Abstract
The Notch signalling pathway is involved in multiple cellular processes and has been recently indicated to modulate the host immune response. However, the role of the Notch pathway in dengue virus (DENV) infection remains unknown. Our study has screened the expression profigle of Notch receptors, ligands and target genes in human monocytes, macrophages and dendritic cells in response to DENV infection. The real-time PCR data showed that Notch ligand Dll1 was significantly induced in DENV-infected monocytes; and receptor Notch4, ligands Dll1 and Dll4, and target Hes1 were dramatically enhanced in DENV-infected macrophages and dendritic cells. In macrophages, induction of Dll1 and Dll4 mediated by DENV2 was increased by treatment with interferon-β (IFN-β), and was impaired by neutralization of IFN-β. The DENV-induced Dll1 and Dll4 expression level was decreased by silencing key innate immune molecules, including Toll-like receptor 3 (TLR3), MyD88, RIG-I and IPS-I. In IFN-receptor-depleted macrophages, the Dll1 and Dll4 induction was significantly alleviated. Functionally, activation of Notch signalling by Dll1 in CD4+ T cells enhanced the expression of a T helper type 1 (Th1) cytokine IFN-γ, while Notch activation in macrophages had no direct effect on replication of DENV. Our data revealed that the expressions of Notch ligands in antigen-presenting cells were differentially induced by DENV via innate immune signalling, which is important for Th1/Th2 differentiation during adaptive immune response.
Keywords: DENV, Dll1, Dll4, IFN-β
Introduction
The Notch pathway is an evolutionarily conserved signalling mechanism that transduces signals between adjacent cells and plays a critical role in cell fate determination during development, tissue homeostasis and stem cell maintenance.1,2 There are five mammalian ligands [Delta-like 1 (Dll1), Dll3, Dll4, Jagged1 and Jagged2], each of which can bind to any of the four Notch receptors (Notch1, 2, 3, 4).2 Once bound by Notch ligand, the intracellular domain of the receptor is released by proteolytic cleavage. The active form of Notch is translocated into the nucleus where it activates target genes, such as basic helic-loop-helix family (Hes1 and Hes5) and hairy and enhancer of split-related family (Hey1 and Hey2).3,4
Recently, the Notch signalling pathway has emerged as an important regulator of the immune system. There is growing evidence that Notch signalling is involved in multiple steps of T-cell and B-cell development, T-cell activation, regulatory T-cell function and T helper cell differentiation.5–11 The Notch signalling pathway regulates the innate immune response.12 Studies showed direct cooperation between the Notch and Toll-like receptor (TLR) pathways in the activation of canonical Notch target genes, as well as inflammatory cytokine genes.13 Expression of Notch ligands and receptors is induced in dendritic cells (DC) and macrophages by TLR ligands and various stimuli, such as virus, bacteria and parasites through myeloid differentiation primary response gene 88 (MyD88)14 and mitogen-activated protein kinase-dependent pathway.15,16 In turn, the activated Notch pathway promoted cytokine production by either positively or negatively modulating the innate immune signalling pathway. For instance, canonical Notch signalling augments or suppresses TLR signalling pathway−mediated cytokine production.12,16–19 In addition, the interaction of Notch receptors and Notch ligands plays a critical role in the antigen-presenting cells (APCs) and T-cell communication.20,21 H1N1 enhanced the expression of Dll1 in macrophages and the induction of Dll1 on macrophages specifically regulated IFN-γ production from CD4+ and CD8+ T cells both in vivo and in vitro.22 Moreover, during respiratory syncytial virus (RSV) infection, Notch ligand Dll4 was up-regulated in bone-marrow-derived DC after RSV infection, and the development of a protective T helper type 1 (Th1) response was biased towards a Th2 type response in RSV-infected mice treated with an anti-Dll4 monoclonal antibody.14 Nonetheless, the expression pattern and role of Notch pathway in response to Dengue virus (DENV) infection remain uninvestigated.
DENV is an arthropod-borne single-stranded RNA virus of the genus Flavivirus. There are five related but distinct serotypes of DENV, known as DENV1, 2, 3, 4 and 5.23,24 The virus is endemic in more than 100 tropical and subtropical countries of the world. Diseases caused by DENV infection, including dengue fever, dengue haemorrhagic fever and dengue shock syndrome, are the most prevalent arthropod-borne viral diseases in subtropical and tropical regions of the world.25 Presently no specific therapies or vaccines are available to treat these diseases or to prevent DENV transmission. The disease severity of DENV infection has been associated with the host's innate immune response, particularly the production of interferons (IFNs).26 Pattern recognition receptors, including TLR3, TLR7, TLR8, retinoic acid inducible gene-I (RIG-I) and melanoma differentiation associated gene 5 (MDA-5) are involved in virus recognition.27–31 The activation signal is transmitted through the adaptor protein Toll/interleukin-1 receptor domain-containing adapter inducing IFN-β (TRIF), MyD88 and IFN-β promoter stimulator 1 (IPS-1). The TLR3-TRIF, TLR7/8-MyD88 and/or RIG-I/MDA-5-IPS-1 signals trigger multiple phosphorylation cascades and activation of IFN regulatory factor 3, nuclear factor-κB and mitogen-activated protein kinase, leading to induction of pro-inflammatory cytokines, chemokines and type I IFNs.32
Interferons not only shape the innate antiviral state, but also regulate the adaptive immune response. Through binding to the IFN-α-receptor (IFN-αR), IFN-α/β activates the Janus kinase/signal transducer and activator of transcription pathway, resulting in an induction of more than 300 interferon-stimulated genes.33 IFN-α/β and IFN-γ affect the activities of other immune cells including macrophages, T cells, DC and natural killer cells by enhancing antigen presentation, cell trafficking and cell differentiation.34–36 More recently, type I IFNs has been found to regulates the expression of Notch ligands through the IFN-αR–Janus kinase/signal transducer and activator of transcription pathway.37
In this study we examined the expression profile of Notch molecules in several major target cells of DENV, including human monocytes, monocyte-derived macrophages (hMDM) and DC. Our data revealed that Notch receptors and ligands were differentially up-regulated by DENV infection. Furthermore, our results showed that the ligand induction is mediated through the IFN-β signalling pathway depending on TLR3, MyD88 RIG-I and IFN-αR.
