Abstract
Intrinsic Notch signaling in intestinal epithelial cells restricts secretory cell differentiation. In gut-associated lymphoid tissue (GALT), stromal cells located beneath the follicle-associated epithelium (FAE) abundantly express the Notch ligand delta-like 1 (Dll1). Here, we show that mice lacking Rbpj—a gene encoding a transcription factor implicated in Notch signaling—in intestinal epithelial cells have defective GALT maturation. This defect can be attributed to the expansion of goblet cells, which leads to the down-regulation of CCL20 in FAE. These data demonstrate that epithelial Notch signaling maintained by stromal cells contributes to the full maturation of GALT by restricting secretory cell differentiation in FAE.
Subject Categories Development & Differentiation; Immunology; Signal Transduction
Keywords: follicle-associated epithelium, gut-associated lymphoid tissues, intestinal epithelial cells, Notch signaling
Introduction
Peyer's patches (PPs) develop in the sterile environment of the fetus. This process necessitates bidirectional signals among the hematopoietic cells, stromal cells, and intestinal epithelial cells (IECs). At embryonic day 15.5 (E15.5) in mice, lymphotoxin α1β2 (LTα1β2)-expressing lymphoid tissue inducer (LTi) cells interact with the surrounding lymphotoxin β receptor (LTβR)-expressing lymphoid tissue organizer (LTo) cells, leading to the induction of chemokines and adhesion molecules involved in the recruitment and organization of lymphocytes 1. Furthermore, in LTo cells, LTβR signaling up-regulates expression of interleukin-7 and receptor activator of nuclear factor-κB ligand (RANKL), a tumor necrosis factor superfamily member, which increases LTα1β2 expression in newly arriving LTi cells 2. This positive feedback loop promotes maturation of the PP anlagen at the late embryonic stage. In addition, LTα1β2-expressing LTi cells transduce epithelial LTβR signaling at E17.5 to induce CCL20 in follicle-associated epithelium (FAE) that harbors microfold/membranous cells 3. FAE-derived CCL20 can be assumed to play a significant role in the later stages of PP development, given that the absence of CCR6 results in fewer lymphocytes in PPs without affecting the number of PPs in the intestine 4–6. This fact suggests the importance of FAE development in the full maturation of PPs. However, little is known about the molecular mechanisms controlling the development and homeostasis of FAE during PP organogenesis.
Notch signaling pathway is an evolutionary conserved mechanism that regulates cell fate decision and development in the various types of cells. Notch ligands are transmembrane proteins termed Jagged (Jag1 and Jag2) and Delta-like (Dll1, 3 and 4). After the ligand binding to Notch receptors on the cell surface of adjacent cells, Notch intracellular domain (NICD) translocates into the nucleus and forms a complex with recombination signal-binding protein for immunoglobulin κ J region (RBP-J). In IECs, the NICD/RBP-J complex activates Notch target genes such as Hes1, which represses the expression of Atoh1, a responsible transcription factor for the differentiation of secretory cell lineages 7. Thus, epithelial Notch activation inhibits the differentiation of secretory cell lineages and plays a critical role in deciding the fate of intestinal epithelial progenitor cells between the secretory and absorptive lineages.
A previous study identified stromal cells expressing the Notch ligand Dll1 beneath FAE of PPs in adult mice 8, although their biological significance was not then elucidated. Here, we show that epithelial Notch signaling contributes not only to the maintenance of the integrity and homeostasis of the FAE but also to the full maturation of gut-associated lymphoid tissue (GALT). Our data demonstrate the biological importance of epithelial–stromal interactions in the development of the gut immune system.
