Abstract
Adenosine monophosphate-activated protein kinase (AMPK) is a key player in maintaining energy homeostasis in response to metabolic stress. Beyond diabetes and metabolic syndrome, there is a growing interest in the therapeutic exploitation of the AMPK pathway in cancer treatment in light of its unique ability to regulate cancer cell proliferation through the reprogramming of cell metabolism. Although many studies support the tumor-suppressive role of AMPK, emerging evidence suggests that the metabolic checkpoint function of AMPK might be overridden by stress or oncogenic signals so that tumor cells use AMPK activation as a survival strategy to gain growth advantage. These findings underscore the complexity in the cellular function of AMPK in maintaining energy homeostasis under physiological versus pathological conditions. Thus, this review aims to provide an overview of recent findings on the functional interplay of AMPK with different cell metabolic and signaling effectors, particularly histone deacetylases, in mediating downstream tumor suppressive or promoting mechanisms in different cell systems. Although AMPK activation inhibits tumor growth by targeting multiple signaling pathways relevant to tumorigenesis, under certain cellular contexts or certain stages of tumor development, AMPK might act as a protective response to metabolic stresses, such as nutrient deprivation, low oxygen, and low pH, or as a downstream effectors of oncogenic proteins, including androgen receptor, hypoxia-inducible factor-1α, c-Src, and MYC. Thus, investigations to define at which stage(s) of tumorigenesis and cancer progression or for which genetic aberrations AMPK inhibition might represent a more relevant strategy than AMPK activation for cancer treatment are clearly warranted.
Keywords: AMPK, metabolic homeostasis, cancer therapy, LKB1, mTORC1, HDAC, Foxo3a, HIF-1α
AMPK
Adenosine monophosphate-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase consisting of the catalytic α, scaffolding β, and AMP-sensing γ subunits, each of which contains two or more isoforms in mammalian cells (α1, α2, β1, β2, γ1, γ2, and γ3) [1]. AMPK was originally discovered in the late 1970s as a kinase that mediated phosphorylating inactivation of key lipogenic enzymes, including acetyl-CoA carboxylase (ACC) [2] and 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase [3], in response to cellular AMP or ADP elevation [4]. Since then, this energy sensor has been recognized as a critical player in maintaining energy homeostasis, at both cellular and whole-body levels, in response to metabolic stresses, such as nutritional deprivation, hypoxia, and oxidative stress, by switching on ATP-generating (catabolic) pathways, i.e., glycolysis, fatty acid oxidation, and mitochondrial biogenesis, while concomitantly turning off ATP-utilizing (anabolic) pathways, i.e., gluconeogenesis, lipogenesis, and protein synthesis [5, 6]. Moreover, AMPK has been shown to mediate the metabolic effects of hormones, such as leptin, adiponection, glucocorticoids, and insulin, on energy balance in multiple peripheral tissues, including liver, skeletal muscle, and hypothalamus [7, 8]. Considering the integral role of AMPK in regulating glucose and lipid homeostasis, AMPK activation represents a therapeutically relevant target for diabetes and the metabolic syndrome [9–12], which constitutes the basis for the use of metformin [13] and thiazolidinediones [14] for the treatment of type 2 diabetes.
More recently, it has been suggested that AMPK acts as a metabolic tumor suppressor in light of its unique ability to regulate cancer cell proliferation through the reprogramming of cell metabolism [11, 15, 16]. For example, inactivating mutations of the gene encoding liver kinase B1 (LKB1), an upstream kinase of AMPK, plays a critical role in the hereditary Peutz-Jeghers syndrome [17] and is associated with sporadic lung and cervical cancers [18–20]. In addition, a recent multilayer integrated analysis of the whole transcriptome of tumors from Asian gastric cancer patients identified PPKAA2, which encodes the AMPKα2 subunit, as one of the most downregulated genes in early-stage gastric carcinogenesis [21]. Under-expression of AMPK-α2 was also reported in hepatocellular carcinoma (HCC) patients, which was statistically associated with an undifferentiated cellular phenotype and poor patient prognosis [22]. From a mechanistic perspective, this increased cancer susceptibility might, in part, be attributable to the dysregulated AMPK-mammalian target of rapamycin (mTOR) signaling pathway [23, 24]. Moreover, epidemiologic evidence links type 2 diabetic patients receiving metformin, a pharmacological activator of AMPK [13], on a long-term basis with reduced risk of cancer [25, 26]. The proof-of-concept of targeting AMPK activation in cancer prevention was supported the ability of metformin to suppress tobacco carcinogen-induced lung tumorigenesis in mice [27], and government records indicate that metformin is currently undergoing multiple clinical trials to reduce cancer risk in various cancer types (www.clinicaltrials.gov).