Materials and methods
Reagents
Antibodies against Dll1 and Dll4 were obtained from Abcam (Cambridge, MA). β-actin antibody was purchased from Sigma-Aldrich (St Louis, MO). Recombinant human IFN-β was from PBL Assay Science (Piscataway, NJ). Interferon-β-neutralizing antibody was purchased from Calbiochem (Darmstadt, Germany). Recombinant Dll1 (rDll1) was from R&D (Minneapolis, MN). Purified recombinant human interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor were obtained from PeproTech (Rocky Hill, NJ). Monoclonal antibodies against CD3 (UCHT1), CD28 (CD28.2), CD1a (HI149), CD11c (HL3), CD14 (rmc5-3), CD16 (3G8), CD11b (M1/70), CD80 (L307.4) and HLA-DR (G46-6) were purchased from BD PharMingen (San Diego, CA). Human peripheral blood samples and AB type serum from healthy donors were obtained from the Guangzhou Blood Centre (Guangzhou, Guangdong, China).
Cell isolation and differentiation
Peripheral blood mononuclear cells were isolated by Ficoll (TBD Sciences, Shanghai, China) density gradient centrifugation. Human monocytes were purified from peripheral blood mononuclear cells by anti-CD14 microbeads (BD PharMingen) according to the manufacturer's directions. Monocytes were cultured in the RPMI-1640 medium (Invitrogen, Carlsbad, CA) with 10% human type AB serum.
CD14+ monocytes were differentiated into macrophages by incubation in RPMI-1640 medium (Invitrogen) with 10% human type AB, and media were replaced every 3 days. At 7 days, macrophages were obtained and used for subsequent experiments.
CD14+ monocytes were differentiated into DC by incubation in the 10% fetal bovine serum (Invitrogen) RPMI-1640 medium with granulocyte–macrophage colony-stimulating factor (50 ng/ml) and IL-4 (20 ng/ml), and media were replaced every 3 days. After incubation for 9 days, monocyte-derived DC were obtained and used for subsequent experiments.
Naive CD4+ T cells (98–99% purity) isolated from peripheral blood mononuclear cells of healthy donors were enriched by magnetic cell sorting (MACS, Miltenyi Biotec, Auburn, CA), using negative selection kits (BD PharMingen) as described by the manufacturer.
FACS analysis
Macrophages and DC were harvested and stained with antibodies specific for their surface molecules: CD14, CD16, CD80, CD11b, CD1a, CD11c and HLA-DR (BD Pharmingen). Appropriate isotype controls were used. Samples were analysed using FACS Calibur Flow Cytometer (Becton-Dickinson, Franklin Lakes, NJ) and CELLQuest software (Becton-Dickinson).
Viruses
The Dengue-1 virus Hawaii strain, Dengue-2 virus New Guinea C strain and Dengue-3 virus H87 strain were provided by the Guangzhou Centres for Disease Control, and propagated in C6/36 cells. Dengue-4 virus strain was not available. C6/36 cells were inoculated with DENV at a multiplicity of infection (MOI) of 0·01, and incubated at 35° for 5–6 days (DENV1), 2–3 days (DENV2) and 6–7 days (DENV3). The supernatants were collected and clarified by centrifugation (1000 g, 5 min). Viral concentrations were titrated on C6/36 cells and viral stocks were stored at −80°. The endotoxin levels in all viral stocks measured by limulus amoebocyte lysate assay (Hou-reagent Inc., Xiamen, China) were below 0·03 EU/ml.
Virus titration
DENV titres in cell supernatants were determined by 50% tissue culture infective dose (TCID50) assay. Samples were serially diluted and inoculated into C6/36 cells in 96-well plates. After 5-day incubation at 35° and 5% CO2, cells were examined for cytopathic effects under a light microscope. The virus titre (TCID50/ml) was calculated using the Reed–Münch method.38 1 TCID50/ml was equivalent to 0·69 plaque-forming units/ml.39
RNAi
The sequences targeted in transient knockdown experiments used validated chemically synthesized short interfering RNAs (siRNAs). The sequences of siRNAs prepared by Invitrogen with dTdT overhangs were as follows: GGAAGAGGUGCAGUAUAUU for RIG-I (siRIG-I), GGUGAAGGAGCAGAUUCAG for MDA-5 (siMDA-5), UAGUUGAUCUCGCGGACGA for IPS-1 (siIPS-1), GGAUGUUUUCGGGCCGCCU for TLR3 (siTLR3), GCAUAUGCCUGAGCGUUUC for MyD88 (siMyD88), GCACAAGGCTTCACGCTTTAA for IFNαR1 (siIFNαR1), GGATTCAGCGGGAACACAACG for IFNαR2 (siIFNαR2) and scrambled siRNA (siNC) was used as a control (siNC). siRNAs were transfected into macrophages by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Neutralizing assay and rDll1 treatment
Macrophages were incubated with IFN-β-neutralizing antibody (500 U/ml; Calbiochem) for 1 hr before DENV2 infection. The media were replaced with fresh medium containing 500 U/ml neutralizing anti-IFN-β after DENV2 adsorption. For rDll1 stimulation experiments, hMDM were grown for 48 hr on 0·2% gelatine-coated plates containing 7·5 μg/ml rDll1 or bovine serum albumin as control.
Reverse-transcription PCR and quantitative PCR
Total RNA was prepared from macrophages using TRIzol reagent (Invitrogen) following the manufacturer's instruction. One microgram of total RNA was reverse transcribed to produce cDNA, and then amplified using SYBR Green Master Mix (Bio-Rad, Hercules, CA) following the manufacturer's protocol as described previously.40,41 The sequences and product size for each pair of PCR primers were listed in Table 1. Quantitative real-time PCR were performed using the CFX96 Real-time PCR System (Bio-Rad). Relative mRNA levels were calculated after normalization to GAPDH.
Table 1.
