Abstract
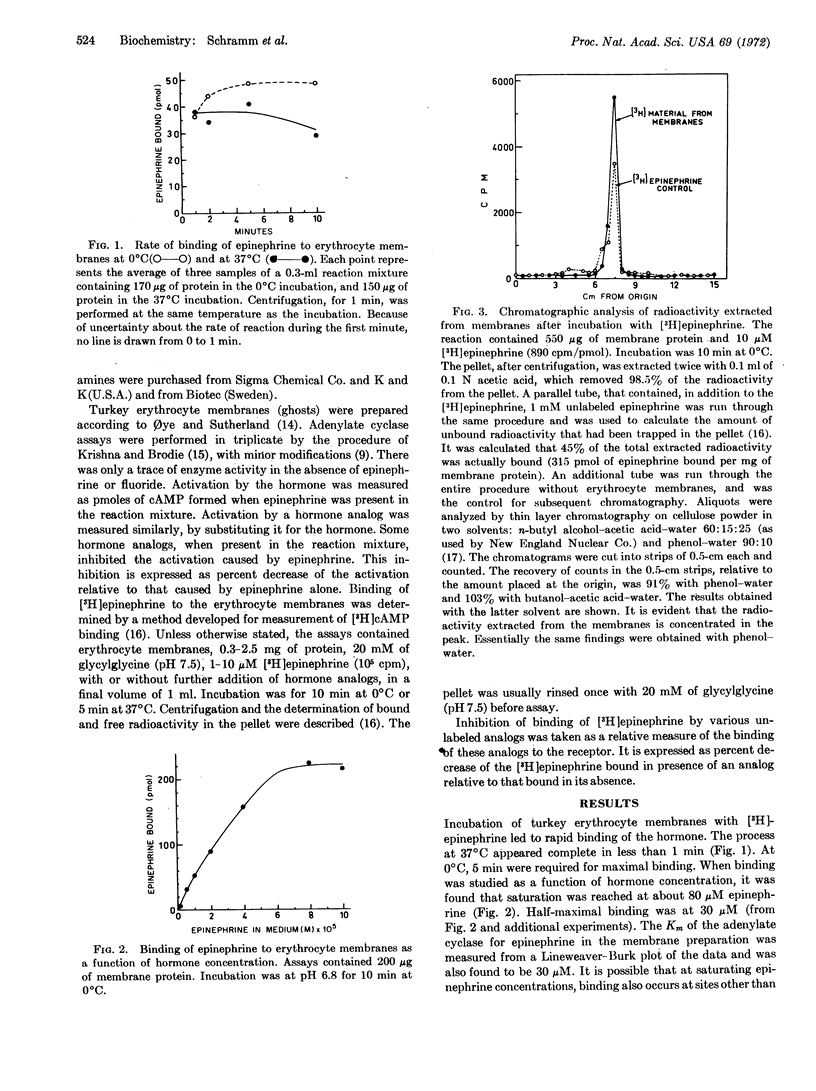
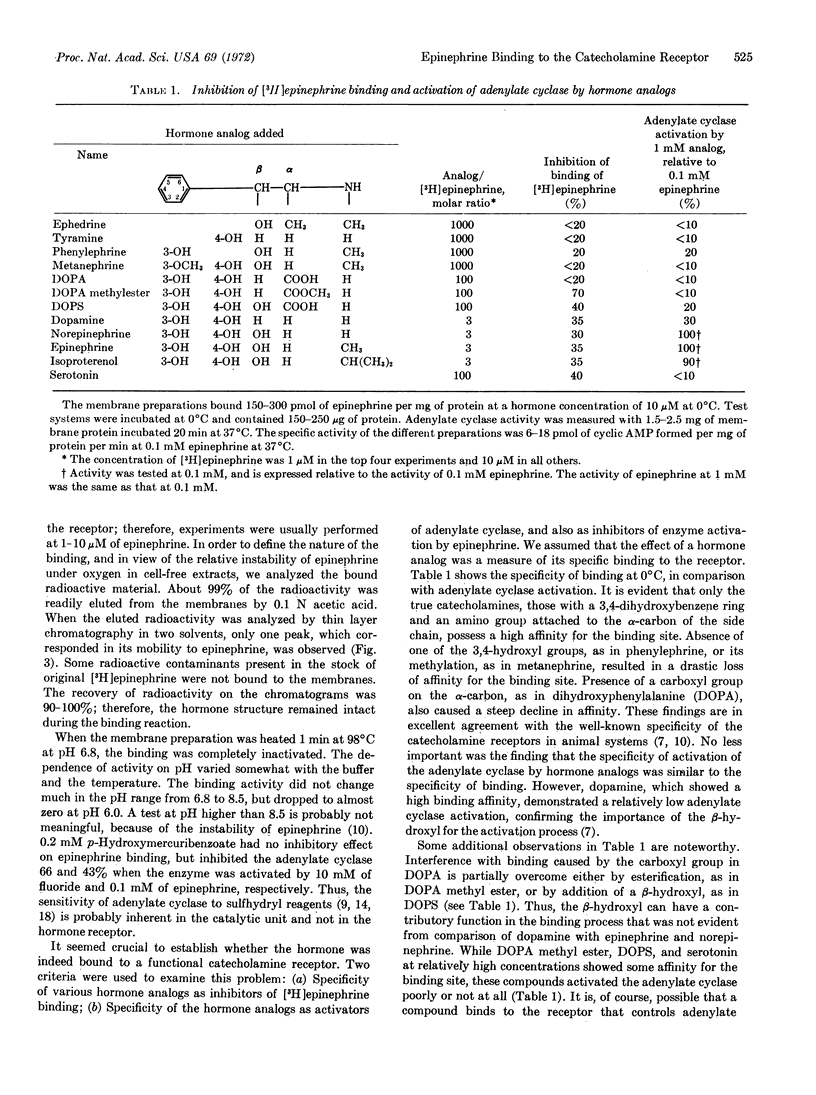
Turkey erythrocyte membranes showed specific binding of [3H]epinephrine. The concentration of hormone required for half-maximal binding (30 μM) was the same as that required for half-maximal activation of the adenylate cyclase located in the same membrane preparation. The binding reaction at 37°C reached completion during the first minute of incubation, which agrees well with the known rapidity of the biological response to catecholamines. Specific binding was abolished by heating the membranes 1 min at 100°C. Chromatography of the bound 3H, after its extraction from the membranes, indicated that the hormone had fully retained its chemical structure. Epinephrine binding was inhibited by the β-adrenergic blocking agent propranolol, which also inhibited the activation of adenylate cyclase by the hormone. The specificity of phenethylamine derivatives in displacing [3H]epinephrine from the binding sites showed that a typical catecholamine receptor was responsible for the binding. Displacement of the bound hormone by analogs lacking the catechol group was more extensive at 37°C than at 0°C. Some of the analogs that displaced epinephrine from the binding site caused only a feeble activation of the adenylate cyclase, but were able to inhibit the activation of the enzyme by epinephrine. Thus, binding to a catecholamine receptor on a membrane preparation is an essential, but insufficient, condition to elicit a response.
Keywords: hormone receptor, β-adrenergic receptor, cyclic AMP, turkey
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Cuatrecasas P., Desbuquois B., Krug F. Insulin-receptor interactions in liver cell membranes. Biochem Biophys Res Commun. 1971 Jul 16;44(2):333–339. doi: 10.1016/0006-291x(71)90604-8. [DOI] [PubMed] [Google Scholar]
