CD4+ IL-17+ cells at any of the doses tested (Fig. 1a).
Figure 1.
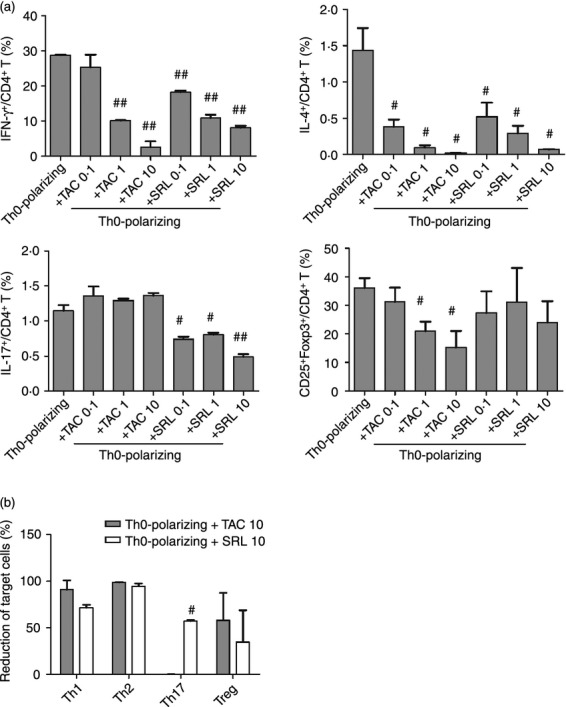
Effects of tacrolimus (TAC) or sirolimus (SRL) on CD4+ T cells isolated from the peripheral blood mononuclear cells (PBMCs) of healthy donors and cultured under T helper type 0 (Th0) -polarizing conditions. Human PBMCs were isolated from healthy subjects (n = 4) and pre-incubated with TAC (0·1, 1, or 10 ng/ml) or SRL (0·1, 1, or 10 ng/ml) as indicated. They were then cultured under Th0-polarizing conditions (anti-CD3, 1 μg/ml and anti-CD28, 1 μg/ml) for 48 hr. The percentage of CD4+ T cells secreting interleukin-17 (IL-17), interferon-γ (IFN-γ), or IL-4 was measured by flow cytometry. (a) The percentages of IFN-γ+/CD4+ T cells, IL-4+/CD4+ T cells, IL-17+/CD4+ T cells, and CD25+FOXP3+ T cells within the PBMC populations isolated from healthy controls. #P < 0·05 and ##P < 0·01 versus culture under Th0-polarizing conditions alone. (b) SRL- and TAC-mediated suppression (%) of IFN-γ+/CD4+ T cells, IL-4+/CD4+ T cells, IL-17+/CD4+ T cells, and CD25+FOXP3+ T cells in the PBMC populations isolated from healthy controls. #P < 0·05 and ##P < 0·01 versus culture under Th0-polarizing conditions alone. Bars represent the mean ± SD.
SRL reciprocally regulates Th17 and Treg cells that were isolated from the PBMCs of KTRs and cultured under Th17-polarizing conditions
Analysis of the IL-17+ and Foxp3+ CD4+ cell populations isolated from KTRs showed that both TAC and SRL (10 ng/ml each) had significant effects in vitro after only 48 hr. Pre-incubation of PBMCs isolated from KTRs with SRL (10 ng/ml) suppressed the production of IL-17-producing CD4+ cells by 70·1 ± 4·7% (versus Th17-polarizing conditions alone) (Fig. 2a). Pre-incubation with SRL suppressed IL-17 mRNA levels below the 70% level under Th17-polarizing conditions; however, SRL increased Foxp3 mRNA levels up to the 50% level (Fig. 2b). SRL (10 ng/ml) suppressed IL-17 protein levels to a greater extent than TAC did (10 ng/ml) [756·3 ± 185·4 (+SRL) and 985·6 ± 398·0 (+TAC) versus 1575·3 ± 858·9 pg/ml (Th17-polarizing conditions alone)]. The same was true for IL-22 levels [199·3 ± 8·0 (+SRL) and 1229·0 ± 994·1 (+TAC) versus 578·6 ± 124·8 pg/ml (Th17-polarizing conditions alone) (Fig. 2c)].
Figure 2.
