Abstract
Background
Cosmetic procedures are growing ever more common, and the use of soft tissue fillers is increasing. Practicing physicians need to be aware of the biological behavior of these products in tissue to enable them to respond to any safety concerns that their patients raise.
Objectives
To provide an overview of the metabolism of 1,4-butanediol diglycidyl ether (BDDE)-crosslinked hyaluronic acid (HA) dermal fillers and to examine the safety of the resulting byproducts.
Methods
A review of available evidence was conducted.
Results
After reaction with HA, the epoxide groups of BDDE are neutralized, and only trace amounts of unreacted BDDE remain in the product (<2 parts per million). When crosslinked HA, uncrosslinked HA, and unreacted BDDE degrade, they break down into harmless byproducts or into byproducts that are identical to substances already found in the skin.
Conclusion
Clinical and biocompatibility data from longer than 15 years support the favorable clinical safety profile of BDDE-crosslinked HA and its degradation products. Given the strength of the empirical evidence, physicians should be confident in offering these products to their patients.
Cosmetic procedures are growing ever more common, and the use of soft tissue fillers is increasing. The technology and biocompatibility of the products has progressed along with this demand, from the use of silicone injections in the 1940s and 1950s and the first animal-derived collagen fillers of the 1980s through to the introduction of hyaluronic acid (HA) fillers in the 1990s.1 HA fillers, according to a survey of plastic surgeons conducted by the International Society of Aesthetic Plastic Surgeons (ISAPS), were the second-most-popular nonsurgical procedure of 2010, with more than two million procedures performed.2 Factors contributing to this popularity include the favorable safety profile of these products, so it is important for physicians to understand what underlies this and to consider the empiric evidence surrounding how these fillers are metabolized and the safety of the byproducts of their degradation.
The three classes of dermal fillers currently in use are3,4:
Absorbable products (temporary; 3–6 months; e.g., HA and collagen fillers).
Slowly absorbable products (temporary; 6–24 months; e.g., HA, calcium hydroxyapatite,5 and l-polylactic acid fillers).
Nonabsorbable products (permanent; >24 months; e.g., polymethyl methacrylate and silicone fillers).
Hyaluronic acid, the most widely used filler substance currently on the market, has a number of advantages over its predecessors. Crosslinked HA fillers have been used for longer than 15 years6 and are considered to be generally well tolerated. They have structural properties similar to those of native tissue, excellent biocompatibility, and good tissue integration.7,8 They have a tunable duration of action spanning the entire range of the temporary filler category (6–24 months), and because of their relatively stable molecular composition, they can be stored without refrigeration for up to 2 years. Because of the hydrophilic nature of HA, these fillers also serve to hydrate the skin, and uniquely among other filler substances, HA can be reversed using hyaluronidase.9
In most commercial products, HA is crosslinked to increase its longevity, and the crosslinking agent used has an important effect on the properties of the final product; 1,4-butanediol diglycidyl ether(BDDE) is the crosslinking agent used in the majority of the market-leading HA fillers, and its stability, biodegradability, and long safety record spanning more than 15 years are what make it the industry standard, ahead of other crosslinkers such as divinyl sulfone and 2,7,8-diepoxyoctane.
In the modern era of enhanced patient awareness of the possible complications associated with cosmetic products, it is essential for practicing physicians to be aware of the biological behavior of these products in tissue to enable them to respond to any safety concerns that their patients raise.
Degradation of HA
Hyaluronic acid is a naturally occurring polymer found in the extracellular matrix, the vitreous humor, and the cartilage. The total quantity of HA found in a 70-kg person is approximately 15 g, and its average turnover rate is 5 g/d. Approximately 50% of the total quantity of HA in the human body is concentrated in the skin, and it has a half-life of 24–48 hours.10
Hyaluronic acid is a polysaccharide that consists of repeating monomers (glucuronic acid and N-acetylglucosamine disaccharide units) linked together in a linear fashion through β-1,4 glycosidic bonds. The degradation of HA can be viewed as a depolymerization process that is mediated by glycosidic bond cleavage, which the dissociation of the polymer chains on a macromolecular level may precede (dissolution and diffusion). The depolymerization of HA has been well characterized in the literature and primarily involves two mechanisms: enzymatic degradation and free radical degradation.
