Abstract
A role for complement, particularly the classical pathway, in the regulation of immune responses is well documented. Deficiencies in C1q or C4 predispose to autoimmunity, while deficiency in C3 affects the suppression of contact sensitization and generation of oral tolerance. Complement components including C3 have been shown to be required for both B-cell and T-cell priming. The mechanisms whereby complement can mediate these diverse regulatory effects are poorly understood. Our previous work, using the mouse minor histocompatibility (HY) model of skin graft rejection, showed that both C1q and C3 were required for the induction of tolerance following intranasal peptide administration. By comparing tolerance induction in wild-type C57BL/6 and C1q-, C3-, C4- and C5-deficient C57BL/6 female mice, we show here that the classical pathway components including C3 are required for tolerance induction, whereas C5 plays no role. C3-deficient mice failed to generate a functional regulatory T (Treg) –dendritic cell (DC) tolerogenic loop required for tolerance induction. This was related to the inability of C3-deficient DC to up-regulate the arginine-consuming enzyme, inducible nitric oxide synthase (Nos-2), in the presence of antigen-specific Treg cells and peptide, leading to reduced Treg cell generation. Our findings demonstrate that the classical pathway and C3 play a critical role in the peptide-mediated induction of tolerance to HY by modulating DC function.
Keywords: complement, dendritic cells, regulatory T cells, rodent, tolerance
Introduction
The complement system, a major component of innate immunity, also has an important regulatory role in adaptive immune responses, where it is involved in both the induction and control of B-cell and T-cell activation.1,2
A role for the classical pathway in immune regulation stems from the observations that, in humans, deficiencies in C1q or C4 predispose to systemic lupus erythematosus.3 The absence of these components causes impaired clearance of apoptotic cells4 and defective B-cell regulation.5,6 C3 has also been reported to be important in the regulation of immune responses, particularly in the suppression of contact sensitization and generation of oral tolerance,7–9 and has been recently demonstrated to have an immunosuppressive role in the differentiation of myeloid suppressor cells.10 Conversely, complement, particularly C3, is required for optimal B-cell immunity11–13 and T-cell priming.2,14,15 Signalling by the complement fragments C3a and C5a through their receptors, C3aR and C5aR, has been implicated in T-cell activation directly16,17 or indirectly through dendritic cell (DC) activation.18–20 The mechanisms dictating the modulatory function of complement in adaptive immunity remain poorly understood and depend on the experimental conditions applied.
Similarly the role of complement in tolerance remains unresolved. Although there is evidence that C3 is needed for the rejection of fully MHC mis-matched kidney grafts,21 we and others have found, using the minor histocompatibility (HY) model of skin graft rejection, that C1q and C3 were required not for rejection, but for the induction of tolerance.23 We previously showed that intranasally (i.n.) administered MHC class II-restricted HY-AbDby peptide led to its presentation by immature DC and the generation of peptide-specific CD4 regulatory T (Treg) cells.24,25 Following skin grafting, or immunization with male cells, the effect of these Treg cells was to impair the presentation of the full cohort of MHC class I and II male epitopes resulting in a diminished cytotoxic T lymphocyte response and graft tolerance.24 These findings were consistent with the notion of a regulatory feedback loop between Treg cells and DC and generation of a population of tolerogenic DC.26,27
Here, we have extended our observations and uncovered some potential mechanisms by which C3 might regulate the induction of peptide-induced tolerance and linked suppression.
Materials and methods
Mice
C57BL/6 (B6) were from Harlan (Bicester, UK). B6.C1qa–/–,28 B6.C3–/–,29 B6.C4–/–29 and B6.C5–/–30 were previously described and backcrossed onto C57BL/6 for 10 generations. Mice with T-cell receptors transgenic for the HY-AbDby peptide (Marilyn mice)31 were provided by Dr O. Lantz (Paris, France). All experiments on animals complied with standard conditions and were covered by a UK Home Office Project License.
Tolerance induction and skin grafting
The HY-AbDby peptide (100 μg diluted in 20 μl PBS) (NAGFNSNRANSSRSS; ThinkPeptides, Sarasota, FL) was administered i.n. on 3 consecutive days to female mice anaesthetized under isoflurane. Control mice received PBS. Ten days later, skin grafting was performed32 using male and female tail skin grafted onto the lateral thorax of syngeneic female recipients. Grafts were scored as rejected when < 10% viable tissue remained. After 100 days, selected mice were injected intraperitoneally with 5 × 106 male splenocytes and 7 days later the HY-specific CD8 T-cell response was measured using HY-DbUty dextramer (Immudex, Copenhagen, Denmark).
Isolation of splenic DC and T-cell proliferation
Splenic DC were isolated using a CD11c-positive selection kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Then, 2·5 × 104 CD4+ Marilyn T cells were added to purified DC and incubated for 72 hr. Wells were pulsed with 0·5 μCi [3H]thymidine for the last 18 hr. Plates were counted using a Wallac Trilux 1450 MicroBeta liquid scintillation counter (PerkinElmer LAS, Seer Green, UK).
