Introduction
Aneurysmal subarachnoid haemorrhage (SAH) is a devastating disease first described in the era of Hippocrates. Despite advances in treatment, 1-month case fatality remains as high as 35 %, with around one-third of survivors needing lifelong care and a further third having residual cognitive impairment that affects functional status and quality of life. The immediate and delayed effects of SAH on outcome have recently been reviewed in Intensive Care Medicine [1]. There have been advances in the treatment of this devastating condition aimed at reducing early (mainly re-bleeding) or late complications, particularly delayed cerebral ischemia (DCI). The importance of treatment in high-volume centres by a multidisciplinary team cannot be over-emphasised [2].
Endovascular coiling of intracranial aneurysms represents a major advance in the treatment of SAH and allows minimally invasive and effective treatment. Flow-diverting stents, with or without coils, are an increasingly employed strategy that allows even complex aneurysm to be treated minimally invasively [3]. Clipping of the aneurysm remains an option in the minority of cases that cannot be secured via the endovascular route. Early aneurysm control reduces the risk of re-bleeding and allows maintenance of higher systemic blood pressure to prevent or treat cerebral hypoperfusion. Once the aneurysm is secured, the intensive care management of SAH involves optimization of systemic physiology, and prevention or treatment of DCI and non-neurological complications. Consensus guidelines on the critical care management of SAH have recently been published [2].
Delayed cerebral ischemia
DCI is a term applied to any neurological deterioration, including focal neurological deficits and altered consciousness, which persists for more than 1 h and cannot be explained by physiological, radiological or laboratory abnormalities. It peaks 4–10 days after the ictus, persists for several days and is second only to the initial hemorrhage in terms of morbidity and mortality. Although DCI has been attributed to cerebral vasospasm, the exact relationship between the two is unclear. DCI can occur in the absence of vasospasm and vice versa, and brain ischemia often involves more than one vascular territory. Other mechanisms contributing to DCI include distal vascular dysautoregulation, micro-thrombi, direct neurotoxic effects, inflammation and cortical spreading depolarizations (SDs) [4]. SDs are pathological events characterized by near-complete sustained depolarization of neurons and astrocytes that result in secondary injury related to mitochondrial damage, accumulation of intracellular calcium and excitotoxicity. SDs, which can only be detected by electrocorticography, have been reported in up to 70 % of SAH patients. They are associated with DCI and worse outcome, and management should focus on controlling variables such as pyrexia, hypoxia, hypoglycemia and systemic hypotension which increase the incidence and duration of SDs [5].
A combination of hypervolemia, hemodilution and hypertension (triple H therapy) has historically been the mainstay of treatment to prevent and treat DCI, but the focus of cardiovascular management after SAH is now on a single H—hypertension [2]. Prophylactic hypervolemia is not effective in increasing CBF or improving neurological outcome, and there is some evidence of harm from overly aggressive filling [6]. Euvolemia is the target for both prophylaxis and treatment of DCI, and hemodilution has no place [2]. Induced hypertension can be effective in reversing DCI, and systemic blood pressure should be increased in a stepwise fashion guided by assessment of neurological function, neuromonitoring or radiological evidence of improved perfusion. The higher blood pressure should be maintained for 2–3 days and gradually weaned while monitoring for deterioration in clinical and neuromonitoring variables [2]. There is preliminary evidence that early goal-directed hemodynamic therapy might reduce the risk of DCI and improve outcome after SAH [7], but large, randomized controlled outcome studies are required before widespread adoption into clinical practice.
Several pharmacological agents which target the diverse pathophysiology of DCI have been investigated, but only nimodipine has been proven to improve outcome [1]. Despite the multiple beneficial effects of statins on vascular function, a recent multicentre randomized phase 3 trial found no benefit of simvastatin on short- or long-term outcome after SAH [8]. In a recent meta-analysis, intravenous magnesium administration was associated with a reduced incidence of DCI but not with beneficial effects on mortality or functional outcome [9]. Endothelin-A antagonists have been studied extensively after SAH because of the key role that endothelin plays in maintaining vascular tone. In randomized controlled trials, the endothelin-A antagonist clazosentan reduced angiographic vasospasm but had no significant effect on outcome [10]. Attention has also focussed on agents affecting the coagulation cascade because of the potential role of microthrombosis in the pathogenesis of DCI. A meta-analysis of seven randomized controlled trials of anti-platelet agents identified a trend towards improved outcome but also a higher risk of intracranial haemorrhagic complications [11].