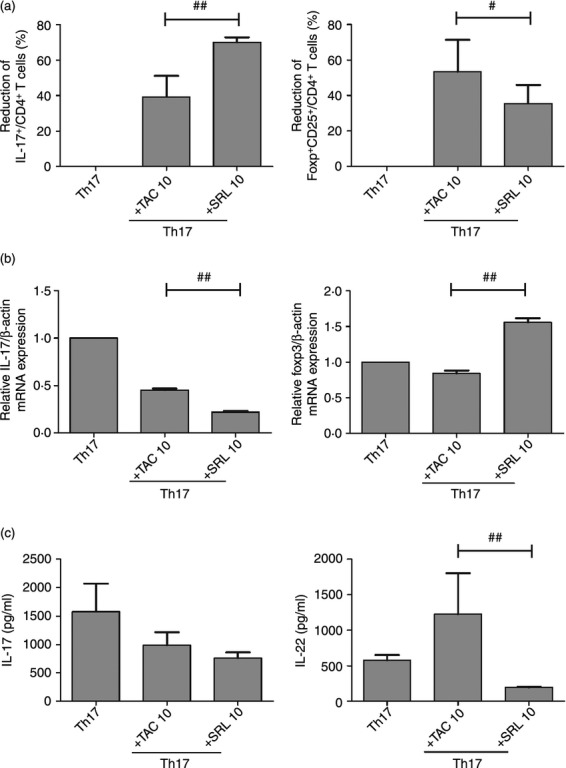
Effects of tacrolimus (TAC) or sirolimus (SRL) on CD4+ T helper type 17 (Th17) cells and CD4+ regulatory T (Treg) cells isolated from the peripheral blood mononuclear cells (PBMCs) of kidney transplant recipients (KTRs) and cultured under Th17-polarizing conditions. PBMCs isolated from KTRs (n = 5) were pre-incubated with TAC (10 ng/ml) or SRL (10 ng/ml) as indicated, and then cultured under Th17-polarizing conditions for 48 hr. The percentage of CD4+ T cells producing interleukin-17 (IL-17) or Foxp3 was measured by flow cytometry. (a) SRL- and TAC-mediated suppression (%) of IL-17+ CD4+ T cells and CD25+ FOXP3+ CD4+ T cells in the PBMC populations isolated from KTRs. #P < 0·05 and ##P < 0·01. (b) The expression of IL-17 and Foxp3 mRNA measured using real-time PCR. ##P < 0·01. (c) Production of IL-17 and IL-22 by Th17 cells and secreted into the culture supernatant. ##P < 0·01. Bars represent the mean ± SD.
SRL mediates its regulatory effects by inhibiting mTOR and STAT3 under Th17-polarizing conditions
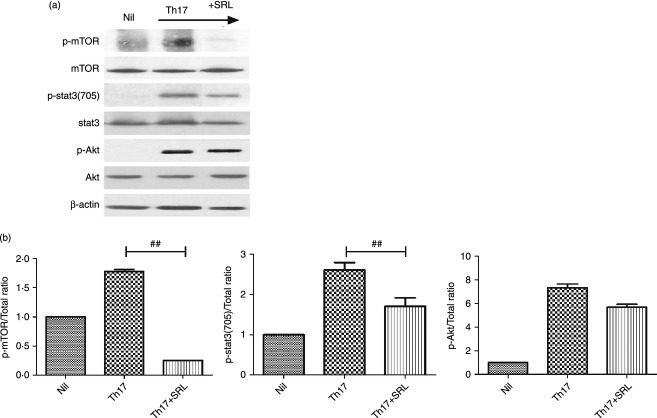
To identify the molecular mechanisms (including STAT3) modulated by mTOR, we investigated whether SRL interferes directly with mTOR and STAT3 signalling. SRL (a specific inhibitor of mTOR) inhibited the phosphorylation of STAT3-705 in CD4+ T cells after 30 min of culture under Th17-polarizing conditions (P = 0·001) (Fig. 3a). In contrast, the phosphorylation status of Akt was not altered by SRL (Fig. 3b). Consistent with these results, SRL abrogated the increase in IL-17 induced by STAT3 inhibition (Fig. 3a, b).
Figure 3.