Adoptive cell transfer
Marilyn CD4+ CD25− T cells were isolated from Marilyn mice using a CD4 negative selection kit (Miltenyi Biotec GmbH), followed by a CD25 selection kit (Miltenyi). Cells were labelled with 5 μm carboxyfluorescein diacetatesuccinimidyl ester (CFSE; Molecular Probes®, Life Technologies, Paisley, UK) according to the manufacturer's instructions. Then 2 × 106 to 5 × 106 labelled cells were injected intravenously into each recipient. Results are shown as proliferation index, calculated using flowjo software.
Flow cytometry
Cells were stained using standard protocols in the presence of saturating anti-FcγRII/III (2.4G2). The following antibodies were used: anti-mouse CD90.1 (OX.7), anti-mouse CD25 (PC61), anti-mouse CD4 (GK1.5), anti-mouse CD40 (3/23), anti-mouse CD86 (GL1), anti-mouse H-2A (2G9) from BD Biosciences (San Diego, CA); anti-rat/mouse forkhead box p3 (Foxp3; FJK-16s), anti-mouse CD8 (53–6.7) from Life Technologies. Data were acquired using a FACSVerse (Becton-Dickinson, Mountain View, CA) and analysed using flowjo software, (TreeStar, Ashland, OR). Foxp3 staining was performed according to the manufacturer (eBioscience, San Diego, CA).
Treg cell generation and suppression assay
CD4+ cells were isolated by negative selection (Miltenyi Biotec GmbH) and cultured in RPM1-1640 at 106 cells/ml in U-bottom wells coated with anti-CD3 antibody (1 μg/ml, 1452C11, Biolegend, San Diego, CA) and anti-CD28 antibody (1 μg/ml; eBioscience); in the presence of recombinant human transforming growth factor-β (TGF-β; 10 ng/ml, PeproTech, Rocky Hill, NJ), anti-interferon-γ antibody (2·5 μg/ml, Biolegend) and r-mouse interleukin-2 (5 ng/ml) for 4 days. The T-cell suppression assay was performed as previously described.33 Proliferation was expressed as a division index, calculated using flowjo software.
Co-culture assays
Bone marrow dendritic cells (BMDC) were generated as previously described.34 Marilyn induced Treg (iTreg) cells were obtained from naive Rag2–/– Marilyn spleen cells (5 × 105 cells/ml) cultured with BMDC (105 cells/ml), HY-AbDby peptide (10 nm), recombinant human TGF-β (2 ng/ml) and retinoic acid (1 μm, Sigma, St Louis, MO) for 5 days. Live cells were recovered on a gradient density (Lympholyte, Cedarlane Laboratories, Burlington, ON, Canada). Staining indicated ≥ 70% Foxp-3+ cells. Fresh BMDC (106 cells/ml) were co-cultured with iTreg cells (106 cells/ml) in the presence or absence of HY-AbDby peptide (1 nm) for 48 hr.
Quantitative real-time RT-PCR
RNA was extracted from cell cultures using RNeasy columns (Qiagen, Hilden, Germany). The cDNA was generated using an iScript cDNA synthesis Kit (BioRad, Hercules, CA). Real-time PCR were performed using specific primers (see Supporting information, Table S1) with SYBR green master mix (Applied Biosystems®, Life Technologies, Paisley, UK) and analysed on a ViiA™7 RealTime PCR system (Applied Biosystem®, Life Technologies). Relative quantities were calculated using the ΔΔCt method.35 Samples that gave no detectable signal were attributed a Ct value of 40; samples were normalized to Gapdh.
Statistical analysis
Comparisons between two groups were performed using two-tailed unpaired Student's t-test. Graft survival was compared using the log-rank test. Statistical significance is defined as P ≤ 0·05.
Results
Early components of the classical pathway are required for induction of nasal tolerance
C57BL/6 (B6) wild-type (WT) female mice can be tolerized to the male HY antigen by i.n. administration of the HY-AbDby peptide,25 but not in the absence of C1q or C3.22 To establish whether the terminal pathway of complement was also required, C5-deficient (B6.C5–/–) female mice received i.n. HY-AbDby peptide and 10 days later were grafted with male skin. Peptide-treated B6.C5–/– females became tolerant, maintaining the male graft as well as WT mice (Fig. 1a). In contrast, as previously reported,22 female mice lacking C1q (B6.C1qa–/–) or C3 (B6.C3–/–) started to lose their grafts with median survival times (MST) of 34 and 37 days, respectively and fewer than 20% retained their graft after 100 days. Syngeneic female grafts were accepted indefinitely. As both C1q36,37 and C37–10 have been shown to independently regulate immune responses, we performed similar experiments with C4-deficient female mice (B6.C4–/–) to investigate whether the early components of the classical pathway were required for tolerance induction. No long-term tolerance was achieved in these mice; they rejected their grafts with an MST of 47 days, which was not statistically different from that of B6.C1qa–/– and B6.C3–/– mice (Fig. 1a). Moreover, these graft survival times were not different from that of non-peptide treated WT mice (MST 31 days). These results therefore indicate a role for the early phase of the classical pathway of complement activation, rather than an independent role for the individual components C1q and C3 or the terminal pathway. To further confirm these findings, some mice were given an in vivo boost with male cells 100 days after grafting and their CD8 T-cell response was measured 7 days later using an HY-peptide-specific (HY-DbUty) dextramer (Fig. 1b). The tolerant WT and B6.C5–/– mice gave a very low anti-HY-specific CD8 T-cell response, whereas the non-tolerant C3-deficient and C4-deficient mice showed large expansions of the HY-DbUty dextramer+ CD8 T-cell population, indicating a failure in the non-tolerant animals to control the cytotoxic response against HY. Hence, the early phase of the classical pathway plays a key role in this model of tolerance. As C3 is central to this, we elected to focus on this molecule to investigate possible mechanisms.