Results and Discussion
Detection of Dll1-expressing LTo cells beneath FAE in PPs
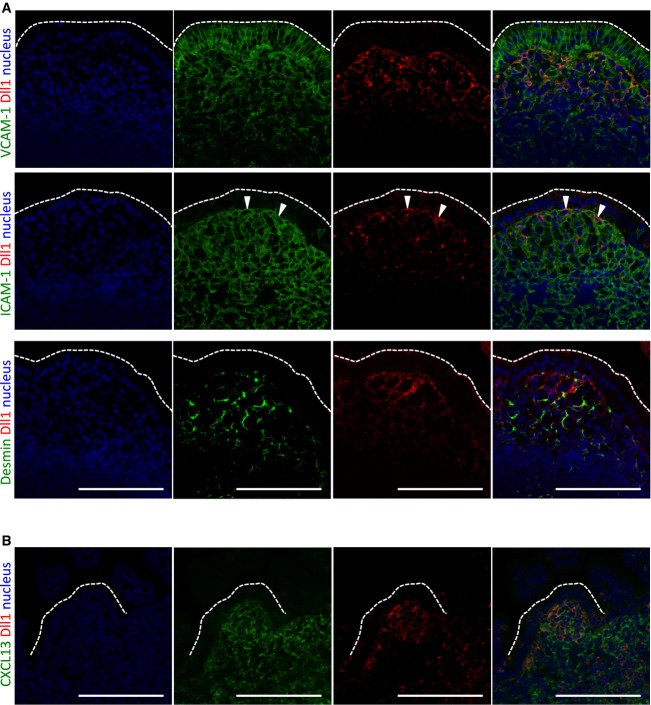
To investigate the role of Notch signaling in GALT formation, we first analyzed the distribution of the Notch ligand Dll1 in PPs using Dll1-lacZ reporter mice 9. Dll1 was expressed in proximity to the FAE of PPs in adult mice (Supplementary Fig S1A). A previous study showed that these Dll1+ cells express mucosal addressin cell adhesion molecule 1 (MadCAM1), a stromal marker 8. In addition, we found that the Dll1+ cells beneath the FAE were positive for vascular cell adhesion molecule 1 (VCAM1) and sometimes positive for intercellular adhesion molecule 1 (ICAM1) but not desmin (Fig1A). Flow cytometry showed that nearly all CD45−Dll1+ stromal cells expressed VCAM1, and approximately 10% of these coexpressed ICAM1 (Supplementary Fig S1B). Thus, it is VCAM1+ and VCAM1+ICAM1+ cells in the subepithelial dome region that send signals to the FAE via Dll1.
Figure 1.
Detection of Delta-like 1 (Dll1)-expressing cells during Peyer's patch (PP) development
A, B PP sections from C57BL/6 mice at adult stage (A) and embryonic day 18.5 (E18.5) (B) were stained for Dll1 (red) together with VCAM1, ICAM1, desmin, or CXCL13 (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Dotted lines show the apical edge of the follicle-associated epithelium (FAE). Scale bars represent 100 μm. Data are representative of two independent experiments with similar results.
We subsequently examined Dll1 expression during PP development at the late embryonic stage. As with a previous study 10, Dll1+ cells were abundant in the PP anlagen at E18.5, and some were in contact with FAE (Supplementary Fig S1C). These cells were identified as LTo cells by their expression of CXCL13, which is important for PP development 11 (Fig1B).
Role of epithelial Notch signaling in integrity of the FAE and maturation of GALT
The expression of the Notch ligand in the subepithelial dome regions raised the possibility that LTo cell-mediated activation of Notch signaling may contribute to the organization of the FAE. To directly address this possibility, we analyzed the FAE of mice with an IEC-specific knockout of the gene coding for RBP-J (RBP-JIEC-KO mice). We have previously reported a marked increase in all three types of secretory epithelial cells in the intestine of RBP-JIEC-KO mice 12. The same proved true for FAE. Although there were very few goblet cells in the FAE of control mice without RBP-J deficiency, RBP-JIEC-KO mice showed abnormal expansion of goblet cells (Fig2A–D). This observation was further confirmed by up-regulation of genes associated with goblet cells (Fig2E), indicating that Notch signaling is essential for the restriction of goblet cell differentiation in FAE.
Figure 2.