Effects of tacrolimus (TAC) or sirolimus (SRL) on the expression of mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription (STAT3) proteins in CD4+ T cells isolated from the peripheral blood mononuclear cells (PBMCs) of healthy donors (n = 3). (a) Immunoblotting of p-mTOR, mTOR, p-STAT3(705), STAT3, p-Akt and Akt in CD4+ T cells pre-treated with SRL (10 ng/ml) and then cultured under T helper type 17 (Th17) -polarizing conditions for 1 hr. (b) Stimulation of CD4+ T cells under Th17-polarizing conditions activated the phosphorylation of p-mTOR, mTOR, p-STAT3(705), STAT3, p-Akt and Akt as detected by Western blotting and shown by the ratio of phosphorylated to total proteins. ##P < 0·01. Bars show the mean ± SD results in three patients, in one of three independent experiments.
Conversion from TAC to SRL increases the immunomodulatory potential of PBMCs in KTRs
After conversion from TAC to SRL, no significant change in clinical and laboratory parameters was detected compared with before conversion and no rejection episode was detected (Table 2). The percentages of different subsets of immune cells within the PBMC population isolated from KTRs were compared both before and after conversion from TAC to SRL. There were no significant differences in the percentages of Th1 cells and CD4+ Treg cells before or after conversion (22·7 ± 7·6 before conversion versus 20·5 ± 13·1% after conversion and 7·8 ± 1·5 before conversion versus 8·1 ± 2·0% after conversion, respectively) (Fig. 4a, d). In contrast, the percentage of Th2 cells was significantly reduced after conversion [1·5 ± 0·3 (before) versus 0·9 ± 0·2% (after); P < 0·01]. The same was true for Th17 cells [1·1 ± 0·5 (before) versus 0·7 ± 0·4% (after); P < 0·01] (Fig. 4b, c).
Table 2.
Clinical outcome of tacrolimus to sirolimus conversion (n = 5)
| Parameter | Pre-conversion | Post-conversion | P value |
|---|---|---|---|
| Complete blood count | |||
| White blood cell (103/µl) | 5·9 ± 2·9 | 6·1 ± 2·6 | 0·66 |
| Haemoglobin (g/dl) | 14·4 ± 1·8 | 13·9 ± 1·9 | 0·13 |
| Platelet (103/µl) | 212·8 ± 29·6 | 221·0 ± 31·5 | 0·17 |
| BUN (mg/dl) | 16·1 ± 3·3 | 14·9 ± 2·8 | 0·51 |
| Serum creatinine (mg/dl) | 1·47 ± 0·28 | 1·47 ± 0·34 | 0·93 |
| eGFR by MDRD (ml/min/1·73 m2) | 58·7 ± 13·8 | 59·9 ± 16·0 | 0·76 |
| Urine protein/urine creatinine | 0·05 ± 0·04 | 0·06 ± 0·03 | 0·65 |
| Mean trough SRL level (ng/ml) | – | 6·4 ± 2·5 | – |
BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; MDRD, modified diet renal disease.
Figure 4.
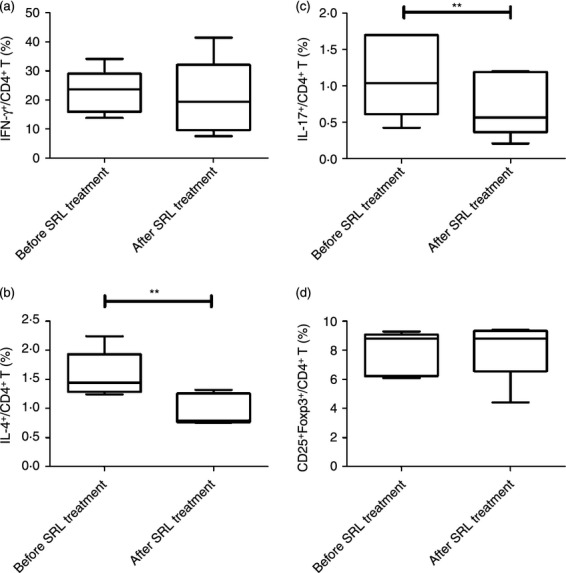
Effect of converting from tacrolimus (TAC) to sirolimus (SRL) on CD4+ T-lymphocyte subpopulations within the peripheral blood mononuclear cell (PBMC) population isolated from kidney transplant recipients (KTRs). Distribution of the T helper type 1 (Th1) [interferon (IFN-γ)], Th17 [interleukin-17 (IL-17)], Th2 (IL-4) and regulatory T (Treg) CD4+ subpopulations within the total PBMC population in KTRs (n = 5) before and after conversion from TAC to SRL. PBMCs from KTRs before and after conversion from TAC to SRL were stimulated for 4 hr in vitro with PMA and ionomycin in the presence of GolgiStop. The percentage of CD4+ T cells producing IFN-γ, IL-4, or IL-17 was measured by flow cytometry. (a) The percentage of IFN-γ+ CD4+ T cells within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. (b) The percentage of IL-4+ CD4+ T cells within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. **P < 0·01 versus before conversion. (c) The percentage of IL-17+ CD4+ T cells within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. **P < 0·01 versus before conversion. (d) The percentage of CD25+ FOXP3+ T cells within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. Bars represent the mean ± SD.