Enzymatic Degradation
A large class of enzymes collectively known as hyaluronidases mediate enzymatic degradation. In humans, the most active enzymes of this class are HYAL1 and HYAL2.10,11 HYAL2 (anchored on the cell membrane) cleaves high-molecular-weight HA (>1 MDa) into 20-kDa fragments. HYAL1 (found in lysosomes) subsequently cleaves these fragments further down to tetrasaccharides, which are then converted to monosaccharides by several enzymes of the hyaluronidase family (e.g., β-glucuronidase, β-N-acetylglucosaminidase). Because these degradation products are native to the human body, they join the natural elimination process.12
Free Radical Degradation
Regardless of magnitude, disturbance to the tissue environment can activate the body’s immune system, leading to a transient inflammatory reaction. Mechanical injury to the tissue caused by the penetration of a needle during filler injection can also lead to such a reaction. Acute tissue inflammation is often linked to a transient increase in free radical activity at the site of the injury. Free radicals are known to degrade biomaterials through oxidation. Several reports in the literature indicate that free radical-mediated degradation of HA proceeds through cleavage of glycosidic bonds.13,14 It has not been investigated whether adjunctive aesthetic treatments, such as laser resurfacing, which is known to promote free radical generation,15 have an effect on the degradation of HA-containing dermal fillers.
Hyaluronic acid catabolism takes place in situ (e.g., in the extracellular matrix), intracellularly, or after transfer to the lymph nodes.16 Long HA chains (polysaccharides) that enzymes and free radicals degrade in situ yield smaller HA units (oligosaccharides). These smaller HA units are further catabolized intracellularly or in the lymph nodes. Eventually, these units enter the circulatory system, where the liver and kidneys eliminate them.12,17
Degradation of BDDE
1,4-Butanediol diglycidyl ether is the crosslinking agent used to stabilize the majority of the HA-based dermal fillers currently available on the market. Its ability to crosslink is attributed to the reactivity of the epoxide groups present at the two ends of the molecule. Under basic (pH > 7) conditions, these epoxide groups preferentially react with the most accessible primary alcohol in the HA backbone, forming an ether bond connection. The superior stability of the ether bond (relative to the ester or amide bond) is one of the reasons that BDDE-crosslinked HA fillers have a clinical duration that can reach or exceed 1 year. In addition, BDDE has a significantly lower toxicity than other ether-bond crosslinking chemistry based agents (e.g., divinyl sulfone), is biodegradable, and has been well studied. All these factors have contributed to BDDE becoming the industry-standard crosslinker.
Although unreacted BDDE has been found to be mutagenic in the Drosophila model organism18, a definitive carcinogenic effect has not been observed in mice.19 Despite this, and because of its mutagenic potential (thought to be the result of the reactive nature of the epoxide groups), the amount of unreacted BDDE in dermal fillers is maintained at trace amounts. For Allergan’s range of HA dermal fillers, this is achieved through a complex purification process, which results in a residual level of unreacted BDDE of <2 parts per million (ppm). This would equate to <0.002 mg of BDDE in 1 mL of HA gel. This trace level, which historically was the limit of detection, has been determined to be safe after a Food and Drug Administration (FDA) safety risk assessment.
Over time, unreacted BDDE undergoes degradation through hydrolysis. The most favorable cleavage sites are the ether bonds in the epoxide groups and in the backbone of the molecule. Degradation of BDDE can produce a number of nonreactive by-products (described below).
Hydrolyzed BDDE
Hydrolyzed BDDE is a diol-ether resulting from the hydrolysis of the epoxide groups in BDDE or the hydrolytic cleavage of crosslinked BDDE. Hydrolyzed BDDE has been shown to be nontoxic and nongenotoxic, even at molar concentrations significantly higher than the concentrations used in commercial fillers (J.X. Roca-Martinez, unpublished data). (These concentrations are defined later in this section.)