Neuromonitoring
In addition to clinical neurological examination in conscious patients, serial transcranial Doppler (TCD) ultrasonography supplemented by vascular and perfusion-imaging studies is the mainstay of monitoring for DCI. While such snapshots in time provide valuable information, multimodal brain monitoring allows real-time bedside assessment of the efficacy of hemodynamic augmentation to prevent or treat DCI. This is particularly important when clinical neurological examination is limited in sedated or poor-grade patients.
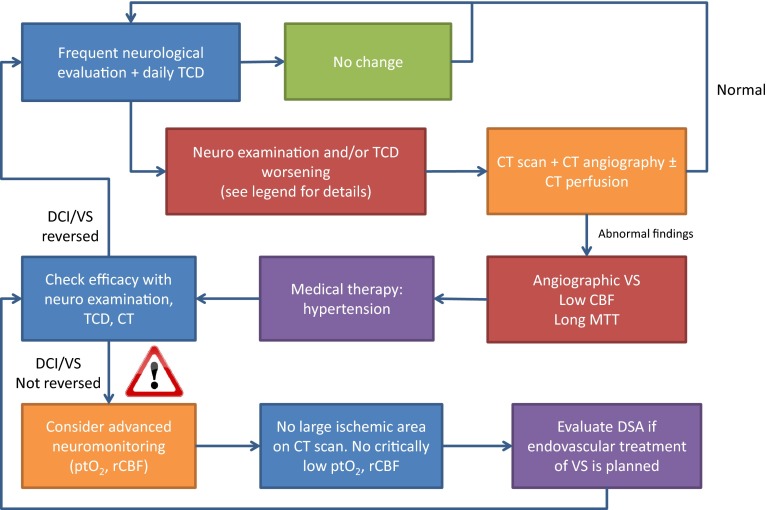
Increased ICP is common after SAH, particularly early after the ictus and in comatose patients [12]. The indications for ICP monitoring are often inter-related with the need to treat obstructive hydrocephalus, so a ventricular catheter is the optimal ICP monitor, being both a diagnostic and therapeutic modality. Intracranial hypertension and lack of ICP response to medical therapy appear to be associated with DCI and poor clinical outcome after SAH, and ICP and CPP monitoring have a role in guiding therapy. Multimodal cerebral monitoring, including brain tissue oxygen tension, TCD, cerebral microdialysis and electrophysiological monitoring in addition to ICP, allows individualized therapy with the aim of preventing or minimizing secondary ischemic injury [13]. Monitoring allows early detection of DCI and identifies end points for cardiovascular augmentation in its management. Changes in brain tissue oxygenation and biochemistry may identify impending or actual cerebral hypoxia/ischemia before changes in other monitoring modalities or clinical status, but it remains to be determined whether interventions directed towards normalization of these variables affects outcome. Data from small studies investigating the efficacy of multimodal neuromonitoring-guided treatment after SAH have been encouraging, but outcome data are lacking [12]. Figure 1 sh ows a practical approach to monitoring-guided management of DCI after SAH.
Fig. 1.