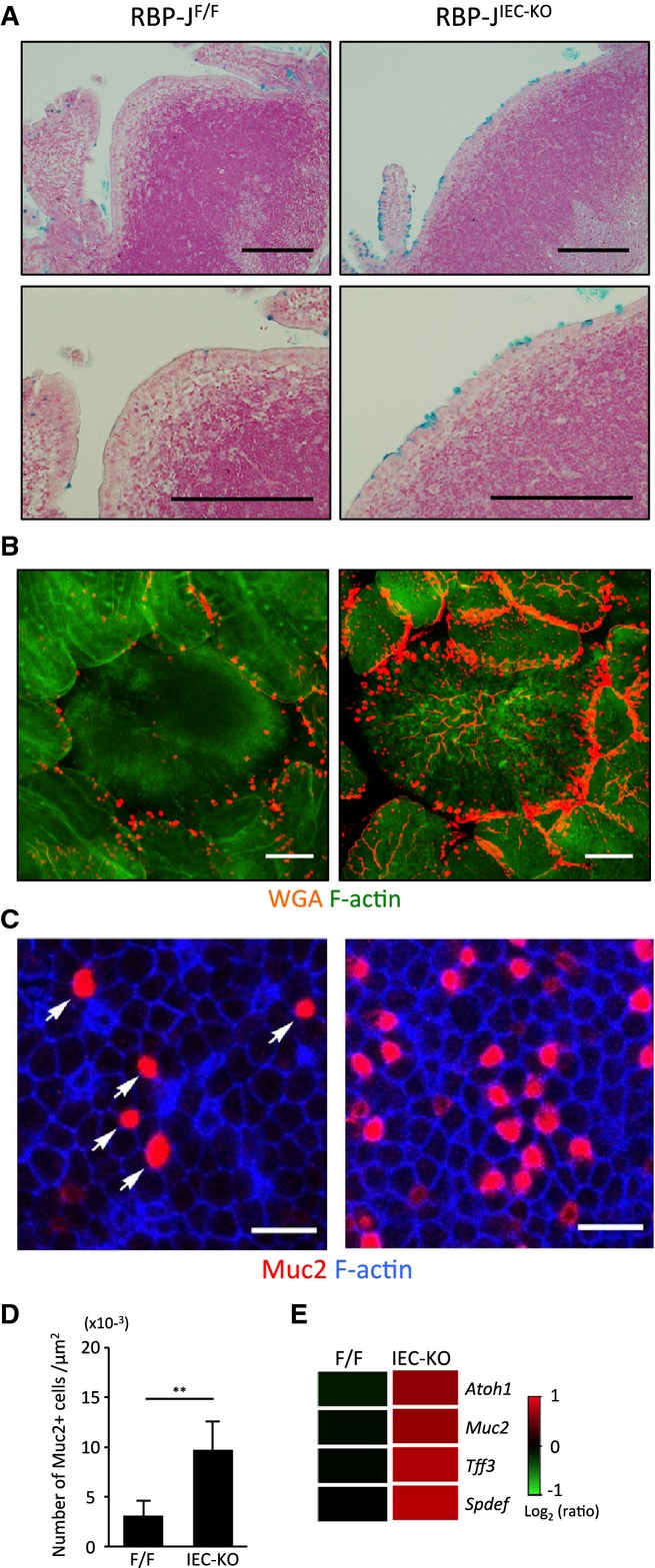
Goblet cell hyperplasia in follicle-associated epithelium (FAE) of RBP-JIEC-KO mice
A Peyer's patch (PP) tissue sections from RBP-JIEC-KO and control (RBP-JF/F) mice were stained with Alcian blue to detect goblet cells, followed by nuclear fast red counterstaining. Scale bars represent 200 μm.
B, C Whole-mount specimens of PPs were stained with wheat germ agglutinin (B) or antibodies against mucin-2 (Muc2) (C) to detect goblet cells. F-actin was stained with phalloidin. Scale bars represent 100 (B) or 20 (C) μm.
D The number of Muc2+ cells in FAE (μm2) was quantified. Data are representative of two independent experiments with similar results. Values are presented as the mean ± standard deviation (n = 3). **P < 0.01, as calculated with the Student's t-test.
E Gene expression profiles of FAE from RBP-JIEC-KO and control mice are shown. The heat map represents expression levels of goblet cell-associated genes.
Source data are available online for this figure.