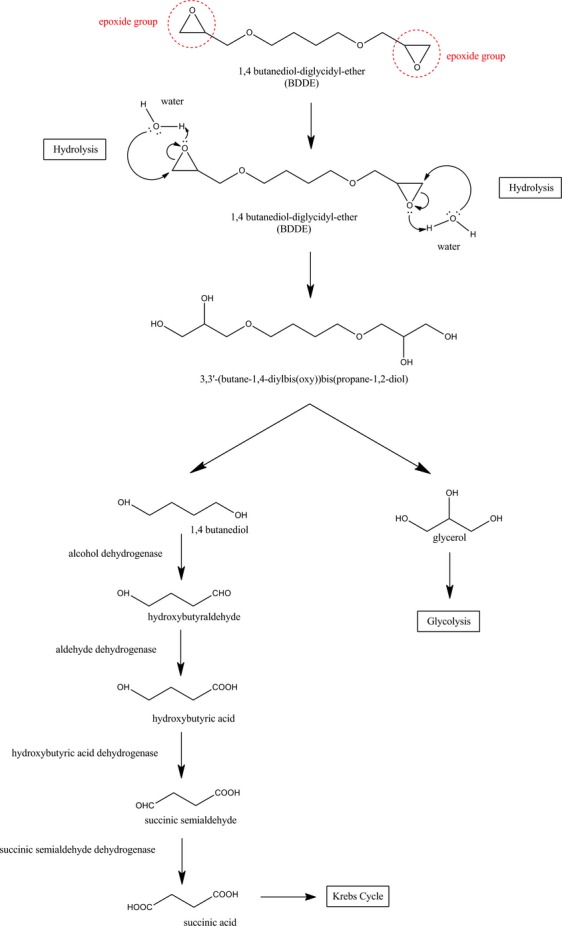
Although the metabolism of hydrolyzed BDDE is not described in the literature, it is understood to proceed through ether bond cleavage by a family of enzymes called cytochromes P450. These enzymes are involved in the oxidative degradation of organic molecules and can catalyze the cleavage of ether bonds into alcohols.20 After degradation, two main products can emerge: glycerol and butanediol. Similar to all diol-ethers, hydrolyzed BDDE is also known to be eliminated in urine.21,22
1,4-Butanediol
1,4-Butanediol is known to be nonmutagenic, nonsensitizing, and a slight irritant.23,24 No carcinogenic potential has been identified by tests performed on its metabolites. Neurotoxic adverse effects were observed in animals with a no observed adverse effect level (NOAEL) of 100 mg/kg per day (determined according to oral administration in mice). The median lethal dose (LD50) of 1,4-butanediol is 1,525 mg/kg (determined according to oral administration in mice).
The metabolic pathway of 1,4-butanediol is summarized in Figure1.25 The process yields succinic acid that is further oxidized through the Krebs cycle. A metabolic study performed using 14C-labeled 1,4-butanediol showed no bioaccumulation and that the majority of 1,4-butanediol was rapidly oxidized into carbon dioxide (CO2).25
Figure 1.
Metabolism of BDDE.
Glycerol
According to a report by the Organization for Economic Co-operation and Development,26 glycerol is nonmutagenic, nonteratogenic, nonsensitizing, and a nonirritant, with a NOAEL of 2,000 mg/kg per day and an LD50 of 4,090 mg/kg (determined according to oral administration in mice). The safety of glycerol is well understood from its clinical role in reducing intracranial pressure (administered by intravenous infusion at a concentration of 24 mg/kg per day).27
Human and animal studies have shown that glycerol kinase phosphorylates glycerol to alpha-glycophosphate in the liver (80–90%) and, to a lesser extent, the kidneys (10–20%). Alpha-glycophosphate is then converted into CO2 and water through the classic metabolic pathway of glycolysis.28 Glycerol is rapidly eliminated through these pathways, with an elimination half-life in humans of approximately 30–45 minutes.29
The concentration of BDDE-derived byproducts that can be present in commercial fillers is so low (<5 mg/mL, determined using nuclear magnetic resonance spectroscopy), that the corresponding annual dose (<1.7 mg/kg per year, based on a 60-kg patient and a clinical dosage of 20 mL/year) is a small fraction of the NOAEL safety thresholds for daily doses of the BDDE metabolites butanediol (100 mg/kg per day) and glycerol (2,000 mg/kg per day). Therefore, and similar to the trace levels of unreacted BDDE, the presence of modified BDDE byproducts in commercial fillers is highly unlikely to result in toxicity.