Primer sequences used in PCR
| Gene name | Sequence (5′–3′) |
|---|---|
| GAPDH F | CCTTCCGTGTCCCCACTG |
| GAPDH R | CGCCTGCTTCACCACCTTC |
| Notch 1 F | TGCCTGTTAGGAGAAGCTGC |
| Notch 1R | GCATACACACTCCGAGAACAC |
| Notch 2 F | CATGCCTGAACCAAGGAACCTG |
| Notch 2 R | AATGGTACACCGCTGACCTTGC |
| Notch 3 F | TGGGCAGTGTGTGGATGAAGAC |
| Notch 3 R | ACGTCGTCCTCACAGTTATCACC |
| Notch 4 F | TGCGATAATGCGAGGAAGATACG |
| Notch 4 R | ATCGGAATGTTGGAGGCAGAAC |
| Dll1 F | AACGAATGCTGCTGAAGAGGAG |
| Dll1 R | AGAGCAACTGTCCATAGTGCAAC |
| Dll3 F | CACCCAGGTCCTTTGAATGCAC |
| Dll3 R | TGGCAGATGTAGGCAGAGTCAG |
| Dll4 F | AATGTGTCATTGCCACGGAGG |
| Dll4 R | AGCTGCCAATCTGATGCCAGAC |
| Jagged1 F | TGATTGCTGCCGTTGCAGAAG |
| Jagged1 R | ACCAAGCAACAGATCCAAGCC |
| Jagged2 F | GCACCTACTGCCATGAGAACATTG |
| Jagged2 R | TGTAGGCATCGCACTGGAACTC |
| Hes1 F | AAGCTGGAGAAGGCGGACATTC |
| Hes1 R | TCGTTCATGCACTCGCTGAAG |
| Hey1 F | AAGGAGAGTGCGGACGAGAATG |
| Hey1 R | TACCAGCCTTCTCAGCTCAGAC |
| IFN-β F | AAACTCATGAGCAGTCTGCA |
| IFN-β R | AGGAGATCTTCAGTTTCGGAGG |
| IFN-γ F | TGGAGACCATCAAGGAAGACA |
| IFN-γ R | GTTCAGCCATCACTTGGATGA |
| IL-4 F | GCTAT-TGATGGGTCTCACCC |
| IL-4 R | CAGGACGTCAAGGTACAGGA |
| IFNαR1 F | TGCCATGCCAGAAGATAGTG |
| IFNαR1 R | TTAGGTGCTCAGGCTTCCAG |
| IFNαR2 F | CCACTCCATTGTACCAACTC |
| IFNαR2 R | TGTGTGCTTCTCCACTCATC |
Western blot
Whole cell extracts were prepared in the presence of 1 mm PMSF and 1% (volume/volume) protease inhibitor cocktail (Sigma, St Louis, MO) as described previously.42,43 Proteins were fractionated by electrophoresis on SDS-10% polyacrylamide gels, transferred to nitrocellulose membranes, blocked, and then probed with an appropriate dilution of primary antibody in PBS containing 3% (weight/volume) skim milk. Western blot detection was performed with IRDye 800 CW conjugated anti-rabbit IgG or IRDye 680 CW conjugated anti-mouse IgG secondary antibody according to the manufacturer's protocols (LI-COR, Lincoln, NE). Immunoreactive bands were visualized using an Odyssey infrared imaging system.
Statistical analysis
Statistical significance was determined by an unpaired, two-tailed Student's t-test; P < 0·05 was considered to be statistically significant.
Results
Expression of Notch molecules is differentially induced by DENV2
To test whether DENV infection regulates the expression of Notch molecules, we measured mRNA expression levels of Notch receptors (Notch1–4), ligands (Dll1, 3, 4 and Jagged 1, 2), and two representative target genes (Hey1 and Hes1) in human CD14+ monocytes. Cells were mock-infected with C6/36 cell culture supernatant or infected with DENV2 for 36 hr, and harvested for real-time PCR analysis. In DENV2-infected monocytes, the expression of Notch ligand Dll1 was increased 25-fold compared with mock-infected cells (P < 0·01, Fig. 1b), but expression of all other Notch molecules was not significantly altered (Fig. 1a–c).
Figure 1.
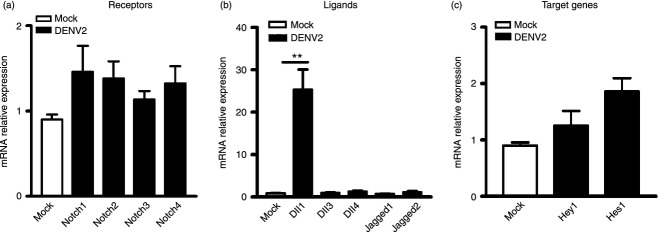
Expression levels of Notch molecules in dengue virus serotype 2 (DENV2) -infected monocytes. Monocytes were infected with DENV2 (multiplicity of infection 4) for 36 hr, and harvested for real-time PCR. Expression of Notch receptors (a), ligands (b) and target genes (c) were analysed and normalized to that of GAPDH in each sample. Data are shown as mean ± standard deviation (SD) of at least three independent experiments; **P < 0·01.
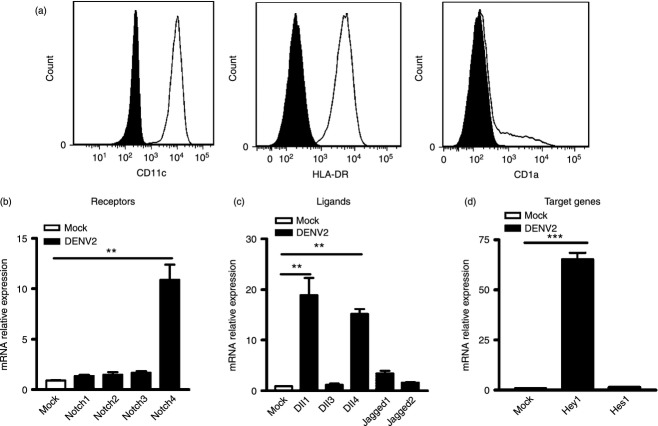
Next, we further compared the expression of Notch molecules in another two DENV target primary cells, hMDM and DC. Macrophages and DC were differentiated from CD14+ monocytes and their phenotypes were assessed by flow cytometry using monoclonal antibody against their surface markers. For hMDM, several typical surface antigens of macrophages, including CD14, CD16, CD11b, HLA-DR and CD80, were chosen. Figure 2(a) showed that the phenotype of the monocyte-derived cells was CD14+ CD11b+ CD16+ HLA-DR+ and CD80−, indicating that these cells possessed features of macrophages. The hMDM were infected by DENV2 and analysed by real-time PCR. In DENV2-infected hMDM, expression levels of Notch4, Dll1 and Dll4 were enhanced by 10-, 70-, and 300-fold, respectively (P < 0·01, P < 0·001, P < 0·001, Fig. 2b,c). And expression of other Notch receptors and ligands was comparable to that in mock-infected cells. In addition, expression of Notch target gene Hes1, but not Hey1 was up-regulated by 90-fold (P < 0·001, Fig. 2d), suggesting that Notch signalling was activated in hMDM by DENV2.
Figure 2.