Unexpectedly, the number of lymphoid follicles per PP was significantly reduced in RBP-JIEC-KO mice compared with controls (Fig3A and B), although the total number of PPs in the small intestine was comparable between the two groups (Fig3B). Likewise, the total number of PP cells in RBP-JIEC-KO mice was decreased by almost half compared with controls (Fig3B). Normal PP microstructure 13 and the composition of the PP immune cell population were maintained in the absence of RBP-J (Fig3C and Supplementary Fig S2). Notably, the frequency of germinal-center B cells was significantly increased in RBP-JIEC-KO mice (Supplementary Fig S2), which might reflect a compensatory reaction to the reduction in lymphoid follicles resulting from RBP-J deficiency. Collectively, these results suggest that IEC-intrinsic Notch signaling is important for the maturation of PPs but does not affect the composition of the PP immune cell population.
Figure 3.
Characterization of gut-associated lymphoid tissue (GALT) in RBP-JIEC-KO mice
A Peyer's patch (PP) tissues were analyzed for lymphoid follicle formation. Asterisks indicate lymphoid follicles. Data are representative of three independent experiments. Scale bars represent 1 mm.
B Surface area, the number of PPs, follicles per PP, and total number of PP lymphocytes were quantified. Values are presented as the mean ± standard deviation (n = 3–4). **P < 0.01, as calculated with the Student's t-test. n.s.: not significantly different.
C Immunofluorescence staining of the PP tissues from RBP-JIEC-KO mice and control littermates was performed using monoclonal antibodies against CD3ε (red) and B220 (green) and polyclonal antibodies against CCL21 or CXCL13 (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Data are representative of three independent experiments. Scale bars represent 0.2 mm.
D, E Whole-mount staining of small intestines from 10- to 14-week-old RBP-JIEC-KO and control mice was performed using a monoclonal antibody against B220. Representative macroscopic views of the serosal side of stained small intestines from RBP-JIEC-KO and control mice are shown (D). Scale bars represent 2 mm. The numbers of immature and mature ILFs in the small intestine were quantified (E). Values are presented as the mean ± standard deviation (n = 3). *P < 0.05, as calculated with the Student's t-test. n.s.: not significantly different.
Source data are available online for this figure.
Isolated lymphoid follicles (ILFs), another constituent of GALT, function as an inductive site for mucosal immune responses 14. ILFs are postnatally generated from cryptopatches through two steps. First, immature ILFs are induced by the action of LTα1β2-expressing LTi cells 15; they then undergo further maturation in response to colonization by commensal microbiota 16. Mature ILFs consist of a single B-cell follicle with a germinal center and FAE. The absence of PPs results in a compensatory increase in the number of mature ILFs 17. We therefore analyzed the development of ILFs in RBP-JIEC-KO mice. The number of mature ILFs was significantly reduced in RBP-JIEC-KO mice, whereas the number of immature ILFs remained unchanged (Fig3D and E). Thus, epithelial Notch signaling appears to be indispensable for the maturation of PPs and ILFs.
Role of epithelial Notch signaling in compartmentalization of lymphoid follicles during PP development
We further explored the role of epithelial Notch signaling in organogenesis of PPs in RBP-JIEC-KO mice. PP anlagen are defined as discrete regions expressing VCAM1 5. Whole-mount immunostaining for VCAM1 showed normal formation of PP anlagen in RBP-JIEC-KO mice at E17.5 (Fig4A); however, follicles showed impaired compartmentalization at the neonatal stage (Fig4B). These observations suggest that epithelial Notch signaling contributes to the compartmentalization of follicles during the later stages of PP development. Thus, the observed reduction in the number of lymphoid follicles in PPs of adult RBP-JIEC-KO mice may be due to a defect in the compartmentalization of lymphoid follicles at the late embryonic stage.
Figure 4.
Compartmentalization of lymphoid follicles during the development of Peyer's patches (PPs) was impaired due to the abnormal expansion of goblet cells in RBP-JIEC-KO mice
A, B Whole-mount staining of the small intestines was performed using an anti-VCAM1 monoclonal antibody to detect PP anlagen and maturing PPs with lymphoid follicle compartmentalization at embryonic day 17.5 (E17.5) (A) and the neonatal stage (B), respectively.
C PP tissue sections were immunostained using polyclonal antibodies against CCL20 followed by Alcian blue.