Growing evidence indicates that tumor cells evolve different mechanisms to develop metabolic adaptations to survive metabolic stresses caused by deprivation of nutrients and oxygen in the microenvironment, and that AMPK, in the capacity of metabolic checkpoint, plays a key role in this molecular adaptation [28, 29]. Although many studies support the tumor-suppressive role of AMPK, emerging evidence suggests that under certain circumstances, cancer cells might also turn the tables by “hijacking” AMPK to gain growth advantage through distinct mechanisms in face of metabolic adversity, which underlies the complicated role of AMPK in regulating tumor metabolism [30–33] (see the “The dark side of AMPK” section for discussion). In light of the rapid advances in the AMPK field, this review aims to provide an overview of recent findings on the functional interplay of AMPK with different cell metabolic and signaling effectors, particularly histone deacetylases (HDACs), in mediating downstream tumor-suppressive or -promoting pathways in different cell systems. The readers are also referred to many recent outstanding reviews for detailed discussion of the functional role of AMPK in regulating metabolic homeostasis and cell growth [6–9, 12, 15, 16, 23, 28, 29].
MULTIFACETED REGULATION OF AMPK ACTIVATION
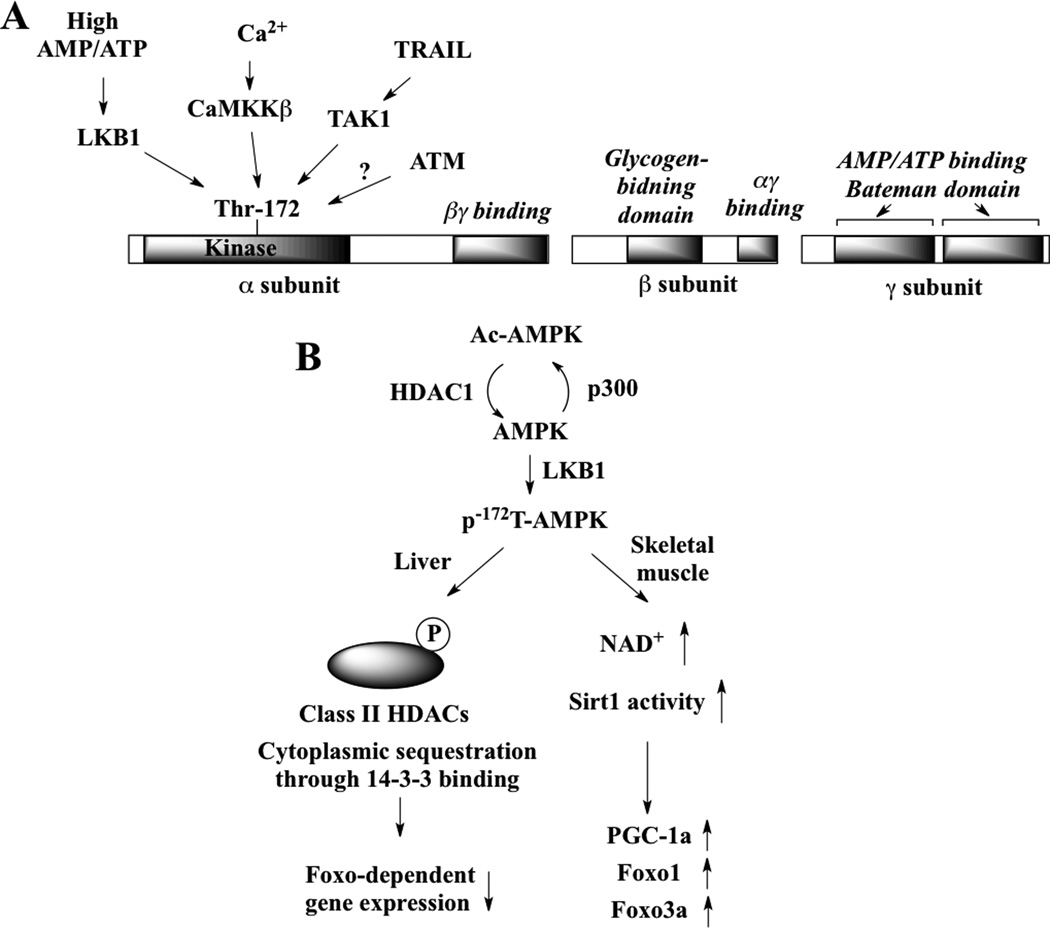
In response to stimuli, AMPK is activated through phosphorylation at Thr-172 within the activation loop of the kinase domain on the AMPKα subunit. This activation is allosterically regulated by AMP and ATP, the positive and negative AMPK modulators, respectively, through binding to the Bateman domain on the AMPKγ regulatory subunit (Fig. 1A) [9, 34, 35]. Moreover, recent evidence showed that glycogen, an indicatior of cellular energy reserves, inhibited AMPK through direct binding to the glycogen-binding domain on the AMPKβ subunit, suggesting that AMPK acts as a glycogen sensor [36].