There was no significant difference in the percentage of CD8+ T naive or CD8+ central memory T cells after conversion from TAC to SRL (n = 5) (58·7 ± 6·3 versus 57·9 ± 3·9% and 19·9 ± 4·7 versus 25·4 ± 4·5%, respectively) (Fig. 5a, b). By contrast, there was a statistically significant reduction in the percentage of CD8+ effector memory T cells after conversion (9·5 ± 2·5 versus 6·2 ± 1·1%; P < 0·01) (Fig. 5c).
Figure 5.
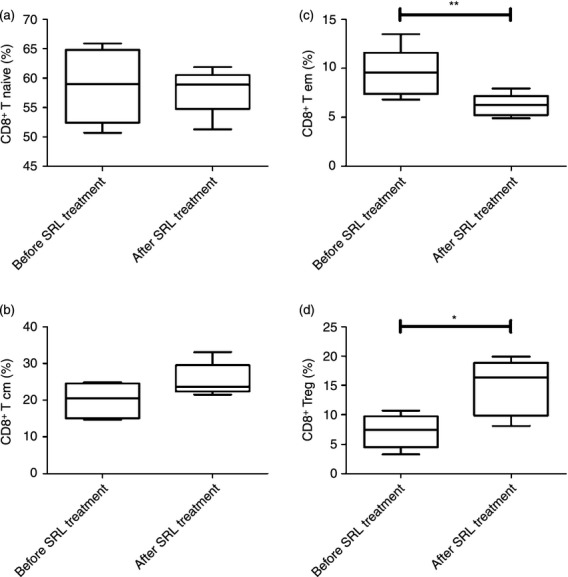
Conversion from tacrolimus (TAC) to sirolimus (SRL) increases the immunomodulatory potential of CD8+ regulatory T (Treg) cells in kidney transplant recipients (KTRs). Distribution of naive, central memory (Tcm), effector memory (Tem) and regulatory CD8+ T-cell subpopulations within the total peripheral blood mononuclear cell (PBMC) population in KTRs (n = 5) before and after conversion from TAC to SRL. PBMCs isolated from KTRs before and after conversion from TAC to SRL were stimulated for 4 hr in vitro with PMA and ionomycin in the presence of GolgiStop. CD8+ lymphocytes were stained with monoclonal antibodies against CD45RA and CCR7, which resulted in the identification of four subsets. (a) The percentage of CD45RA+ CCR7+ CD8+ T cells (Tnaive/CD8+ T) within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. (b) The percentage of CD45RA− CCR7+ CD8+ T cells (Tcm/CD8+ T) within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. (c) The percentage of CD45RA− CCR7− CD8+ Tcells (Tem/CD8+ T) within the PBMC populations isolated KTRs before and after conversion from TAC to SRL. **P < 0·01 versus before conversion. (d) The percentage of CD8+ Treg/CD8+ cells (CD8+ CCR7+ CD8+ T) within the PBMC populations isolated from KTRs before and after conversion from TAC to SRL. *P < 0·05 versus before conversion. Bars represent the mean ± SD.
Patients receiving SRL showed a significant increase in the percentage of CD8+ Treg cells (CD8+ CCR7+) after conversion to SRL (1·6 ± 0·6 versus 3·6 ± 0·7%; P < 0·01) (Fig. 5d).