D Whole-mount immunofluorescence staining of follicle-associated epithelium (FAE) from RBP-JIEC-KO mice was performed using polyclonal antibodies against CCL20 (green), Muc-2 (red), and F-actin (blue). Arrowheads represent goblet cells.
E Whole-mount specimens of the small intestine were immunostained using an anti-VCAM1 monoclonal antibody (left); tissue sections were then prepared from these specimens and stained with Alcian blue to detect goblet cells (right).
F The number of follicles per PP in 2-week-old mice was quantified. Values are presented as the mean ± standard deviation (n = 3–4). *P < 0.05, **P < 0.01, as calculated with one-way analysis of variance. n.s.: not significantly different.
Data information: Scale bars represent 200 (A, B, E left panel), 100 (C, D left panel, E right panel), or 20 (D center and right panels) μm. Data are representative of two (A, B, D, E) or three (C) independent experiments with similar results.
Source data are available online for this figure.
Role of epithelial Notch signaling in FAE-specific chemokine expression and FAE cell proliferation
A reduced number of lymphoid follicles in PPs has also been observed in CCR6-deficient mice 6,18. CCR6 is expressed by the immune cells in PPs, including B cells and subsets of innate lymphoid cells, dendritic cells, and T cells 19. The ligand for CCR6, CCL20, is specifically expressed by FAE from E17.5 onward over the course of lymphoid follicle compartmentalization 4,5. LTα1β2-expressing LTi cells in the PP anlagen induce CCL20 expression in FAE by activating LTβR signaling in epithelial cells at the late embryonic stage 4. Therefore, CCL20 appears to play an important role in the maturation and compartmentalization of lymphoid follicles by recruiting CCR6-expressing immune cells at this stage. The CCL20-CCR6 axis is also important for ILF maturation 20. We therefore analyzed the expression of CCL20 in PP FAE of RBP-JIEC-KO mice. Immunostaining of PPs showed remarkably attenuated expression of CCL20 in FAE in RBP-JIEC-KO mice (Fig4C), along with expansion of goblet cells completely devoid of CCL20 expression (Fig4D). The lack of CCL20 expression in goblet cells might be due to the lower expression of LTβR in secretory epithelial cells than in absorptive enterocytes, although further investigation is required to prove this possibility. Taken together, these observations imply that Notch-dependent suppression of goblet cell differentiation ensures constitutive CCL20 expression, which is a prerequisite for maturation of PP follicles.
To assess the efficiency of CCL20 induction in villus epithelium, we treated RBP-JIEC-KO mice with LTβR-agonistic antibodies. We observed significantly lower induction of CCL20 in RBP-JIEC-KO mice than in control mice (Supplementary Fig S3A), supporting the notion that goblet cell hyperplasia results in reduction of CCL20 expression. Indeed, goblet cell hyperplasia coincided with impaired formation of FAE and lymphoid follicles at both neonatal and adult stages (Supplementary Fig S3B and C). In contrast, RBP-J knockdown in T84 human absorptive epithelial cells did not interfere with LTα1β2-dependent CCL20 induction (Supplementary Fig S3D). Therefore, Notch signaling appears dispensable for the intracellular signaling pathway regulating CCL20 expression, although it is required for the restriction of goblet cell expansion in FAE. Notch-dependent inhibition of goblet cell differentiation seems to be a prerequisite for full FAE maturation.
Partial rescue of PP compartmentalization defect by deletion of Atoh1 in RBP-JIEC-KO mice
Impaired GALT maturation associated with goblet cell hyperplasia in RBP-JIEC-KO mice may result from aberrant activation of the Atoh1 transcription factor due to lack of RBP-J-dependent Hes1 expression. To test this hypothesis, we compared PP formation between Atoh1-deficient and Atoh1-sufficient RBP-JIEC-KO mice (Atoh1/RBP-JIEC-DKO and Atoh1+/+/RBP-JIEC-KO, respectively). The defect in the compartmentalization of PP follicles in Atoh1+/+/RBP-JIEC-KO mice was at least partly improved in Atoh1/RBP-JIEC-DKO mice (Fig4E). We confirmed that Atoh1/RBP-JIEC-DKO mice were devoid of goblet cells and died around weaning age (Fig4E), consistent with a previous report on Atoh1IEC-KO mice 21. We therefore analyzed lymphoid follicle formation in PPs at 2 weeks of age. Consistent with the observation at neonatal stage, deletion of the Atoh1 gene at least partially rescued the reduction of lymphoid follicles in RBP-JIEC-KO mice (Fig4F). These results confirmed that epithelial Notch signaling-dependent repression of Atoh1 contributes to the maturation of PPs.