Degradation of BDDE-Crosslinked HA
In the previous sections, we reviewed scientific data suggesting that uncrosslinked HA, BDDE, and their metabolites are clinically safe at the concentrations used in HA fillers. After the crosslinking reaction, the chemical structures of BDDE and HA are modified to various degrees. In this section, we review additional preclinical data, which demonstrate that these molecular changes do not pose additional safety risks.
Degradation of Modified BDDE
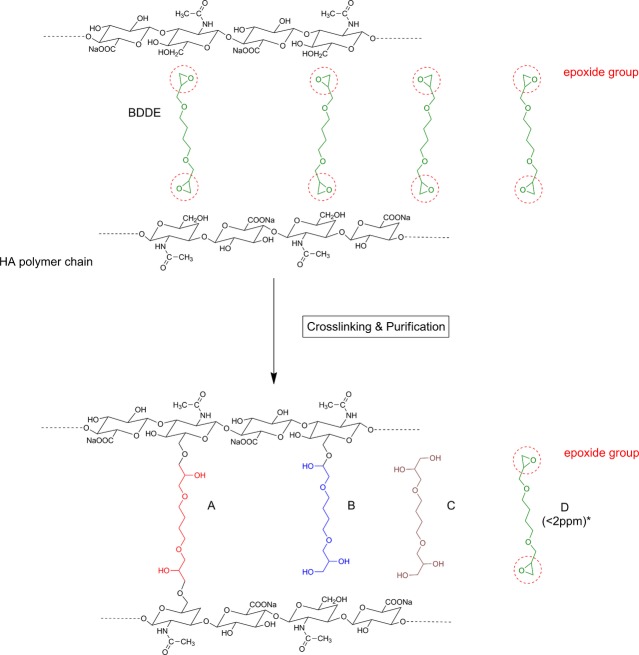
After the crosslinking reaction, BDDE can be present in different chemical states. As illustrated in Figure2, BDDE preferentially reacts with the primary alcohol groups in the HA backbone.
Figure 2.
Schematic showing the crosslinking reaction of hyaluronic acid chains with BDDE. The epoxide groups in BDDE preferentially react with the primary hydroxyl groups in the HA backbone, resulting in “fully reacted crosslinker” (A) or “pendant crosslinker” (B). BDDE that has not reacted with HA can be present in its hydrolyzed form “deactivated crosslinker” (C) or its native form “residual crosslinker” (D). *Since the sum of all four forms of BDDE is <5 mg/mL, or 5,000 ppm, the trace levels of the native form D (<2 ppm) represent a minute fraction of that sum.
The different states in which BDDE can be present in the final product are summarized here
(A) Fully reacted crosslinker: A BDDE molecule that has reacted with HA on both ends.
(B) Pendant crosslinker: A BDDE molecule that has reacted with HA on one end only.
(C) Deactivated crosslinker: A BDDE molecule that has reacted with H2O (hydrolyzed BDDE).
(D) Residual crosslinker: A BDDE molecule that has not reacted with HA or H2O.
As seen earlier, the risk linked to the presence of residual crosslinker is almost completely eliminated through purification of the crosslinked product. FDA-approved fillers are manufactured with an extremely tight design specification of <2 ppm unreacted BDDE in the finished product.
Because of the absence of the reactive epoxide groups, it is highly unlikely that any of the other chemical states of BDDE result in additional toxicity risks. Under the basic (pH > 7) conditions of the crosslinking reaction, the majority of the epoxide groups “open” to link to HA through an ether bond or to hydrolyze into an alcohol. It is also likely that any trace amounts of residual crosslinker in the finished product will continue to hydrolyze even under neutral conditions. This epoxide transformation is not reversible, and experimental evidence confirms that BDDE cannot be regenerated in the finished product (J.X. Roca-Martinez, unpublished data).
Because the ether bond-based chemistry is preserved in the fully reacted, pendant, and deactivated crosslinkers, it is thought that the in vivo metabolism of these molecules is similar to that of BDDE (by ether-bond cleavage). As discussed earlier, these metabolites (1,4-butanediol and glycerol) have been extensively described in the literature and are considered to be well tolerated in vivo.