CD8+ CCR7+ T cells suppress the proliferation of CD4+ T cells in KTRs
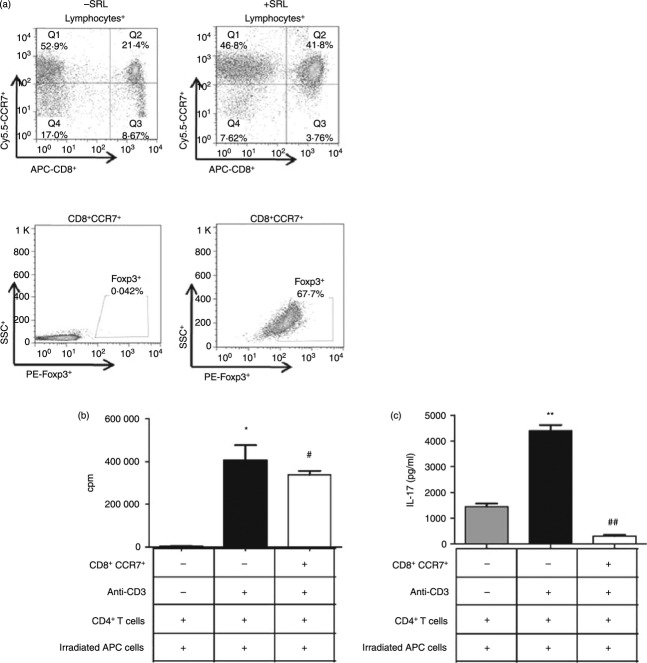
Finally, to ascertain the functional profile of CD8+ Treg cells induced by a combination of suboptimal T-cell receptor stimulation, IL-15-mediated STAT5 signalling and SRL treatment, we examined CD8+ Treg cells cultured for 6 days in the absence or presence of anti-CD3+ IL-15+ SRL using an in vitro suppression assay (Fig. 6). Co-culture of naive CD4+ T cells with CD8+ Treg cells showed that CD8+ Treg cells suppressed the clonal expansion of conventional CD4+ T cells (Fig. 6a, b). The regulatory function of CD8+ Treg cells ensured the retention of a fraction of the CD4+ T cells. Inducible CD8+ Treg cells inhibited allogeneic CD4+ T cells and down-regulated IL-17 (CD8+ CCR7+, 305·1 ± 88·2 versus anti-CD3, 4407·5 ± 436·8 pg/ml) production by allogeneic CD4+ T cells (Fig. 6c). Allogeneic CD4+ T cells stimulated by anti-CD3 were susceptible to suppression by CD8+ Treg cells.
Figure 6.
Suppression of effector T-cell proliferation by CD8+ regulatory T (Treg) cells induced with sirolimus (SRL). (a) Peripheral blood mononuclear cells (PBMCs) were collected from healthy donors (n = 4), plated at a density of 2 × 105 per well, and then stimulated with an anti-CD3 antibody, interleukin-15 (IL-15; 50 ng/ml), and SRL. On Day 3, the cells were harvested, stained with antibodies specific for CD8, CCR7 and Foxp3 or isotype, and then subjected to flow cytometry. (b) CD8+ Treg-mediated suppression of the proliferation of effector T cells within the PBMC population isolated from healthy donors was measured using a [3H]thymidine incorporation assay. Briefly, CD4+ T cells were stimulated with anti-CD3 (1 μg/ml) and T-cell-depleted irradiated antigen-presenting cells (APC) in the absence or presence of CD8+ Treg cells (CD8+ CCR7+) that were differentiated by exposure to 1 μg/ml plate-bound anti-CD3, IL-15 and SRL. The cells were cultured for 3 days. [3H]Thymidine was added for the final 8 hr before harvesting. The incorporation of [3H]thymidine into the CD4+ T cells was measured using a liquid β-scintillation counter. CD8+ Treg cells were isolated as CD8+ CCR7+ cells. The purity of all the T-cell subsets was > 95% as determined by FACS analysis. *P < 0·05 versus Nil; #P < 0·05 versus anti-CD3. (c) IL-17 levels in the supernatants of the cultures described in (b). **P < 0·01 versus Nil; ##P < 0·01 versus anti-CD3. Bars represent the mean ± SD.
Discussion
The results of the present study clearly demonstrate that SRL regulates the human Th17/Treg axis favourably in KTRs. Unlike TAC, SRL suppressed Th17 cells in vitro, and conversion from TAC to SRL not only suppressed Th17 cells but also up-regulated Treg cells in vivo. These findings provide the rationale for conversion from TAC to SRL in KTRs.
Mammalian TOR is an important regulator of helper T-cell differentiation.31–35 CD4+ T cells lacking or deficient in mTOR fail to differentiate into effector cells or into Foxp3+ regulatory cells under appropriate skewing conditions.23 In addition, mTOR inhibition abrogates the reprogramming of CD4+ Foxp3+ Treg cells into pathogenic Th1/Th17 effector cells.36 Therefore, we hypothesized that mTOR inhibition by SRL may suppress Th17 cells in human KTRs. To test our hypothesis, we isolated PBMCs from both healthy controls and KTRs and compared the suppressive effects of SRL and TAC on the Th17 pathway under both Th0- and Th17-polarizing conditions. The results showed that TAC did not suppress, while SRL effectively suppressed all effector T-cell subsets, including Th17 cells at clinically relevant concentrations.