Defect in induction of mucosal immune responses in RBP-JIEC-KO mice
Because GALT plays a pivotal role in immunosurveillance at the mucosal surface by inducing antigen-specific immunoglobulin A production 13, we investigated the contribution of epithelial Notch signaling to the mucosal immune response. After oral immunization with ovalbumin (OVA) in the presence of cholera toxin as a mucosal adjuvant, the amount of OVA-specific immunoglobulin A in feces was significantly decreased in the RBP-JIEC-KO mice compared with control mice (Fig5), demonstrating that maturation of GALT supported by epithelial Notch signaling is essential for the efficient induction of antigen-specific mucosal antibody responses.
Figure 5.
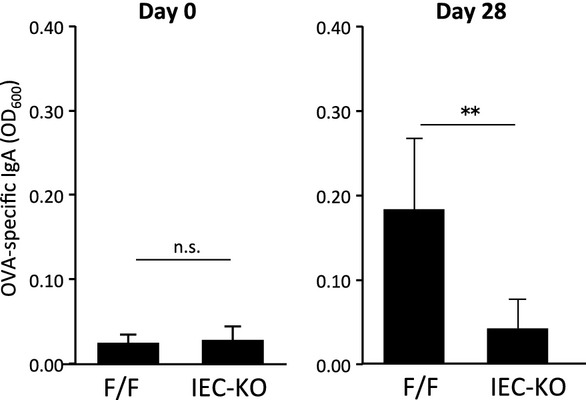
Attenuated antigen-specific mucosal immune response in RBP-JIEC-KO mice
RBP-JIEC-KO and control mice were administered ovalbumin (OVA) orally in the presence of cholera toxin as a mucosal adjuvant. The levels of OVA-specific immunoglobulin A in fecal suspensions were determined at days 0 and 28 by enzyme-linked immunosorbent assay. Values are presented as the mean ± standard deviation (n = 6–9 for day 0; n = 5–6 for day 28). **P < 0.01, as calculated with the Student's t-test. n.s.: not significantly different.
Source data are available online for this figure.
On the basis of these observations, we propose the following model of the role of epithelial Notch signaling in PP formation (Supplementary Fig S4). First, LTo cells in PP anlagen express Dll1 to inhibit the differentiation of secretory epithelial cells in the proto-FAE region via activation of Notch signaling prior to the compartmentalization of lymphoid follicles. This mechanism facilitates the formation of FAE. Therefore, Dll1+ stromal cells function as “FAE organizers” at the late embryonic stage. Considering that the FAE harbors microfold cells and serves as a portal for mucosal antigen uptake, the restriction of goblet cell differentiation by Dll1+ stromal cells may also contribute to the efficient uptake of mucosal antigen into lymphoid follicles by reducing the mucous layer overlying the FAE. Although further investigation will be required to uncover the mechanism of Dll1 expression by LTo cells in PP anlagen, our finding provides novel mechanistic insight into the involvement of Dll1+ stromal cells and epithelial Notch signaling in the histogenesis of GALT.
Materials and Methods
Detailed descriptions of our methods can be found in the Supplementary Methods.
Animal experiments
Mice carrying a floxed Rbpj allele (RBP-JF/F) 22 were obtained from RIKEN BioResource Center (Yokohama, Japan). Atoh1F/F mice and Dll1-lacZ reporter mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). To obtain RBP-JIEC-KO and RBP-J-Atoh1IEC-KO mice, we crossed RBP-JF/F and RBP-J-Atoh1F/F mice with Villin-Cre transgenic mice obtained from The Jackson Laboratory. C57BL/6 mice were purchased from CLEA Japan Inc. (Tokyo, Japan). These mice were maintained under specific-pathogen-free conditions in the animal facilities of Yokohama City University, the Institute of Medical Science at the University of Tokyo, and RIKEN Yokohama Institute. All animal experiments were performed using protocols approved by the animal studies committees of Yokohama City University, the Institute of Medical Science at the University of Tokyo, and RIKEN Yokohama Institute.