Degradation of Modified HA
As described earlier, BDDE reacts mainly with the primary alcohol in the HA backbone (Figure2). Because the glycosidic bonds in the polysaccharide backbone are maintained after the reaction, it is thought that crosslinked HA is susceptible to the same in vivo degradation mechanisms as uncrosslinked HA (described earlier), and several reports in the literature have confirmed that crosslinked HA is amenable to the same enzymatic, hydrolytic, and oxidative degradation processes that break down native HA in the human body.30 The hyaluronidase family of enzymes degrades HA by specifically cleaving the glycosidic bonds between glucuronic acid and acetylglucosamine in the HA backbone. Because these bonds remain unaffected after the crosslinking reaction, it is thought that crosslinked HA is also susceptible to enzymatic degradation. Two separate studies using a variety of BDDE-crosslinked HA fillers with different physicochemical properties showed that the BDDE modification does not interfere with the natural enzymatic degradation mechanisms of HA.31,32 Similarly, it has been shown that the BDDE modification also does not affect the susceptibility of uncrosslinked HA to oxidative degradation (D. Stroumpoulis, unpublished data). More specifically, using size exclusion chromatography, it was shown that crosslinked HA is fully susceptible to free radical-induced oxidative degradation.
Biocompatibility of BDDE-Crosslinked HA fillers
In addition to clinical safety data spanning longer than 15 years, a substantial body of biocompatibility data exists for BDDE-crosslinked HA fillers. As early as the 1980s, reports on acute and chronic biocompatibility of crosslinked HA were published after testing in the vitreous cavity of the eye and viscosupplementation of the osteoarthritic synovial fluid.33,35,34 Furthermore, animal studies have successfully provided information on topics such as length of tissue reaction (acute, subchronic, or chronic), type of reaction (systemic vs local tissue toxicity), hypersensitivity, and genotoxicity.
For the majority of the commercially available fillers, much of these data were generated in compliance with regulatory agencies to gain market approval. Using Juvéderm products (Allergan) as an example, Table1 summarizes a typical biocompatibility profile of crosslinked HA fillers. The tests shown in Table1 were performed in conformance with international standards (International Standards Organization (ISO) 10993) as mandated by regulatory agencies. Based on these results, no evidence of acute, subchronic, or chronic inflammation; sensitivity; irritation; intracutaneous reactivity; systemic toxicity; hypersensitivity; or genotoxicity were observed for these products.
Table 1.
Biocompatibility Test Results for 1,4-Butanediol Diglycidyl Ether–Containing Products
| Biocompatibility Test Objective | Test Standard | Results |
|---|---|---|
| Tests for in vitro cytotoxicity | ISO 10993–5 | No evidence of cytotoxicity |
| Tests for irritation and delayed-type hypersensitivity | ISO 10993–10 | No evidence of skin irritation (erythema, edema, necrosis) or delayed dermal contact sensitization |
| Tests for acute systemic toxicity | ISO 10993–11 | No evidence of systemic toxicity for up to 4 days after implantation |
| Tests for subchronic toxicity | ISO 10993–11 | No evidence of toxicity or signs of irritation or inflammation for up to 3 months after implantation |
| Tests for local effects after implantation | ISO 10993–6 | No evidence of irritation and minor local tissue reaction for up to 4 weeks and 12 weeks after muscle implantation |
| Tests for genotoxicity: Ames test | ISO 10993–3 | No evidence of genotoxicity |
| Tests for genotoxicity: Micronucleus Test | ISO 10993–3 | No evidence of genotoxicity |
| Tests for genotoxicity: Chromosomal Aberration Test | ISO 10993–3 | No evidence of genotoxicity |
| Test for chronic toxicity: 12-month study, multiple injections | ISO 10993–11 | No evidence of systemic toxicity or local irritation for up to 12 months after implantation |
ISO, International Standards Organization.
These data, from multiple regulatory agency-mandated preclinical tests, demonstrate that this BDDE-containing range of products has a favorable safety profile. The products used in all of these tests were from the Juvéderm range.
Furthermore, chronic toxicity and subchronic toxicity data (according to ISO 10993–11) were used to determine what constitutes a safe dose of Juvéderm products in animals. The human equivalent yearly dose was then determined to be equal to 20 mL per 60 kg of body weight (according to ISO 10993–17 Guidelines and the FDA’s Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers).
These preclinical results provided the first evidence of the safety of BDDE-crosslinked HA products and paved the way to the first clinical studies.