Figure 1.
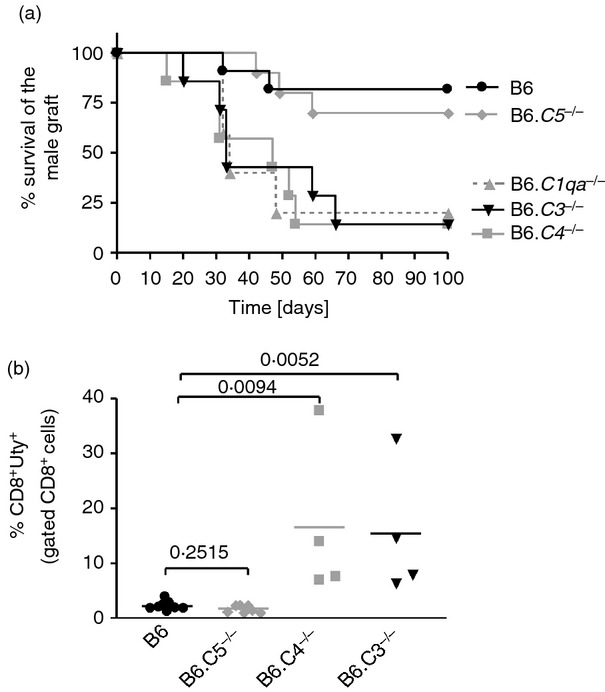
Impaired induction of tolerance in mice lacking early components of the classical complement pathway. B6 wild-type (WT; black circle), B6.C1qa–/–- (grey triangles), B6.C4–/– (grey squares), B6.C3–/– (black triangles) and B6.C5–/– (grey diamonds) female mice were given HY-AbDby peptide (100 μg for 3 days) intranasally. (a) Survival of syngeneic male skin grafts transplanted on peptide-treated female WT (n = 11), B6.C1qa–/– [n = 5, median survival time (MST) 34 days, P = 0·0141], B6.C4–/– (n = 7, MST 47 days, P = 0·0045), B6.C3–/– (n = 7, MST 33 days, P = 0·0030), B6.C5–/– (n = 10, P = 0·6039). Syngeneic female grafts were accepted indefinitely. Statistical analysis was performed using the log-rank method comparing separately each complement-deficient strain to peptide-treated WT B6. (b) 100 days after skin grafting, selected mice were injected intraperitoneally with 5 × 106 male splenocytes and 7 days later spleen cell suspensions were analysed for HY-specific CD8 T-cell responses (HY-DbUty dextramer+ CD8 T cells) by flow cytometry. B6 (n = 9), B6.C5–/– (n = 7), B6.C4–/– (n = 4), B6.C3–/– (n = 4). Statistical analysis: t-test.
C3 does not affect peptide dissemination in vivo
The initial phase in this model of tolerance induction is the presentation of the peptide by antigen-presenting cells, most likely DC. Twenty-four hours after the i.n. administration the peptide is found in CD11c-positive DC in spleen, and in peripheral and cervical draining lymph nodes.24 To determine whether complement affected the peptide dissemination and/or presentation, B6 WT and B6.C3–/– female mice were given i.n. HY-AbDby peptide. Twenty-four hours later splenic DC were isolated and used to stimulate HY-AbDby-specific CD4+ T cells from TCR transgenic Marilyn mice (Marilyn T cells). The purity, percentage of CD8+/CD8− DC and expression levels of CD40, CD86 and H2-Ab were similar (data not shown). Regardless of their complement status splenic, DC from the peptide-treated mice were equally able to stimulate the proliferation of the Marilyn T cells (Fig. 2); hence complement does not influence the distribution or initial presentation of the peptide.
Figure 2.