Expression levels of Notch molecules in dengue virus serotype 2 (DENV2) -infected human monocyte-derived macrophages (hMDM). Expression of CD14, CD16, CD11b, CD80 and HLA-DR in cells was measured by FACS (a). Human MDM were infected with DENV2 and harvested for real-time PCR. Expression of Notch receptors (b), ligands (c) and target genes (d) was analysed and normalized to that of GAPDH in each sample. Data are shown as mean ± SD of at least three independent experiments; **P < 0·01; ***P < 0·001.
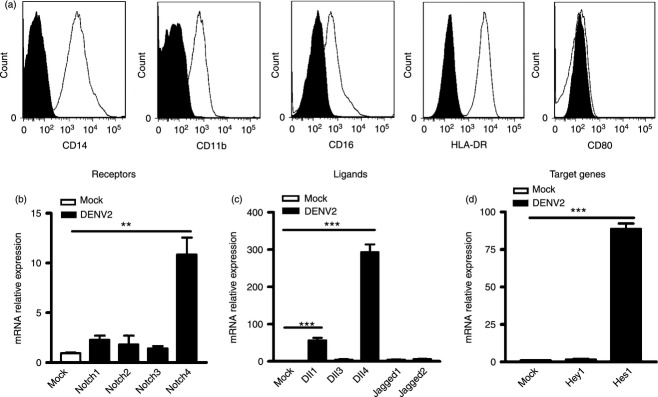
For monocyte-derived DC, their phenotype was validated by flow cytometry using surface antigens of DC including CD11c, CD1a and HLA-DR. These cells showed a typical immature DC expression pattern: CD11c+ CD1alow HLA-DR+ (Fig. 3a). Upon DENV2 infection, the induction pattern of Notch receptors and ligands in DC was similar to that seen in hMDM: Notch4, Dll1 and Dll4 were dramatically up-regulated while others were comparable to control cells (Fig. 3b,c). Interestingly, target gene Hey1 instead of Hes1, was induced in DC (P < 0·001, Fig. 3d), which was different from that seen in hMDM.
Figure 3.
Expression levels of Notch molecules in dengue virus serotype 2 (DENV2) -infected dendritic cells (DC). Expression of CD11c, CD1a and HLA-DR in cells was measured by FACS (a). DC were infected with DENV2 and harvested for real-time PCR. Expression of Notch receptors (b), ligands (c) and target genes (d) was analysed and normalized to that of GAPDH in each sample. Data are shown as mean ± SD of at least three independent experiments; **P < 0·01;***P < 0·001.
Taken together, these data indicated that DENV2 infection resulted in differential induction of Notch molecules, and activation of Notch signalling in monocytes, macrophages and DC.
Expression levels of Dll1 and Dll4 are induced in hMDM by DENV2 in a time- and dose-dependent manner
As both hMDM and DC belong to APC, and the induction patterns of Notch molecules in them were similar, we chose hMDM for all following assays. First, we examined the expression level of Notch ligands Dll1 and Dll4 following DENV2 infection at different times and MOIs. Real-time PCR data showed that in DENV2-infected cells, expression levels of Dll1 were gradually increased by 15-, 30- and 50-fold at 24, 36 and 48 hr post infection (p.i.), respectively (P < 0·01, P < 0·001, P < 0·001, Fig. 4a). And the expression levels of Dll4 were also gradually increased by 10-, 90-, 200- and 460-fold at 12, 24, 36 and 48 hr p.i., respectively (P < 0·01, P < 0·001, P < 0·001, P < 0·001, Fig. 4b). At MOI 0·1, 1 and 4 of DENV infection, Dll1 expression levels were induced by 10-fold and 45-fold, respectively (P < 0·05, P < 0·001, Fig. 4c). Expression of Dll4 was induced by 150-fold and 450-fold, respectively (P < 0·001, Fig. 4d). In addition, the expression levels of Dll1 and Dll4 proteins were assessed by Western blot. As shown in Fig. 4(e), Dll1 and Dll4 expression levels were significantly increased in hMDM after DENV2 infection. These data indicated that both mRNA and protein levels of Dll1 and Dll4 were increased by DENV2 infection in a dose-dependent manner.
Figure 4.
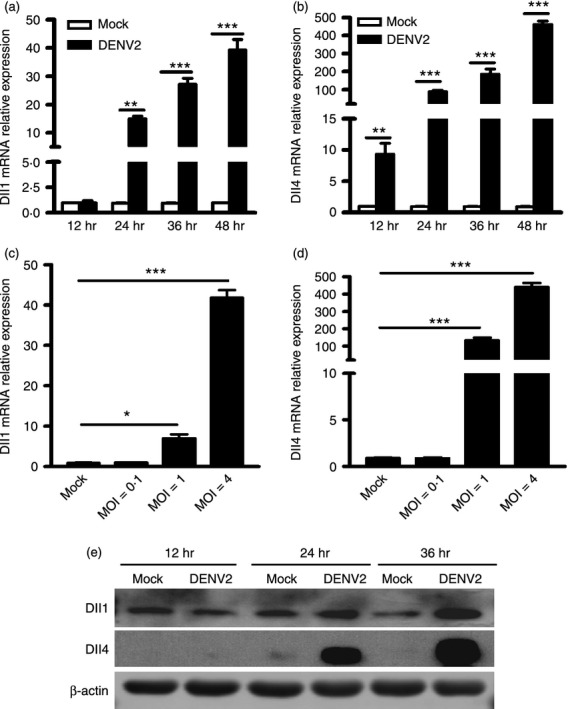
Dengue virus (DENV) enhances expression levels of Dll1 and Dll4 in a time- and dose-dependent manner. (a, b) Macrophages were infected with DENV2 at a multiplicity of infection (MOI) 4, for 12, 24, 36 and 48 hr. (c, d) Macrophages were infected with DENV2 at MOIs of 0·1, 1 and 4 for 36 hr. Cells were harvested for RNA extraction and real-time PCR to measure the mRNA expression levels of Dll1 and Dll4, and GAPDH as an internal control. (e) Western blot to detect the expression of Dll1 and Dll4 at the indicated times. Data are shown as mean ± SD of at least three independent experiments; *P < 0·05; **P < 0·01; ***P < 0·001.