Clinical Safety of HA Fillers
Hyaluronic acid has a long history of cosmetic use; in 1989, Balasz and Denlinger described the first HA developed as a dermal filler,1,36 and HA dermal fillers have been used for soft tissue augmentation since the 1990s.1 The first BDDE-crosslinked HA filler was made available in Europe in 1996, and since then the safety profile of this type of product has been extensively studied. A literature search revealed that, in more than 50 studies conducted over the past 15 years in more than 9,000 patients, BDDE-crosslinked HA fillers have been reported to have a favorable safety profile and be generally well tolerated.
Summary
Hyaluronic acid is a naturally occurring polymer with a degradation pathway that is well understood. Because the half-life of HA in the skin is only a few days, crosslinking is typically employed to stabilize the HA matrix and provide a clinical duration in soft tissue filling that can reach or exceed 1 year. This duration is predominately achieved using epoxide-based crosslinking chemistry and specifically BDDE as the crosslinking agent. BDDE is the crosslinker used in the majority of the market-leading HA fillers. After reaction with HA, the epoxide groups of BDDE are neutralized, and only trace amounts of unreacted BDDE remain in the product (<2 ppm). These trace amounts, which the FDA has determined to be below the level that is safe after a safety risk assessment, are prone to hydrolysis that ultimately yields CO2 and water. Crosslinked HA is expected to follow a degradation pathway that is similar to that of uncrosslinked HA and unreacted BDDE, because the crosslinking reaction does not affect the backbone chemistry of these molecules. When the product degrades, it breaks down into harmless byproducts or into byproducts that are identical to substances already found in the skin.
Clinical and biocompatibility data spanning more than 15 years support the favorable clinical safety profile of BDDE-crosslinked HA and its degradation products. Since the launch of the first BDDE-crosslinked HA dermal filler for cosmetic use in 1996, more than 50 studies have been conducted with more than 9,000 patients that have reported on the safety and tolerability of this type of product. This legacy of safety may explain why two million people in 2010 chose to undergo cosmetic procedures with HA dermal fillers, the majority of which use BDDE crosslinking. Given the strength of the empirical evidence, physicians should be confident in offering these products to their patients.
References
- Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer Adherence. 2011;5:133–9. doi: 10.2147/PPA.S11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAPS. International Survey on Aesthetic/Cosmetic Procedures Performed in 2010. ISAPS, December 2011. Available from: http://www.isaps.org/files/html-contents/ISAPS-Procedures-Study-Results-2011.pdf Accessed January 2, 2013.
- Afssaps. Topical Report: Injectable products to fill wrinkles. Afssaps, June 2011. Available from: http://www.afssaps.fr/var/ansm_site/storage/original/application/28d68bbb0f7ee427f4836b15cf107a1b.pdf Accessed January 2, 2013.
- De Boulle K. Management of complications after implantation of fillers. J Cosmet Dermatol. 2004;3:2–15. doi: 10.1111/j.1473-2130.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- Radiesse Instructions for Use. Merz Aesthetics, Inc, October 2012 Available from: http://www.radiesse.com/en-US/downloads/RADIESSE_Wrinkle_Filler_Instructions_for_Use.pdf Accessed January 2, 2013.
- Gold MH. What’s new in fillers in 2010? J Clin Aesthet Dermatol. 2010;3:36–45. [PMC free article] [PubMed] [Google Scholar]
- Newman J. Review of soft tissue augmentation in the face. Clin Cosmet Investig Dermatol. 2009;2:141–50. doi: 10.2147/ccid.s3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67–72. doi: 10.1055/s-0029-1220645. [DOI] [PubMed] [Google Scholar]
- Kim J-E, Sykes JM. Hyaluronic acid fillers: history and overview. Facial Plast Surg. 2011;27:523–8. doi: 10.1055/s-0031-1298785. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–25. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- Stern R, Kogan G, Jedrzejas M, Šoltés L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25:537–57. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Fraser JRE, Laurent TE. Turnover and metabolism of hyaluronan. In: Evered D, Wheelan J, editors. The Biology of hyaluronan Ciba Found Symp. Vol. 143. 1989. pp. 41–59. [PubMed] [Google Scholar]
- Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16:1718–45. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, Davies MJ. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem J. 2004;381:175–84. doi: 10.1042/BJ20040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alster TS, West T. Effect of topical vitamin C on postoperative carbon dioxide laser resurfacing erythema. Dermatol Surg. 1998;24:331–4. doi: 10.1111/j.1524-4725.1998.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Laurent UB, Dahl LB, Reed RK. Catabolism of hyaluronan in rabbit skin takes place locally, in lymph nodes and liver. Exp Physiol. 1991;76:695–703. doi: 10.1113/expphysiol.1991.sp003536. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A, Hellström S. The role of liver and kidneys in the removal of circulating hyaluronan an experimental study in the rat. Connect Tissue Res. 1990;24:219–24. doi: 10.3109/03008209009152150. [DOI] [PubMed] [Google Scholar]
- Foureman P, Mason JM, Valencia R, Zimmering S. Chemical mutagenesis testing in drosophila IX. Results of 50 coded compounds tested for the national toxicology program. Environ Mol Mutagen. 1994;23:51–63. doi: 10.1002/em.2850230109. [DOI] [PubMed] [Google Scholar]
- Ciba-Geigy Corp. 1987. A cutaneous carcinogenicity study with mice on the diglycidyl ether of 1,4-butanediol.