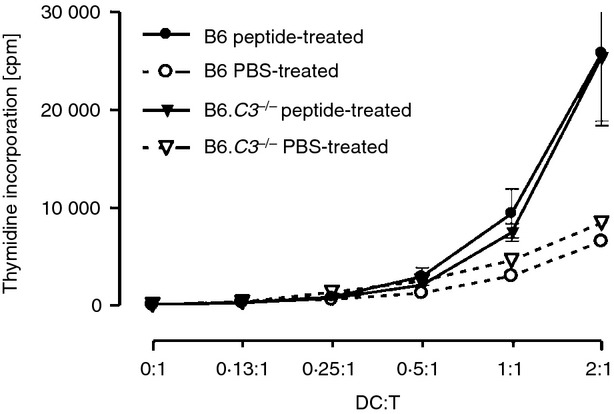
C3 deficiency does not alter the distribution of the peptide. B6 wild-type (WT; n = 3) and B6.C3–/– (n = 3) female mice were given the HY-AbDby peptide twice (100 μg at time 0 and 6 hr later) intranasally, control mice received PBS. Twenty-four hours after the first peptide dose, splenic dendritic cells (DC) were isolated and cultured with Marilyn T cells (25 000 T cells/well) at the ratios indicated. Proliferation was assessed by pulsing with [3H]thymidine for the final 18 hr of the 72 hr incubation. Data are shown as mean ± SEM, Student's t-test. (DC : T ratio 2 : 1 P = 0·9704, 1 : 1 P = 0·5030).
The early phase of tolerance induction is defective in B6.C3–/– mice
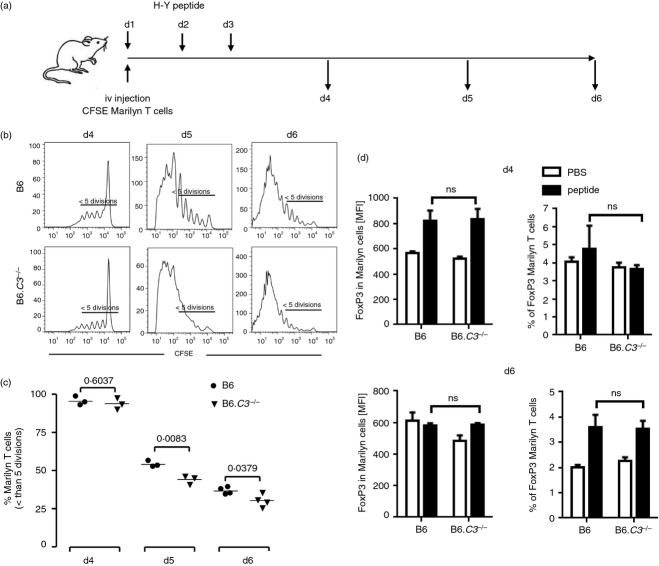
To study the early phase of tolerance induction, concomitantly with i.n. peptide administration, we adoptively transferred intravenously CFSE-labelled Marilyn CD4 T cells to act as indicators of the endogenous anti-HY response. This protocol has been previously shown to have no effect on peptide-mediated tolerance induction.24 Proliferation was assessed at days 4, 5 and 6 (Fig. 3a).
Figure 3.
Increased antigen-specific T-cell proliferation in response to intranasal (i.n.) peptide delivery in B6.C3–/– mice. 2 × 106 CFSE-labelled Marilyn CD90.1 CD4+ CD25− T cells were injected intravenously into female recipient B6 wild-type (WT) or B6.C3–/– mice (three or four animals per group). Mice received i.n. three doses of the HY-AbDby peptide. Splenocytes were recovered 4, 5 and 6 days after the first peptide dose and proliferation of Marilyn T cells (gated as CD4+, CD90.1+) was analysed by flow cytometry. (a) Experimental schedule. (b) Representative data of gated Marilyn T-cell proliferation. (c) Percentage of Marilyn T cells with less than five divisions. (d) Foxp3 expression [mean fluorescence intensity (MFI)] in Marilyn T cells (left panel) and percentage of Foxp3+ Marilyn T cells (right panel). Results from control mice receiving PBS are indicated by open columns; those from peptide-treated mice are shown by filled columns. Data are shown as mean ± SEM. Statistical analysis by unpaired t-test; P-values as indicated; ns, non-significant.
No differences in proliferation were observed at day 4, confirming that complement does not play a role in the early distribution and presentation of the peptide. By day 5 Marilyn T cells transferred into B6.C3–/– mice had divided extensively, and by day 6 most of the cells were CFSE negative. In contrast, Marilyn T cells transferred into WT mice demonstrated a significantly slower rate of proliferation (Fig. 3b,c).
To determine whether complement affected Treg cell induction in the adoptively transferred Marilyn T cells, we examined Foxp3 expression at days 4 and 6 (Fig. 3d). At day 4 the mean fluorescence intensity of Foxp3 in the Marilyn T cells was increased compared with the PBS-treated controls,38 irrespective of the complement status of the recipient mice (Fig. 3d, left hand panel). The percentage of Foxp3+ Marilyn T cells was similar. By day 6, the percentage of Foxp3+ Marilyn T cells had increased in the peptide-treated compared with the PBS-treated mice, again regardless of the complement status of the hosts (Fig. 3d, right hand panel). Hence, although C3 was required for the peptide-mediated regulation of proliferation of the adoptively transferred Marilyn T cells, it appeared to have little effect on the induction of Foxp3+ Treg cells.