Next, expression levels of Dll1 and Dll4 in response to other serotypes of DENV were examined. The hMDM were infected with DENV1 and DENV3 at an MOI of 1 and analysed for Dll1 and Dll4 expression by real-time PCR. As shown in Fig. 5(a,b), upon DENV1 and DENV3 infection, Dll1 and Dll4 expression levels were significantly higher than mock-infected cells (all P < 0·05, Fig. 5a,b). Next, to investigate whether the expression of Dll1 and Dll4 was dependent on the replication of DENV, we measured the mRNA levels of Dll1 and Dll4 in macrophages following active, heat- or UV- inactivated DENV2 stimulation. The expression of Dll1 and Dll4 was significantly increased following active DENV2 stimulation, whereas these ligands were not induced or were weakly induced in mock-infected, heat-inactivated or UV-inactivated DENV2-infected cells (Fig. 5c,d). These data suggest that the expression of Dll1 and Dll4 was not DENV serotype-specific, but depended on DENV production.
Figure 5.
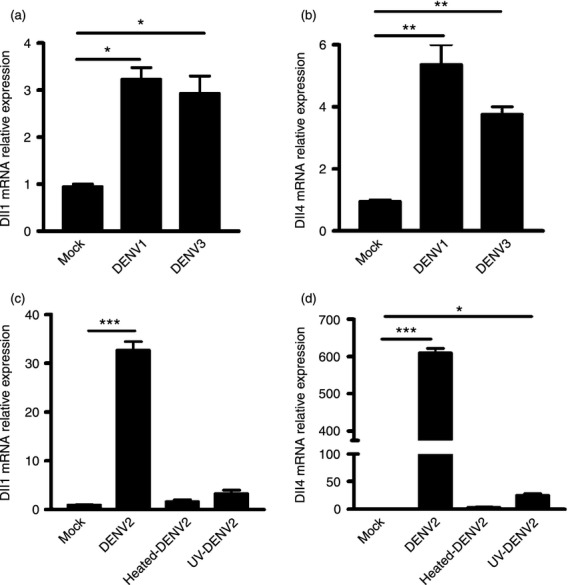
Expression of Dll1 and Dll4 is enhanced by dengue virus serotype 1 (DENV1) and DENV3 and depends on DENV replication. Human monocyte-derived macrophages (hMDM) were stimulated with DENV1 and DENV3 at a multiplicity of infection of 1 for 36 hr, the mRNA expression levels of Dll1 (a) and Dll4 (b) was examined by real-time PCR and normalized to that of GAPDH in each sample. The hMDM were infected with DENV2, UV- or heat-inactivated DENV2. Cells were harvested at 36 hr post-infection for RNA extraction. Expression of Dll1 (c) and Dll4 (d) was examined by real-time PCR and normalized to that of GAPDH in each sample. Data are shown as mean ± SD of at least three independent experiments; *P < 0·05; **P < 0·01; ***P < 0·001.
The DENV-induced Dll1 and Dll4 expression depends on IFN-β and IFNαR signalling pathway
To probe the underlying mechanism of Dll1 and Dll4 induction after DENV infection, we investigated whether type I IFN is involved. The hMDM were stimulated with rIFN-β or control vehicle (ctrl). Following rIFN-β stimulation, expression levels of Dll1 and Dll4 were elevated by 16-fold and 35-fold (all P < 0·01, Fig. 6a) compared with control cells. Next, we examined the protein expression levels of Dll1 and Dll4 following rIFN-β stimulation. As shown in Fig. 6(b), the protein levels of Dll1 and Dll4 were significantly enhanced in rIFN-β-treated cells. To further test whether DENV infection up-regulates Dll1 and Dll4 expression in an IFN-β-dependent manner, Dll1 and Dll4 expression was detected in the presence of IFN-β-neutralizing antibody in DENV2-infected hMDM for 36 hr. As shown in Fig. 6c,d, the mRNA and protein expression levels of Dll1 and Dll4 following DENV2 stimulation in hMDM were largely abrogated in anti-IFN-β-treated hMDM compared with control cells (all P < 0·05).
Figure 6.
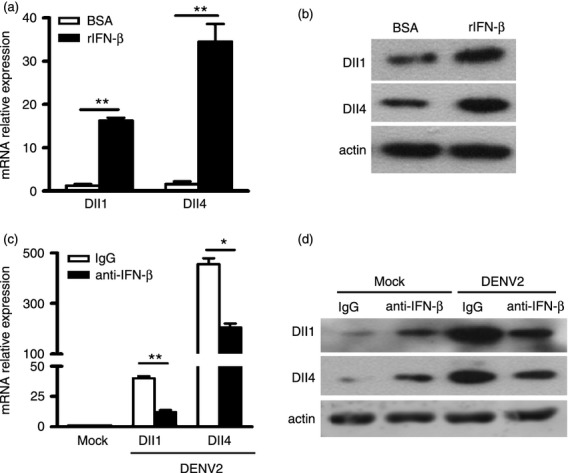
Dll1 and Dll4 expression is dependent upon interferon-β (IFN-β). (a) Human monocyte-derived macrophages (hMDM) were stimulated with rIFN-β (500 Units/ml) for 24 hr or vehicle-treated (ctrl). Dll1 and Dll4 mRNA expression was quantified by real-time PCR and normalized to that of GAPDH in each sample. (b) Protein levels of Dll1 and Dll4 were examined by Western blot. (c, d) Macrophages were infected with dengue virus serotype 2 (DENV2) (multiplicity of infection 4) for 36 hr in the absence or presence of the neutralizing antibody of IFN-β for 24 hr. Dll1 and Dll4 mRNA and protein expression was examined. Data are shown as mean ± SD of at least three independent experiments; *P < 0·05; **P < 0·01.
To further validate that type I IFN is involved in induction of Dll1 and Dll4, siRNAs against receptors of type I IFN, IFNαR1 and IFNαR2, were transfected into hMDM, followed by DENV2 infection. The real-time PCR data showed that transfection of siIFNαR1 and siIFNαR2 resulted in more than 50% reduction of IFNαR1 and IFNαR2 expression (all P < 0·05, Fig. 7a,b), validating the efficacy of siIFNαR1 and siIFNαR2. Next, Dll1 and Dll4 expression levels in IFNαR1 and IFNαR2 sufficient and deficient cells following DENV2 infection were measured by real-time PCR. As shown in Fig. 6(c,d), IFNαR1 and IFNαR2 depletion dramatically decreased the mRNA levels of Dll1 and Dll4 in response to DENV2 infection. These data revealed that DENV2 infection up-regulated expression of Dll1 and Dll4, and the presence of IFN-β was essential for the induction of Dll1 and Dll4.