The Th17 subset is an independent lineage of Th cells in both humans and mice. These cells are unique in terms of cytokine profile, transcriptional regulation and biological function,37,38 but Th17 cells retain potential developmental plasticity.39,40 Both Treg and Th17 cells produce a distinct set of transcription factors related to their differentiation.41,42 STAT3 and RORc are involved in Th17 differentiation, whereas STAT5 and Foxp3 help in the differentiation of Treg cells.43 STAT3 and STAT5 bind to the same DNA motif competitively. Therefore, activation of STAT3 or STAT5 determines whether the Th cells differentiate into Th17 or Treg cells.17 The present study shows that SRL inhibited the phosphorylation of STAT3-705 in CD4+ T cells, suggesting an interaction between STAT3 and mTOR; previous studies show that rapamycin inhibits STAT3 activation in both DCs and cancer cells.44,45 This suggests that the SRL-mediated reduction in STAT3 activation is closely associated with differentiation into Treg cells rather than into effector Th17 cells. Interestingly, we found that SRL-mediated inhibition of STAT3 also increased Foxp3 activity. Therefore, it is possible that reduction in STAT3 activation by SRL activated STAT5, considering the opposing effects of STAT3 and STAT5 observed in animal models.17 The results of our study in KTRs confirm that such duality also exists in humans, during conversion from TAC to SRL.
Based on in vitro study, we evaluated if conversion from TAC to SRL suppressed the Th17 pathway in KTRs. We examined the T-cell subsets within the PBMC population before and after conversion. Similar to the in vitro data, the proportion of Th17 cells decreased significantly after conversion to SRL. At the molecular level, SRL treatment decreased the expression of IL-17 and increased the expression of Foxp3. In addition, SRL treatment suppressed both IL-17 and IL-22 production by Th17 cells. Hence, conversion from TAC to SRL favourably regulated the Th17/Treg pathway in KTRs. Similar results have been observed in liver transplant recipients, where conversion from TAC to SRL increased the systemic regulatory T and dendritic cell populations as well as the immunoregulatory proteogenomic signatures.46 Taken together, conversion from TAC to SRL is beneficial for regulating the Th17 pathway in patients with solid organ transplants.
One of the interesting findings of the present study is that SRL conversion significantly increased the proportion of CD8+ CCR7+ T cells (CD8+ Treg cells).24 It is well known that CD8+ Treg cells suppress CD4+ T-cell activation and proliferation in animal models of autoimmune disease and transplantation.24,47,48 Therefore, we tested whether CD8+ Treg cells suppress CD4+ cell proliferation in humans by co-culture of CD8+ Treg cells with effector CD4+ T cells. We found that SRL induced the expansion of CD8+ Treg cells in vitro as well as in vivo, which inhibited the proliferation of effector CD4+ T cells. We further examined the effects of SRL on the differentiation of CD8+ Treg cells and its regulatory function in vitro. As shown in Figure 6, SRL induced the differentiation of CD8+ T cells into Foxp3+ CCR7+ CD8+ T cells, and isolated CD8+ Treg cells had a significant suppressive effect on effector CD4+ T cells. Taken together, SRL exerts its suppressive effects on CD4+ effector T cells by inducing CD8+ Treg cells in animals as well as in humans.
One may argue that the CD8+ CCR7+ Treg cells identified in the present study overlap with previously described human Treg cells, wherein they express the classical regulatory marker, Foxp3.24,49 However, their naive phenotype and mechanism of action suggest that they represent a novel CD8 subset. They also suppress CD4+ T-cell activation and proliferation by interfering with the very early steps of T-cell receptor activation.24 In addition, this cell type has an important characteristic, namely it can be reliably induced by stimulation by very low doses of T-cell receptor cross-linking anti-CD3 antibodies.24 The regulation of CD4+ T cells (including Th17 cells) by CD8+ CCR7+ Treg cells has been reported in a mouse model of autoimmune disease and organ transplantation.47,48 Therefore, this is also the first report stating that human CD8+ Treg cells are not different from the CD8+ Treg cells previously described in mice.