The early phase of the induction of linked tolerance is impaired in B6.C3–/– mice
To examine the early phase of induction of linked tolerance, to the additional HY epitopes expressed on male tissue, B6 WT and B6.C3–/– mice were given an intraperitoneal injection of male splenocytes 10 days after receiving three doses of HYAbDby peptide or control PBS. At the same time, CFSE-labelled Marilyn T cells were injected intravenously (Fig. 4a). At day 3 there was minimal proliferation by the Marilyn T cells, even from the peptide-treated mice. However, by days 5 and 6 a marked increase in the proliferation of Marilyn T cells in response to the immunization with male cells was found in the control PBS-treated mice regardless of their complement status as well as in the peptide-treated B6.C3–/– mice. In contrast, there was significantly less proliferation in the peptide-treated WT mice compared with the PBS-treated controls. (Fig. 4b).
Figure 4.
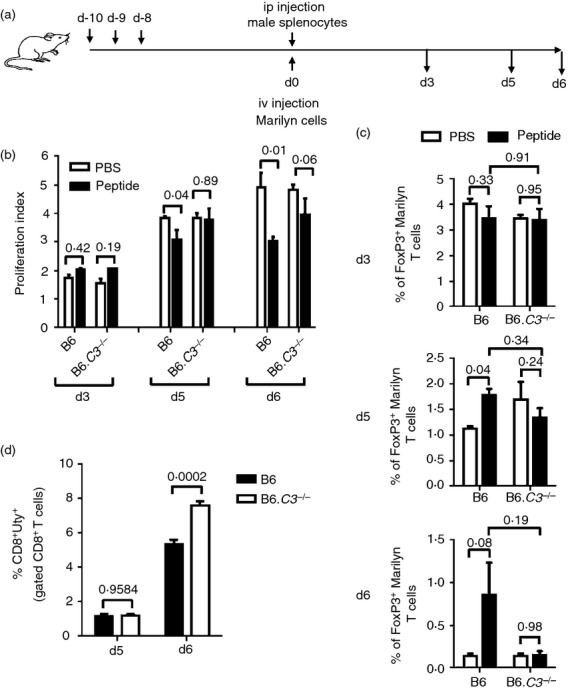
Induction of antigen-specific regulatory T (Treg) cells in peptide-treated mice after immunization with male cells. Ten days after intranasal (i.n.) treatment with three doses of peptide (100 μg), female B6 wild-type (WT) or B6.C3–/– mice (three or four animals per group) were challenged intraperitoneally with 5 × 106 male splenocytes. The antigen-specific CD4 T-cell response was monitored by infusing 2 × 106 CFSE-labelled Marilyn CD90.1 CD4+ CD25− T cells intravenously. Three, 5 and 6 days after the challenge, splenocytes were recovered and the proliferation of Marilyn T cells (gated as CD4+ CD90.1+) was analysed by flow cytometry (a) Experimental schedule. (b) Proliferation indices of Marilyn T cells. (c) Percentage of Foxp3+ Marilyn T cells. Results from mice treated with PBS (open column) and peptide (filled column) are shown. Bars indicate SEM; P-values of unpaired t-test are indicated. (d) Five and 6 days after the challenge, splenocytes were recovered and analysed for HY-specific CD8 T-cell responses (HY-DbUty dextramer+ CD8 T cells) by flow cytometry. Bars indicate SEM. P-values of unpaired t-test are indicated.
Endogenous Treg cells are expanded in peptide-treated mice following immunization with male antigen.24,39 We therefore examined the percentage of Foxp3+ cells within the adoptively transferred Marilyn T-cell population following encounter with male antigen. At day 3 there was no expansion of Foxp3+ cells. However, by days 5 and 6 the percentage of Foxp3+ Marilyn T cells was increased in the peptide-treated WT mice compared with PBS-treated controls, whereas in B6.C3–/– mice no such induction was observed (Fig. 4c).
We also examined expansion of endogenous HY-specific CD8 effector T cells. On day 5 there was no expansion of HY-specific CD8 T cells within the CD8 population from either peptide-treated WT or B6.C3–/– mice. However, by day 6 we observed expansions that were significantly greater in the B6.C3–/– compared with WT animals (Fig. 4d).
Taken together, these findings indicate that in C3-deficient mice the generation of Treg cells is impaired, perhaps because of a defect in the induction of the tolerogenic loop. Consistent with this, both the adoptively transferred Marilyn T cells and the endogenous HY-specific CD8 T cells divided more extensively in the peptide-treated C3-deficient mice than in the corresponding WT controls.