Monitoring-guided management of delayed cerebral ischemia after subarachnoid haemorrhage. The figure shows a practical approach to monitoring-guided management of DCI after subarachnoid hemorrhage. The starting point is a frequent neurological evaluation and daily TCD, with clinically significant changes defined as new focal deficit or altered consciousness and TCD mean flow velocity >120 cm/s, increase >50 cm/s in 24 h, and/or a Lindegaard index (MCA/ICA blood flow velocity ratio) >6. If the neurological examination or TCD indicates a worsening state, a reasonable approach is to search for a potential reversible cause with a CT scan, CT angiography and perfusion CT. If angiographic vasospasm is present and CBF is reduced and/or MTT increased, a trial of stepwise-induced hypertension is recommended. If this strategy reverses DCI, close monitoring with maintenance of the higher blood pressure for 2–3 days is recommended. If hypertension alone does not reverse DCI, advanced neuromonitoring and further imaging prior to interventional radiological treatment should be considered in salvageable patients. CBF cerebral blood flow, CT computerized tomography, DCI delayed cerebral ischemia, DSA digital subtraction angiography, ICA internal carotid artery, MCA middle cerebral artery, MTT mean transmit time, ptO 2 brain tissue oxygen tension, rCBF regional cerebral blood flow, TCD transcranial Doppler ultrasonography, VS vasospasm
Seizures
Using surface electroencephalography (EEG), seizures are recorded in around 8 % of patients after SAH but invasive EEG monitoring identifies seizure activity in almost 40 %. Continuous EEG monitoring is the only reliable way to detect subclinical seizures, and it may also predict DCI many hours in advance of clinical symptoms [14]. Actual seizures must be treated aggressively but universal prophylaxis remains controversial. Three days of treatment offers similar seizure prevention and better outcome than longer-term therapy [2]. Phenytoin has been associated with cognitive effects and poor outcome and, although of equal efficacy in controlling seizures, levetiracetam is often chosen for the management of early-onset seizures because of its more favourable side-effect profile [15].
Conflicts of interest
G.C. is Deputy Editor and M.S. Section Editor of Intensive Care Medicine. No other conflicts declared.
Contributor Information
M. Smith, Phone: +44 (0)20 3448 4711, Email: martin.smith@uclh.nhs.uk
G. Citerio, Email: giuseppe.citerio@unimib.it
References
- 1.Macdonald RL, Diringer MN, Citerio G. Understanding the disease: aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2014 doi: 10.1007/s00134-014-3483-5. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN, Bleck TP, Claude HJ, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211–240. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 3.Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44:442–447. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 5.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankbaar JW, Slooter AJ, Rinkel GJ, Schaaf IC. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care. 2010;14:R23. doi: 10.1186/cc8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke. 2014;45:1280–1284. doi: 10.1161/STROKEAHA.114.004739. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13:666–675. doi: 10.1016/S1474-4422(14)70084-5. [DOI] [PubMed] [Google Scholar]
- 9.Reddy D, Fallah A, Petropoulos JA, Farrokhyar F, Macdonald RL, Jichici D. Prophylactic magnesium sulfate for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2014;21:356–364. doi: 10.1007/s12028-014-9964-0. [DOI] [PubMed] [Google Scholar]
- 10.Vergouwen MD, Algra A, Rinkel GJ. Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2012;43:2671–2676. doi: 10.1161/STROKEAHA.112.666693. [DOI] [PubMed] [Google Scholar]
- 11.Dorhout Mees SM, van den Bergh WM, Algra A, Rinkel GJ. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;4:CD006184. doi: 10.1002/14651858.CD006184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoerle T, Lombardo A, Colombo A, et al. Intracranial pressure after subarachnoid hemorrhage. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 13.Sarrafzadeh AS, Vajkoczy P, Bijlenga P, Schaller K. Monitoring in neurointensive care–the challenge to detect delayed cerebral ischemia in high-grade aneurysmal SAH. Front Neurol. 2014;5:134. doi: 10.3389/fneur.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondziella D, Friberg CK, Wellwood I, Reiffurth C, Fabricius M, Dreier JP. Continuous EEG monitoring in aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2014 doi: 10.1007/s12028-014-0068-7. [DOI] [PubMed] [Google Scholar]
- 15.Karamchandani RR, Fletcher JJ, Pandey AS, Rajajee V. Incidence of delayed seizures, delayed cerebral ischemia and poor outcome with the use of levetiracetam versus phenytoin after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:1507–1513. doi: 10.1016/j.jocn.2014.03.009. [DOI] [PubMed] [Google Scholar]