Figure 7.
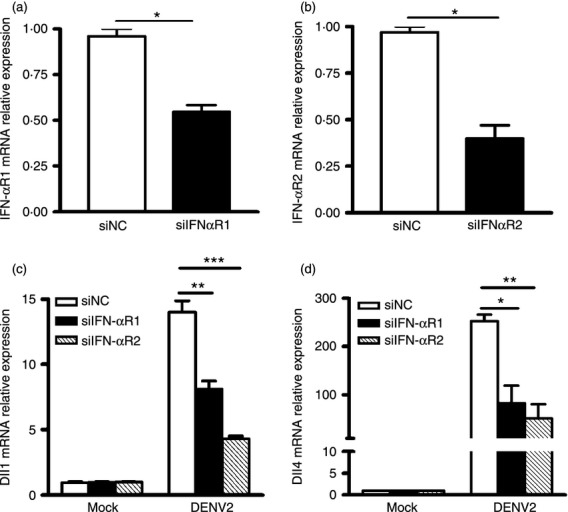
Interferon-β (IFN-β) signalling pathway is involved in the induction of Dll1 and Dll4. Macrophages were transfected with IFNαR1 short-interfering RNA (siIFNαR1), IFNαR2 siRNA (siIFNαR2) or control siRNA (siNC). The cells were then incubated with dengue virus serotype 2 (DENV2) (multiplicity of infection 4) for 36 hr. The expression level of IFNαR1 or IFNαR2 (a, b) Dll1 and Dll4 (c, d) were examined by real-time PCR and normalized to that of GAPDH in each sample. Data are shown as mean ± SD of at least three independent experiments; *P < 0·05; **P < 0·01; ***P < 0·001.
TLR3, MyD88 and RIG-I/IPS-1 pathways are involved in the regulation of Dll1 and Dll4
As the signalling pathway leading to type I IFN induction can be either a TLR/MyD88 or an RLR/IPS-1 axis, we then distinguished which signalling pathway is responsible for Dll1 and Dll4 induction. The siRNA against TLR3 (siTLR3), MyD88 (siMyD88), RIG-I (siRIG-I), MDA-5 (siMDA-5) or IPS-1 (siIPS-1) was transfected into hMDM, followed by DENV2 challenge. The efficacy of siTLR3, siMyD88, siRIG-I, siMDA-5 or siIPS-1 resulted in more than 80% depletion of corresponding proteins in hMDM (data not shown). Expression levels of Dll1 and Dll4 in TLR3-, MyD88-, RIG-I-, MDA-5- or IPS-1-silencing cells were examined by real-time PCR. The induction levels of Dll1 (P < 0·05, P < 0·01, P < 0·05, P < 0·05, Fig. 8a) and Dll4 (all P < 0·05, Fig. 8b) mediated by DENV2 were significantly reduced in TLR3, MyD88, RIG-I and IPS-1 knockdown cells. In contrast, in MDA-5 knockdown cells, the expression levels of Dll1 and Dll4 were comparable to those in control cells (Fig. 8a,b). Next, expression levels of IFN-β in these siRNA transfected cells were examined by real-time PCR. Figure 8(c) shows that the IFN-β levels mediated by DENV2 infection were significantly decreased in TLR3-, MyD88-, RIG-I- and IPS-1- silencing cells, but not in MDA-5-silencing cells (all P < 0·01). Our results suggest that DENV-induced Dll1 and Dll4 expression is TLR3-, MyD88- and RIG-I/IPS-1-dependent.
Figure 8.
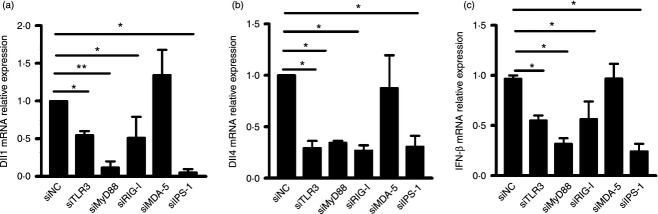
The Dll1 and Dll4 induction is dependent on Toll-like receptor 3 (TLR3), myeloid differentiation primary response gene 88 (MyD88), retinoic acid inducible gene-I (RIG-I) and interferon-β promoter stimulator 1 (IPS-1), but not melanoma differentiation associated gene 5 (MDA-5). Macrophages were transfected with siTLR3, siMyD88, siRIG-I, siMDA-5, siIPS-1 or control siRNA (siNC) for 48 hr. Cells were harvested for RNA extraction. The expression levels of Dll1 and Dll4 (a, b), interferon-β (IFN-β) (c) in siTLR3-, siMyD88-, siRIG-I-, siMDA-5-, and siIPS-1-transfected cells were examined by real-time PCR and normalized to that of GAPDH in each sample. Data are shown as mean ± SD of at least three independent experiments; *P < 0·05; **P < 0·01.
Activation of Notch pathway does not affect the replication of DENV2 in macrophages
To test whether the Notch pathway has a biological function in viral replication, we activated Notch pathway in macrophages using rDll1 protein, and compared the viral replication levels by real-time PCR or TCID50 assay. As shown in Fig. 9(a), the mRNA level of Notch target gene Hes1 was increased sixfold in hMDM cultured on rDll1-coated plates compared with control (P < 0·01), indicating that Notch pathway was activated. The viral RNA levels (Fig. 9b) and viral yields (Fig. 9c) were comparable between the rDll1-treated cells and control cells, suggesting that the activation of Notch pathway in macrophages does not have a direct impact on the viral replication.
Figure 9.
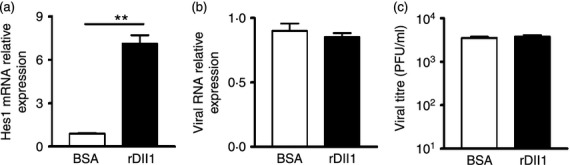
Activation of Notch signalling pathway has no effect on the replication of dengue virus serotype 2 (DENV2). RNAs were extracted from DENV2-infected macrophages treated with BSA or rDll1. The levels of Hes1 mRNA (a) and DENV RNA (b) were analysed by real-time PCR. Supernatants from DENV2-infected macrophages cultured on BSA- or rDll1-coated plates for 48 hr were harvested for virus titration. (c) DENV2 titres were examined by TCID50. Data are shown as mean ± SD of at least three independent experiments; **P < 0·01.