C3 deficiency in T cells does not affect their capacity to become Treg cells
Administration of the peptide to B6.C3–/– mice resulted in a defective induction of Treg cells within the adoptively transferred C3-sufficient Marilyn T cells (Fig. 4c). However, it was not possible to evaluate the endogenous HY-peptide specific C3-deficient Treg cells in vivo or ex vivo. Therefore we assessed whether C3 deficiency could affect the in vitro induction of Treg cells from naive CD4 T cells. Using a standard protocol40 we found that the generation of iTreg cells from WT and B6.C3–/– mice was similar (see Supporting information, Fig. S1A). In addition, both iTreg populations were functionally equivalent in suppression assays, irrespective of whether the responder T cells were WT (see Supporting information, Fig. S1B) or C3-deficient (Fig. S1C). These results suggest that there is no inherent inability in the B6.C3–/– T cells to become Treg cells.
Induction of tolerogenic DC appears to be impaired in C3-deficient mice
Although it is well established that DC make up a central component of the tolerance-inducing pathway, the identification and characterization of these cells in vivo have proven to be difficult. It has recently been reported that the generation of Treg cells from naive T cells, a process known as infectious tolerance, was a result of the induction of amino-acid-metabolizing enzymes in the antigen-presenting DC. In addition, culture of in-vitro-generated Treg cells with BMDC in the presence of cognate peptide induced a similar set of amino-acid-consuming enzymes, arginase 1 (Arg-1), nitric oxide synthase 2 (Nos-2), interleukin-4-induced 1 (Il4i-1) and indolamine 2,3-dioxygenase (Indo-1) in the DC.41 Using a similar approach, we cultured WT and B6.C3–/– BMDC with Foxp3+ Marilyn iTreg cells. This induced C3 expression (see Supporting information, Fig. S2) and an increase in Nos-2 transcription in the WT DC compared with DC alone. Transcription was further increased in the presence of cognate peptide (Fig. 5). When C3-deficient DC were used, the expression of the Nos-2 gene in the presence of peptide was significantly lower (Fig. 5). Arg-1 expression was up-regulated following incubation of the DC with Marilyn iTreg cells, but the presence of the cognate peptide did not further increase its expression, as was reported previously.41 However, there was no significant difference in expression of Arg-1 between the WT and C3-deficient DC. We found no obvious differences in the expression of IL-4i or Indo-1 in the co-culture of DC and Marilyn iTreg cells with or without peptide compared with DC alone. Again the absence of C3 did not alter the expression of these genes.
Figure 5.
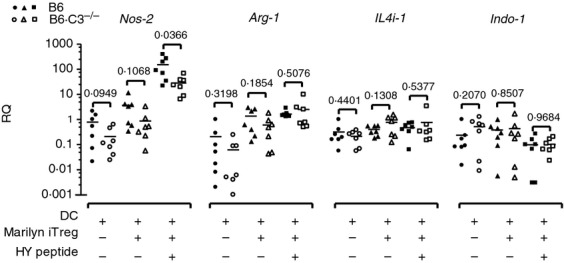
Induction of amino acid-consuming enzymes in dendritic cells (DC) by inducible regulatory T (iTreg) cells. Bone-marrow-derived dendritic cells (BMDC) from B6 wild-type (WT; closed symbols) or B6.C3–/– mice (open symbols) were cultured either alone or with induced Marilyn Treg cells (ratio 1 : 1), in the absence or in the presence of 1 nm HY-AbDby peptide for 48 hr. Samples were analysed for gene expression by real-time PCR using specific primers for Nos-2, Arg-1, IL4i-1 and Indo-1. Relative expression was normalized to Gapdh. Data are represented as relative quantification (RQ); each dot represents a separate biological replicate (seven per group). P-values are indicated (t-test).
Discussion
The role of complement in the regulation of immune responses remains controversial. Here we have extended our previous studies and show that, in addition to C1q and C3, C4 is also needed for peptide-induced tolerance, confirming the key role of the early components of the classical pathway. The finding that B6.C5–/– mice can be tolerized as well as WT demonstrates that the terminal pathway is not involved.
We and others have shown that this model of peptide-induced tolerance involves the induction of a dominant regulatory mechanism mediated by Foxp3+ CD4 Treg cells and resulting in impaired expansions of HY-specific CD4 and CD8 T cells.24,25,39 The lack of tolerance in C3-deficient mice was accompanied by increased proliferation of the adoptively transferred HY-specific CD4 T cells and expansion of endogenous HY-specific CD8 T cells. This correlated with a lower frequency of Foxp3+ CD4 Treg cells within the adoptively transferred indicator Marilyn T cells. Collectively, these results suggest that the generation of Treg cells and the subsequent Treg–DC tolerogenic loop26,27 was defective in the absence of C3. Using naive CD4 T cells we were unable to demonstrate a role for C3 in the induction of Treg cells in vitro. Moreover, the cells from both WT and B6.C3–/– animals were equally suppressive, demonstrating that in the presence of exogenous TGF-β, there is no intrinsic defect in the ability of B6.C3–/– T cells to differentiate into functional Foxp3+ Treg cells. Our observations that there were fewer Foxp3+ Marilyn T cells, which are complement-sufficient, following adoptive transfer into peptide-treated B6.C3–/– recipients immunized with male cells, pointed to a defect within the DC rather than the T-cell populations.