Whole-mount staining
The whole-mount specimens of PPs were fixed with BD Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA) and then subjected to immunostaining to detect goblet cells and CCL20 expression in FAE. In separate experiments, the small intestinal serosa was removed using filter paper and tweezers under monitoring with a stereoscopic microscope, and the specimens were subjected to immunostaining of B220 to detect ILFs. For the detailed experimental procedure, see Supplementary Methods. ILFs can be classified on the basis of the size of B220 clusters and the presence or absence of FAE. Mature ILFs can be distinguished from the luminal side of the intestinal epithelium by the presence of FAE; immature ILFs, which lack FAE, are not so easily distinguished 17,23.
Statistical analysis
Differences between two groups were analyzed with the Student's t-test. When variances were not homogeneous, the data were analyzed with the Mann–Whitney U-test. Differences among more than two groups were analyzed by one-way analysis of variance followed by the Dunnett's test.
Acknowledgments
We would like to thank Dr. Tasuku Honjo for providing RBP-JF/F mice, Dr. Jeffrey L. Browning for the anti-LTβR antibody, and Dr. Peter D. Burrows for critical reading and English editing of the manuscript. We also thank Dr. Takashi Kanaya, Dr. Daisuke Takahashi, and Ms. Toshi Jinnohara for valuable comments and suggestions.
Funding
This study was supported in part by grants from the Japan Society for the Promotion of Science (24117723 to KH, 24249029 to HO, and 252667 to YO), Japan Science and Technology Agency (KH), RIKEN RCAI Young Chief Investigator program (KH), Uehara Memorial Foundation (KH), Mochida Memorial Foundation for Medical and Pharmaceutical Research (KH), Toray Science Foundation (KH), and Takeda Science Foundation (HO).
Author contributions
YO contributed to study design, acquisition and analysis of a large part of the data, and writing of the manuscript. SK contributed to acquisition and analysis of data. GN, KI, YN, YM, YFur, and YFuj contributed to acquisition of data. ME, MS, and TI contributed to the provision of experimental protocols. KS contributed to the provision of experimental protocols and interpretation of data. HY contributed to the provision of materials. KH contributed to study concept, analysis and interpretation of data, and writing of the manuscript and obtained funding. HO contributed to interpretation of data and critical editing of the manuscript and obtained funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
for this article is available online: http://embor.embopress.org
Supplementary Information
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
References
- Nishikawa S-I, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Naito A, Inoue J-I, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- Rumbo M, Sierro F, Debard N, Kraehenbuhl J-P, Finke D. Lymphotoxin beta receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004;127:213–223. doi: 10.1053/j.gastro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Hashi H, Yoshida H, Honda K, Fraser S, Kubo H, Awane M, Takabayashi A, Nakano H, Yamaoka Y, Nishikawa S. Compartmentalization of Peyer's patch anlagen before lymphocyte entry. J Immunol. 2001;166:3702–3709. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–1240. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005;206:177–189. doi: 10.1111/j.0105-2896.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- Hrabĕ de Angelis M, McIntyre J, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- Okuda M, Togawa A, Wada H, Nishikawa S-I. Distinct activities of stromal cells involved in the organogenesis of lymph nodes and Peyer's patches. J Immunol. 2007;179:804–811. doi: 10.4049/jimmunol.179.2.804. [DOI] [PubMed] [Google Scholar]
- Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y, Takahashi D, Ebisawa M, Kakiguchi K, Yonemura S, Jinnohara T, Kanaya T, Fujimura Y, Ohmae M, Hase K, et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J Immunol. 2012;188:2427–2436. doi: 10.4049/jimmunol.1101128. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kawamoto S, Maruya M, Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol. 2010;107:153–185. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2:478–485. doi: 10.1038/mi.2009.114. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutiérrez J, Torres M, Martínez-A C, Mérquez G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–R45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;29:283–292. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY-C, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5