Activation of Notch pathway by Dll1 promotes a Th1 differentiation
As our data clearly showed that Dll ligands, but not Jagged ligands were increased in hMDM and DC, and both hMDM and DC function as APC to help T-cell activation and differentiation, we further investigated whether Dll ligands play a role in T-cell differentiation by stimulating naive CD4+ T cells with rDll1 or BSA, and measuring the expression of a Th1 cytokine (IFN-γ) and a Th2 cytokine (IL-4). Expression of the Notch target gene Hes1 was increased eightfold in CD4+ T cells treated with rDll1 (P < 0·01, Fig. 10a), validating the idea that the Notch pathway was activated by Dll1 protein. In the rDll-incubated T cells, the expression level of IFN-γ was enhanced fivefold (Fig. 10b), whereas the level of IL-4 (Fig. 10c) was comparable to control cells. The data suggested that Dll1 can specifically promote the production of Th1 cytokine.
Figure 10.
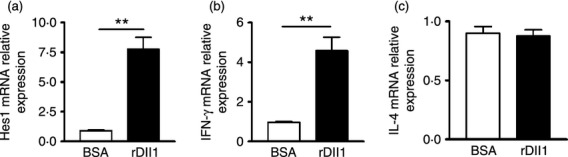
Notch activation by Dlls in T cells increases the expression of T helper type 1 cytokine. Naive CD4 T cells were stimulated with rDll1 for 48 hr, and harvested for real-time PCR to detect the expression levels of Hes1 (a), interferon-γ (IFN-γ) (b) and interleukin-4 (IL-4) (c). Data are shown as mean ± SD of at least three independent experiments; **P < 0·01.
Discussion
Notch signalling has been indicated to play critical roles in the immune response against viral invasion. The present study for the first time investigated the relationship between Notch and DENV. Our data demonstrated that the expression of Notch molecules is differentially regulated by DENV infection, and provided further investigations into the signalling molecules that are involved in the induction of Notch ligands.
Our work first screened the expression pattern of Notch molecules in three major in vivo target cells of DENV, namely monocytes, hMDM and DC, and found that Notch molecules are differentially regulated by DENV. In monocytes, only Notch ligand Dll1 was highly induced; whereas in both hMDM and DC, we observed that Notch receptors and more ligands are up-regulated, and the Notch signalling pathway is activated by DENV infection. This finding is in keeping with previous observations with other viruses: influenza virus induces expression of Dll1 but not Dll4;22 and RSV induces expression of Dll4 in bone marrow-derived DC.14 The differences of Notch molecule induction and Notch signalling activation between monocytes and APC (hMDM and DC) gives another hint that Notch signalling is required for APC action. Altogether, we concluded that the regulation of Notch molecules is virus-specific and cell-specific.
Importantly, several lines of evidence demonstrate that the induction of Dll1 and Dll4 mediated by DENV is closely associated with IFN-β. First, in the DENV-infected macrophage cells, the up-regulation of Dll1 and Dll4 expression was seen until 24 hr post-infection, which is later than the time when IFN-β was induced. Second, both ligand induction and IFN-β induction were dependent on the innate immune molecules, including TLR3, MyD88, RIG-I and IPS-I, but not MDA-5. Third, direct treatment of macrophages with IFN-β led to a high induction of Dll1 and Dll4, while neutralization of IFN-β led to impairment of Dll1 and Dll4 induction, strongly suggesting that IFN-β is one of the regulators of Notch ligands. Finally, the cells depleted of IFN-αR failed to induce Dll1 and Dll4 expression in response to DENV2. These data illustrates that the DENV-triggered Dll1 and Dll4 expression depends on TLR3-, MyD88- and RIG-I-mediated IFN-β signalling pathways.
Not only does DENV induce the expression of Notch molecules, but it also activates the Notch signalling in APC. Considering that both of the Notch receptors and ligands are constitutively expressed on the surface of APC, we deduced that the activation of Notch signalling could be a comprehensive outcome from the interaction between either basal or inducible proteins of Notch receptors and Notch ligands.
Functionally, we did not observe a direct antiviral role of Notch activation by Dll1 in infected macrophages, but the Dll1-activated Notch signalling specifically up-regulates the expression of Th1 cytokine. This observation agrees with other work showing the role of different ligands in Th1 and Th2 differentiation: Dll ligands in the APC surface lead to a differentiation of Th0 cells toward Th1,20,44,45 while Jagged ligands predominantly promote a Th2 differentiation.20,46,47 Therefore, we proposed that in response to DENV infection, APC specifically up-regulate the expression of Dll1 and Dll4 ligands, to promote a Th1 differentiation and shape a cell-mediated immune response to combat intracellular DENV particles. In addition, the activation of Notch signalling might have other influences as the induction of target genes inevitably alters host cell physiology.
In summary, our study for the first time demonstrated that expression of some Notch receptors, ligands and target genes was up-regulated in DENV-infected APC. Moreover, we found that induction of ligands Dll1 and Dll4 by DENV infection is dependent on IFN-β, and these ligands play some role in T-cell activation and differentiation. Therefore, the Notch signalling pathway is an important bridge to connect innate and adaptive immune responses to DENV infection. The role of the Notch signalling pathway in DENV infection needs further investigation, to advance our understanding of the immune response against DENV and to provide new approach for the prevention and clinical treatment of DENV diseases.
Acknowledgments
YYL, XH and PZ conceived and designed the experiments and wrote the paper. YYL, JYP and SYW performed the experiments and YYL, SYW analysed the data. This work was supported by the National Natural Science Foundation of China (81261160323, 81171576, 81371794), Guangdong Innovative Research Team Programme (NO. 2009010058), Guangdong Province Universities and Colleges Pearl River School Funded Scheme (NO. 2009), and the Guangdong Natural Science Foundation. (S2013010016454).
Glossary
- APCs
an antigen-presenting cells
- DCs
dendritic cells
- DENV
dengue virus
- Dll
Delta-like
- Hes
helic-loop-helix family
- Hey
hairy and enhancer of split-related (HESR) family
- hMDMs
human monocyte-derived macrophages
- IFN
interferon
- IFNαR
IFNα- receptor
- IPS-1
interferon-β promoter stimulator 1
- MDA-5
melanoma differentiation associated gene 5
- Notch 1
Notch receptors 1
- p.i.
post infection
- PAMPs
pathogen-associated molecular pattern
- PRRs
pattern recognition receptors
- RIG-I
retinoic acid inducible gene-I
- siRNAs
short interfering RNAs
- TLR
Toll-like receptor
Disclosures
There are no financial or commercial conflicts of interest.