How does the classical pathway of complement affect tolerance induction? It has recently been shown that all the subcomponents of the C1 complex (C1q, C1r, C1s) are not only synthesized by, but are also present on the surface of human monocytes,42 supporting the idea of local complement activation via the classical pathway on the surface on monocytes and possibly other bone-marrow-derived cells. Although we found that both C1q and C4 were needed for tolerance induction, C3 deficiency gave the same phenotype, indicating that C3, with its associated regulatory function7–9 may play a central role in mediating tolerance in this model. Following the cognate interaction between DC and CD4 T cells, both cell types can produce C3, although T cells to a far less extent (17 and see Supporting information, Fig. S2). A possible scenario would therefore be that local production of complement causing the activation of C3 would lead to the generation of C3 activation fragments, C3a and C3b.
Despite evidence suggesting that C3a and C5a signalling through their respective C3aR and C5aR receptors is important for T-cell activation16,17,19,20 and that this signalling leads to suppression of Treg cell generation,43 our results and those of others23 clearly show that C3 is required for tolerance induction. Our observation that C5 deficiency did not affect peptide-induced tolerance also argues against a role for C5aR signalling. In addition, the C3-deficient mice used in our study cannot generate any intracellular C3a fragment as they lack the corresponding genetic region,29 making it unlikely that the effects we observed were mediated by intracellular or extracellular C3aR. Nonetheless, the possibility of a C3-mediated intracellular signalling mediated by other C3 fragments cannot be excluded.44
We have previously shown that following i.n. peptide administration, a population of Treg cells with up-regulated expression of Foxp3, CTLA4, programmed cell death 1 and lymphocyte activation gene 3 as well as interferon-γ and interleukin-1324 was generated. Expression of these molecules causes down-modulation of DC maturation and co-stimulatory capacity45,46 and the induction of the amino acid catabolizing enzymes indolamine 2,3-dioxygenase47,48 and Arginase 1.49 This gives rise to tolerogenic DC, which in turn interact with naive CD4 T cells recognizing cognate MHC peptide and causing down-regulation of the T-cell mammalian target of rapamycin (mTOR) signalling pathway, up-regulation of Foxp3 and further Treg cell generation.50,51 Hence establishing a tolerogenic loop.26,27
Related to this, here we have identified a potential explanation for the defective ability of the B6.C3–/– DC to induce non-responsiveness: an impaired induction of Nos2 expression following culture with in vitro-generated HY-specific Treg cells in the presence of the cognate peptide. Nos2 encodes inducible nitric oxide synthase 2 (iNOS), an enzyme shown to have an important role in the reduction of available arginine, which is crucial for the induction of infectious tolerance through the mTOR signalling pathway.41,50 Although NO generation by iNOS has been associated with pro-inflammatory responses, there is now good evidence that NO has direct immunoregulatory effects.52 Given the importance of iNOS in immune regulation, it is plausible that C3 contributes to tolerance induction in the intranasal peptide model by modulating its expression.
In summary, our results have shown that the complement classical pathway, and particularly C3, is important for the induction of tolerance to HY by the intranasal administration of a single MHC class II-restricted HY peptide. The defect is likely to be related to the inability of complement-deficient mice to establish the Treg–DC tolerogenic loop required for the induction and maintenance of tolerance. We found no evidence of a defect in the ability of C3-deficient T cells to generate Treg cells using established in vitro protocols arguing against an intrinsic T-cell defect. Rather the finding that B6.C3–/– BMDC produced significantly less iNOS following incubation with T cells and cognate antigen, suggests that the defect is at the level of the antigen-presenting cells. In the absence of C3, the induction of amino-acid-depleting enzymes in DC is impaired, causing reduced Treg cell generation, possibly as a result of impaired down-regulation of the mTOR pathway.
Acknowledgments
We thank the staff of the Biological Services Unit at our institution for the care of the animals involved in this study. This work was supported by the Wellcome Trust (grant number 088517). L. B was supported by a Swiss National Science Foundation fellowship and a Novartis Foundation fellowship.
Glossary
- BMDC
bone-marrow-derived dendritic cells
- B6
C57BL/6
- CFSE
carboxyfluorescein diacetatesuccinimidyl ester
- DC
dendritic cells
- Foxp3
Forkhead box p3
- i.n.
intranasal
- iTreg
induced regulatory T
- MST
median survival time
- iNOS
nitric oxide-synthase-2
- TGF-β
transforming growth factor-β
- Treg
regulatory T
- WT
wild-type
Author contributions
LF-J participated in research design, performance of the research, data analysis and writing of the paper. GSL, LB, MS, KM, JCZ and RM participated in performance of the research. J-GC participated in research design. ES participated in research design and writing of the paper. MB and DS participated in research design, data analysis and writing of the paper.
Disclosures
The authors have no financial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Primer sequences for real-time RT-PCR.
Figure S1. Induction of regulatory T cells in vitro.
Figure S2. C3 expression in dendritic cell–T-cell co-culture conditions.