- Gerbal-Chaloin S. 2000. Regulation de l’expression des cytochromes P450 de la sous-familler 2C dans des cultures primaires d’hépatocytes humains. Thèse Médecine: Université de Montpellier 1. Extrait.
- Ethers de glycol—quels risques pour la santé? INSERM; 1999. INSERM. Généralités; pp. 1–13. ISBN 2-85598-761-X, ISSN 1264-1782: [Google Scholar]
- Ethers de glycol—quels risques pour la santé? INSERM; 1999. Métabolisme et toxicocinétique chez l’animal; pp. 21–39. ISBN 2-85598-761-X, ISSN 1264-1782: [Google Scholar]
- Ishikawa K. 2000. pp. 1–60. 1,4-butanediol. OECD SIDS CAS N° 110-63-4 .
- NICNAS. 2009. pp. 1–25. 1,4-butanediol. Existing chemical hazard assessment report ISBN 978-0-9803124-7-8.
- Irwin RD. 1996. pp. 1–44. NTP summary report on the metabolism, disposition, and toxicity of 1,4-butanediol. National toxicology program toxicity report series (54)
- Robertson S. 2002. pp. 1–178. Glycerol. OECD SIDS CAS N° 56-81-5:
- Pitlick WH, Pirikitakuhlr P, Painter MJ, Wessel HB. Effect of glycerol and hyperosmolality on intracranial pressure. Clin Pharmacol Ther. 1982;31:466–71. doi: 10.1038/clpt.1982.61. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Griffin SE. Glycerol: biochemistry, pharmacokinetics and clinical and practical applications. Sports Med. 1998;26:145–67. doi: 10.2165/00007256-199826030-00002. [DOI] [PubMed] [Google Scholar]
- Frank MSB, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy. 1981;1:147–60. doi: 10.1002/j.1875-9114.1981.tb03562.x. [DOI] [PubMed] [Google Scholar]
- Larsen NE, Pollak CT, Reiner K, Leshchiner E, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129–34. doi: 10.1002/jbm.820270903. [DOI] [PubMed] [Google Scholar]
- Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804–9. [Google Scholar]
- Sall I, Ferard G. Comparison of the sensitivity of 11 crosslinked hyaluronic acid gels to bovine testis hyaluronidase. Polym Degrad Stab. 2007;92:915–9. [Google Scholar]
- Weiss C, Levy HJ, Denlinger JL, Suros JM, et al. The role of Na-hylan in reducing post-surgical tendon adhesions: Part I. Bull Hosp Jt Dis Orthop Inst. 1986;46:9–15. [PubMed] [Google Scholar]
- Weiss C, Suros JM, Michalow A, Moore M, et al. The role of Na-hylan in reducing post-surgical tendon adhesions Part II. Bull Hosp Jt Dis Orthop Inst. 1987;47:31–9. [PubMed] [Google Scholar]
- Malson T, Lindqvist BL. 1987. Gel of crosslinked hyaluronic acid for use as a vitreous humor substitute. United States Patent n° 4 716 154.
- Balazs EA, Denlinger JL. Clinical uses of hyaluronan. Ciba Found Symp. 1989;143:265–75. doi: 10.1002/9780470513774.ch16. [DOI] [PubMed] [Google Scholar]