- De Robertis E. Molecular biology of synaptic receptors. Science. 1971 Mar 12;171(3975):963–971. doi: 10.1126/science.171.3975.963. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., O'Brien R. D. Autoinhibition of acetylcholine binding to Torpedo electroplax; a possible molecular mechanism for desensitization. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2006–2007. doi: 10.1073/pnas.68.9.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Pharmacological characteristics of adrenergic receptors. Fed Proc. 1970 Jul-Aug;29(4):1352–1361. [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E. A fraction of the ventricular myocardium that has the specificity of the cardiac beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1773–1777. doi: 10.1073/pnas.68.8.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- Michaelson I. A., Taylor P. W., Jr, Richardson K. C., Titus E. Uptake and metabolism of dl-norepinephrine by subcellular particles of rat heart. J Pharmacol Exp Ther. 1968 Apr;160(2):277–291. [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Salomon Y., Schramm M. A specific binding site for 3',5' cyclic AMP in rat parotid microsomes. Biochem Biophys Res Commun. 1970 Jan 6;38(1):106–111. doi: 10.1016/0006-291x(70)91090-9. [DOI] [PubMed] [Google Scholar]
- Schramm M., Naim E. Adenyl cyclase of rat parotid gland. Activation by fluoride and norepinephrine. J Biol Chem. 1970 Jun;245(12):3225–3231. [PubMed] [Google Scholar]
- Tomasi V., Koretz S., Ray T. K., Dunnick J., Marinetti G. V. Hormone action at the membrane level. II. The binding of epinephrine and glucagon to the rat liver plasma membrane. Biochim Biophys Acta. 1970 Jul 7;211(1):31–42. doi: 10.1016/0005-2736(70)90120-3. [DOI] [PubMed] [Google Scholar]
- WEISS B., ROSSI C. V. Separation of catecholamines by paper chromatography. Nature. 1962 Jul 14;195:178–178. doi: 10.1038/195178a0. [DOI] [PubMed] [Google Scholar]