References
- 1.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41:141–6. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 3.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–31. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 6.Ferry H, Potter PK, Crockford TL, Nijnik A, Ehrenstein MR, Walport MJ, Botto M, Cornall RJ. Increased positive selection of B1 cells and reduced B cell tolerance to intracellular antigens in c1q-deficient mice. J Immunol. 2007;178:2916–22. doi: 10.4049/jimmunol.178.5.2916. [DOI] [PubMed] [Google Scholar]
- 7.Hammerberg C, Katiyar SK, Carroll MC, Cooper KD. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J Exp Med. 1998;187:1133–8. doi: 10.1084/jem.187.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purwar R, Baumer W, Niebuhr M, Tschernig T, Kietzmann M, Werfel T. A protective role of complement component 3 in T cell-mediated skin inflammation. Exp Dermatol. 2011;20:709–14. doi: 10.1111/j.1600-0625.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 9.Pekkarinen PT, Vaali K, Jarva H, et al. Impaired intestinal tolerance in the absence of a functional complement system. J Allergy Clin Immunol. 2013;131:1167–75. doi: 10.1016/j.jaci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CC, Chou HS, Yang HR, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760–8. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev (Orlando) 1976;32:93–120. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–49. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 13.Molina H, Holers VM, Li B, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 15.Suresh M, Molina H, Salvato MS, Mastellos D, Lambris JD, Sandor M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J Immunol. 2003;170:788–94. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 16.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–66. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-γ-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, Sacks SH, Zhou W. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a–C3aR interaction. Blood. 2008;111:2452–61. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Anderson KJ, Peng Q, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–94. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 21.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–7. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 22.Baruah P, Simpson E, Dumitriu IE, et al. Mice lacking C1q or C3 show accelerated rejection of minor H disparate skin grafts and resistance to induction of tolerance. Eur J Immunol. 2010;40:1758–67. doi: 10.1002/eji.200940158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel G, Brown K, Phillips R, Peng Q, Zhou W, Sacks SH, Wong W. Donor specific transplant tolerance is dependent on complement receptors. Transpl Int. 2013;26:99–108. doi: 10.1111/tri.12006. [DOI] [PubMed] [Google Scholar]
- 24.Derbyshire K, Addey C, Coe D, et al. Molecular mechanisms of induction of antigen-specific allograft tolerance by intranasal peptide administration. J Immunol. 2011;186:5719–28. doi: 10.4049/jimmunol.1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai JG, James E, Dewchand H, Simpson E, Scott D. Transplantation tolerance induced by intranasal administration of HY peptides. Blood. 2004;103:3951–9. doi: 10.1182/blood-2003-11-3763. [DOI] [PubMed] [Google Scholar]
- 26.Min WP, Zhou D, Ichim TE, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol. 2003;170:1304–12. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 27.Yates SF, Paterson AM, Nolan KF, Cobbold SP, Saunders NJ, Waldmann H, Fairchild PJ. Induction of regulatory T cells and dominant tolerance by dendritic cells incapable of full activation. J Immunol. 2007;179:967–76. doi: 10.4049/jimmunol.179.2.967. [DOI] [PubMed] [Google Scholar]
- 28.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 29.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–4. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wetsel RA, Fleischer DT, Haviland DL. Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5′-exon. J Biol Chem. 1990;265:2435–40. [PubMed] [Google Scholar]
- 31.Braun MY, Grandjean I, Feunou P, Duban L, Kiss R, Goldman M, Lantz O. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166:4879–83. doi: 10.4049/jimmunol.166.8.4879. [DOI] [PubMed] [Google Scholar]
- 32.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385–402. [Google Scholar]
- 33.Carlucci F, Fossati-Jimack L, Dumitriu IE, et al. Identification and characterization of a lupus suppressor 129 locus on chromosome 3. J Immunol. 2010;184:6256–65. doi: 10.4049/jimmunol.0901463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci USA. 2012;109:E3160–7. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teh BK, Yeo JG, Chern LM, Lu J. C1q regulation of dendritic cell development from monocytes with distinct cytokine production and T cell stimulation. Mol Immunol. 2011;48:1128–38. doi: 10.1016/j.molimm.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–68. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc Natl Acad Sci USA. 2008;105:3479–84. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–6. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 41.Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–60. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosszu KK, Valentino A, Ji Y, et al. Cell surface expression and function of the macromolecular c1 complex on the surface of human monocytes. Front Immunol. 2012;3:38. doi: 10.3389/fimmu.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liszewski MK, Kolev M, Le Friec G, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–57. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–82. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 46.Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 47.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 48.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat Rev Immunol. 2007;7:817–23. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 49.Wei LH, Jacobs AT, Morris SM, Jr, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C248–56. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 50.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology. 2013;140:391–8. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22:552–9. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 53.Bobe P, Benihoud K, Grandjon D, Opolon P, Pritchard LL, Huchet R. Nitric oxide mediation of active immunosuppression associated with graft-versus-host reaction. Blood. 1999;94:1028–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences for real-time RT-PCR.
Figure S1. Induction of regulatory T cells in vitro.
Figure S2. C3 expression in dendritic cell–T-cell co-culture conditions.