References
- 1.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 3.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–55. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 4.Fischer A, Gessler M. Delta-Notch–and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–96. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyne GF, Dallman MJ, Champion BR, Lamb JR. Notch signalling in the regulation of peripheral immunity. Immunol Rev. 2001;182:215–27. doi: 10.1034/j.1600-065x.2001.1820118.x. [DOI] [PubMed] [Google Scholar]
- 6.Harman BC, Jenkinson EJ, Anderson G. Microenvironmental regulation of Notch signalling in T cell development. Semin Immunol. 2003;15:91–7. doi: 10.1016/s1044-5323(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MJ, Champion B, Lamb JR. Notch signalling in the peripheral immune system. Novartis Found Symp. 2003;252:268–76. discussion 276-68. [PubMed] [Google Scholar]
- 8.McKenzie GJ, Young LL, Briend E, Lamb JR, Dallman MJ, Champion BR. Notch signalling in the regulation of peripheral T-cell function. Semin Cell Dev Biol. 2003;14:127–34. doi: 10.1016/s1084-9521(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt TM, Zuniga-Pflucker JC. Thymus-derived signals regulate early T-cell development. Crit Rev Immunol. 2005;25:141–59. doi: 10.1615/critrevimmunol.v25.i2.40. [DOI] [PubMed] [Google Scholar]
- 10.Basson MA, Zamoyska R. The CD4/CD8 lineage decision: integration of signalling pathways. Immunol Today. 2000;21:509–14. doi: 10.1016/s0167-5699(00)01711-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Zhou J, Cheng PY, Gabrilovich D. Notch ligand Jagged-1 negatively regulates dendritic cell differentiation during emergency myelopoiesis by inhibiting Wingless signaling. J Immunol. 2012;188:111–4. [Google Scholar]
- 12.Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE, Kaufmann SH, Osborne BA. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174–83. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- 13.Foldi J, Chung AY, Xu H, et al. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. J Immunol. 2010;185:5023–31. doi: 10.4049/jimmunol.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaller MA, Buranaruk C, Rengpipat S, Fauq AH, Golde TE, Kaufmann SH, Osborne BA. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med. 2007;204:2925–34. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh F, Irvine KM, Lovelace E, et al. Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling. Immunology. 2009;127:326–37. doi: 10.1111/j.1365-2567.2008.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao PN, Wei SC, Huang MT, Lee MC, Chou HC, Chen CY, Hsieh WS. Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J Biomed Sci. 2011;18:56. doi: 10.1186/1423-0127-18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Wang C, Liu Z, Liu X, Han C, Cao X, Li N. Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor κB activation. J Biol Chem. 2012;287:6208–17. doi: 10.1074/jbc.M111.310375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Chung AY, Wu I, et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-γ pathways. Immunity. 2008;29:691–703. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Zhu J, Smith S, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642–50. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 21.Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35:2443–51. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Allen RM, William F, et al. The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog. 2011;7:e1002341. doi: 10.1371/journal.ppat.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell PK, Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967;99:291–6. [PubMed] [Google Scholar]
- 24.Normile D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- 25.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, He L, Li Y, Wang T, Feng L, Jiang L, Zhang P, Huang X. miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J Infect. 2013;67:329–41. doi: 10.1016/j.jinf.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YT, Chang SY, Lee CN, Kao CL. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell Microbiol. 2009;11:604–15. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 28.Conceicao TM, El-Bacha T, Villas-Bôas CS, Coello G, Ramírez J, Montero-Lomeli M, Da Poian AT. Gene expression analysis during dengue virus infection in HepG2 cells reveals virus control of innate immune response. J Infect. 2010;60:65–75. doi: 10.1016/j.jinf.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Gilfoy FD, Mason PW. West Nile virus-induced interferon production is mediated by the double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81:11148–58. doi: 10.1128/JVI.00446-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos HJ, Gale M., Jr RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr Opin Virol. 2011;1:167–76. doi: 10.1016/j.coviro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 33.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 34.Biron CA. Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–90. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 35.Knop J, Penick EC, L Mortensen E, Nickel EJ, Gabrielli WF, Jensen P, Mednick SA. Prediction of mortality at age 40 in Danish males at high and low risk for alcoholism. Acta Psychiatr Scand. 2004;110:476–82. doi: 10.1111/j.1600-0447.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 36.Chalise J, Narendra S, Paudyal B, Magnusson M. Interferon α inhibits antigen-specific production of proinflammatory cytokines and enhances antigen-specific transforming growth factor β production in antigen-induced arthritis. Arthritis Res Ther. 2013;15:R143. doi: 10.1186/ar4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, Pfeffer LM. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem. 2013;288:26167–76. doi: 10.1074/jbc.M113.477950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 39.Maul A. Aspects statistiques des méthodes de quantification en virologie. 1991. pp. 143–71. In Virologie des milieux hydriques, Tec & Doc - Lavoisier.
- 40.Deng Q, Sun M, Yang K, Zhu M, Chen K, Yuan J, Wu M, Huang X. MRP8/14 enhances corneal susceptibility to Pseudomonas aeruginosa infection by amplifying inflammatory responses. Invest Ophthalmol Vis Sci. 2013;54:1227–34. doi: 10.1167/iovs.12-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu M, Li D, Wu Y, Huang X, Wu M. TREM-2 promotes macrophage-mediated eradication of Pseudomonas aeruginosa via a PI3K/Akt pathway. Scand J Immunol. 2014;79:187–96. doi: 10.1111/sji.12148. [DOI] [PubMed] [Google Scholar]
- 42.Wu MH, Peng A, Sun M, et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating toll-like receptor signaling and Th1/Th2-type immune responses. Infect Immun. 2011;79:2709–16. doi: 10.1128/IAI.00144-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K, Yin L, Nie X, et al. β-Catenin promotes host resistance against Pseudomonas aeruginosa keratitis. J Infect. 2013;67:584–94. doi: 10.1016/j.jinf.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 44.Skokos D, Nussenzweig MC. CD8– DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204:1525–31. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180:1655–61. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol. 2008;180:7931–7. doi: 10.4049/jimmunol.180.12.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worsley AG, LeibundGut-Landmann S, Slack E, Phng LK, Gerhardt H, Reis e Sousa C, MacDonald AS. Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo. Eur J Immunol. 2008;38:1043–9. doi: 10.1002/eji.200737335. [DOI] [PubMed] [Google Scholar]