The results of this study clearly show that SRL has a beneficial effect on the Th17/Treg axis. However, we must consider that SRL treatment did not up-regulate Foxp3 regulatory T cells, which are otherwise induced by SRL treatment in KTRs. The reason for this may be related to the concomitant use of mycophenolate mofetil.50,51 Additionally, there was marked variability between individuals in the mRNA levels of IL-17 and Foxp3 cytokines. Therefore, we calculated the relative changes in values rather than absolute changes to minimize individual variation.
In conclusion, SRL suppressed Th17 cells in vitro, and conversion from TAC to SRL not only suppressed Th17 cells but also up-regulated Treg cells in vivo. These findings provide the rationale for conversion from TAC to SRL in KTRs.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: A092258 and HI13C1704).
Glossary
- SRL
Sirolimus
- TAC
Tacrolimus
- KTRs
kidney transplant recipients
- Th0
T helper cells
- Th1
Type 1 helper T cells
- Th2
Type 2 helper T cells
- Th17
IL-17-producing helper T cells
- Treg
regulatory T cells
- CNIs
calcineurin inhibitors
- PBMC
peripheral blood mononuclear cell
- mTOR
mammalian target of rapamycin
- STAT3
Signal transducer and activator of transcription 3
- STAT5
Signal transducer and activator of transcription 5
- RORc
RAR-related orphan receptor gamma
Conflict of interest
None.
Disclosures
The authors declare no competing financial interests.
References
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–14. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–9. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Calvo-Turrubiartes M, Romano-Moreno S, Garcia-Hernandez M, Chevaile-Ramos JA, Layseca-Espinosa E, Gonzalez-Amaro R, Portales-Perez D. Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol. 2009;21:43–9. doi: 10.1016/j.trim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+ CD25+ FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82:550–7. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 8.Akimova T, Kamath BM, Goebel JW, Meyers KE, Rand EB, Hawkins A, Levine MH, Bucuvalas JC, Hancock WW. Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant. 2012;12:3449–61. doi: 10.1111/j.1600-6143.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–51. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 10.Mas VR, Archer KJ, Scian M, Maluf DG. Molecular pathways involved in loss of graft function in kidney transplant recipients. Expert Rev Mol Diagn. 2010;10:269–84. doi: 10.1586/erm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mas V, Maluf D, Archer K, et al. Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation. 2007;83:448–57. doi: 10.1097/01.tp.0000251373.17997.9a. [DOI] [PubMed] [Google Scholar]
- 12.Braun RK, Martin A, Shah S, et al. Inhibition of bleomycin-induced pulmonary fibrosis through pre-treatment with collagen type V. J Heart Lung Transplant. 2010;29:873–80. doi: 10.1016/j.healun.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Syrjala SO, Keranen MA, Tuuminen R, Nykanen AI, Tammi M, Krebs R, Lemstrom KB. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic preservation in the rat. J Heart Lung Transplant. 2010;29:1047–57. doi: 10.1016/j.healun.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Decraene A, Willems-Widyastuti A, Kasran A, De Boeck K, Bullens DM, Dupont LJ. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable cystic fibrosis patients. Respir Res. 2010;11:177. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung BH, OH HJ, Piao SG, Sun IO, Kang SH, Choi SR, et al. Higher infiltration by Th17 cells compared with regulatory T cells is associated with severe acute T-cell-mediated rejection. Exp Mol Med. 2011;43:630–37. doi: 10.3858/emm.2011.43.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisitkun P, Claudio E, Ren N, Wang H, Siebenlist U. The adaptor protein CIKS/ACT1 is necessary for collagen-induced arthritis, and it contributes to the production of collagen-specific antibody. Arthritis Rheum. 2010;62:3334–44. doi: 10.1002/art.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual M, Swinford RD, Ingelfinger JR, Williams WW, Cosimi AB, Tolkoff-Rubin N. Chronic rejection and chronic cyclosporin toxicity in renal allografts. Immunol Today. 1998;19:514–9. doi: 10.1016/s0167-5699(98)01324-3. [DOI] [PubMed] [Google Scholar]
- 19.Beveridge T, Calne RY. Cyclosporine (Sandimmun) in cadaveric renal transplantation. Ten-year follow-up of a multicenter trial. European Multicentre Trial Group. Transplantation. 1995;59:1568–70. [PubMed] [Google Scholar]
- 20.Bertani T, Ferrazzi P, Schieppati A, et al. Nature and extent of glomerular injury induced by cyclosporine in heart transplant patients. Kidney Int. 1991;40:243–50. doi: 10.1038/ki.1991.206. [DOI] [PubMed] [Google Scholar]
- 21.Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, Brindley DJ, Loukas A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biosyst. 2011;7:1367–75. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzman SD, O'Gorman WE, Villarino AV, Gallo E, Friedman RS, Krummel MF, Nolan GP, Abbas AK. Duration of antigen receptor signaling determines T-cell tolerance or activation. Proc Natl Acad Sci USA. 2010;107:18085–90. doi: 10.1073/pnas.1010560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+ CD45RA+ CCR7+ FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. 2012;189:2118–30. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+ CD25+ Foxp3+ regulatory T cells. Blood. 2011;118:2342–50. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robb RJ, Lineburg KE, Kuns RD, et al. Identification and expansion of highly suppressive CD8+ FoxP3+ regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 27.Stallone G, Infante B, Schena A, Battaglia M, Ditonno P, Loverre A, Gesualdo L, Schena FP, Grandaliano G. Rapamycin for treatment of chronic allograft nephropathy in renal transplant patients. J Am Soc Nephrol. 2005;16:3755–62. doi: 10.1681/ASN.2005060635. [DOI] [PubMed] [Google Scholar]
- 28.McMahon G, Weir MR, Li XC, Mandelbrot DA. The evolving role of mTOR inhibition in transplantation tolerance. J Am Soc Nephrol. 2011;22:408–15. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- 29.Diekmann F, Campistol JM. Conversion from calcineurin inhibitors to sirolimus in chronic allograft nephropathy: benefits and risks. Nephrol Dial Transplant. 2006;21:562–8. doi: 10.1093/ndt/gfi336. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Lara MA, Carracedo J, Ramirez R, Martin-Malo A, Rodriguez M, Madueno JA, Aliama P. The imbalance in the ratio of Th1 and Th2 helper lymphocytes in uraemia is mediated by an increased apoptosis of Th1 subset. Nephrol Dial Transplant. 2004;19:3084–90. doi: 10.1093/ndt/gfh382. [DOI] [PubMed] [Google Scholar]
- 31.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mammalian target of rapamycin inhibition on CD4+ CD25+ Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flechner SM, Kurian SM, Solez K, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant. 2004;4:1776–85. doi: 10.1111/j.1600-6143.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 34.Larson TS, Dean PG, Stegall MD, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006;6:514–22. doi: 10.1111/j.1600-6143.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 35.Hackstein H. Rapamycin and dendritic cells: keep on movin’. Transplantation. 2006;82:739–40. doi: 10.1097/01.tp.0000235438.11132.8f. [DOI] [PubMed] [Google Scholar]
- 36.Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, Olivier M, Piccirillo CA. Inflammation-driven reprogramming of CD4+ Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo. PLoS One. 2012;7:e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Rowell E, Wilson CB. Programming perpetual T helper cell plasticity. Immunity. 2009;30:7–9. doi: 10.1016/j.immuni.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Lochner M, Peduto L, Cherrier M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J Exp Med. 2008;205:1381–93. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120:103–14. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitsky J, Mathew JM, Abecassis M, et al. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology. 2013;57:239–48. doi: 10.1002/hep.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leavenworth JW, Tang X, Kim HJ, Wang X, Cantor H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J Clin Invest. 2013;123:1382–9. doi: 10.1172/JCI66938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li XL, Menoret S, Bezie S, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010;185:823–33. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- 49.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 50.Lim DG, Koo SK, Park YH, Kim Y, Kim HM, Park CS, Kim SC, Han DJ. Impact of immunosuppressants on the therapeutic efficacy of in vitro-expanded CD4+ CD25+ Foxp3+ regulatory T cells in allotransplantation. Transplantation. 2010;89:928–36. doi: 10.1097/TP.0b013e3181d3c9d4. [DOI] [PubMed] [Google Scholar]
- 51.Wu T, Zhang L, Xu K, Sun C, Lei T, Peng J, et al. Immunosuppressive drugs on inducing Ag-specific CD4+ CD25+ Foxp3+ Treg cells during immune response in vivo. Transpl Immunol. 2012;27:30–8. doi: 10.1016/j.trim.2012.05.001. [DOI] [PubMed] [Google Scholar]