Fig. (1).
(A) Domain structure of the three subunits of the AMPK heterotrimer. Each of the two AMP/ATP-binding Bateman domains in the γ subunit is composed of two tandem repeats of a CBS motif. AMPK can be activated by four different upstream kinases under different physiological conditions. (B) Interplay between AMPK and different HDACs, as both upstream and downstream effectors, in regulating the activity of AMPK, which is discussed in the text.
At least four different upstream kinases have been identified to mediate Thr-172 AMPK phosphorylation in response to various stimuli in different cell systems, including the aforementioned LKB1 [37–40], calmodulin kinase kinase (CaMKK)β [41–43], TGF-β-activated kinase (TAK)1 [44, 45], and ataxia telangiectasia mutated (ATM) [46]. Under physiological conditions, each of these kinases plays a distinct role in regulating AMPK activation in different cellular contexts (Table 1).
Table 1.
Characteristic features of the upstream kinases of AMPK
| Kinase | Physiological function |
|---|---|
| LKB1 | Constitutively switched-on in cells. Under conditions of low cellular energy, i.e., high cellular AMP/ATP ratios, AMP binding causes conformational changes, which allows LKB1 to phosphorylate AMPKα [47]. |
| CaMKK | An alternative AMPK kinase in LKB-1-deficient cell lines, which is activated in response to elevated cytosolic Ca2+ levels [41–43]. Recent evidence suggests that CaMKK-induced AMPK activation plays an important role in Ca2+-regulated glucose and fatty acid metabolism in contracting skeletal muscle [48]. In addition, CaMKK plays a crucial role in T cell antigen receptor-induced rapid activation of AMPK in response to Ca2+ signaling in T lymphocytes [49]. |
| TAK1 | An upstream kinase of the MAP-kinase signaling pathway involved in cardiac biology and disease. It was found that inhibition of TAK1 in mice by a cardiac-specific dominant-negative mutation evoked electrophysiological and biochemical properties reminiscent of human Wolff-Parkinson-White syndrome, arising from mutations in AMPK [50]. TAK1 was found to mediate TRAIL-induced autophagy in MCF-10A breast epithelial cells by targeting AMPK phosphorylation [45]. |
| ATM | A major player in response to DNA double-strand breakage. It was reported that ATM phosphorylates AMPKα in etoposide-treated HeLa cells [46]. However, a separate study indicated that ROS-induced ATM activation increased AMPK phosphorylation via an LKB1-dependent mechanism [51]. |
Among these kinases, LKB1 and CaMKK represent major kinases responsible for AMPK activation in response to decreased AMP/ATP ratios and elevated intracellular Ca2+, respectively, to regulate energy homeostasis in peripheral tissues [8]. The physiological roles of TAK1 and ATM in regulating AMPK phosphorylation, however, are not as well defined. For example, TAK1, a cytokine-activated kinase, was found to mediate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced AMPK activation and autophagy in MCF-10A breast and human immortalized retinal pigment epithelial cells independently of LKB1 and CaMKKβ [45]. However, TAK1 activation by the cytokines tumor necrosis factor or interleukin-1β failed to induce AMPK activation or autophagy in these cells [45]. Together, these findings suggest that TAK1 is essential, but not sufficient for the effective activation of AMPK. With regard to ATM, although this DNA damage-induced kinase has been reported to directly target AMPKα phosphorylation to increase mitochondrial biogenesis in etoposide-treated HeLa cells [46], it has also been reported that ROS-induced ATM activation led to LKB1-dependent AMPK activation and subsequent mTORC1 inhibition [51]. This discrepancy underscores a cellular context-dependent role of ATM in mediating AMPK activation.
In addition, Akt has also been shown to be a negative regulator of AMPK [52, 53], which might, in part, be mediated through decreases in cellular AMP/ATP ratios [53]. More recently, a unique AMP/Ca2+-independent mechanism linking AMPK activation with HDAC1-mediated deacetylation was reported [54] (Fig. 1B). Specifically, a recent genetic interaction profiling analysis revealed the enzyme-substrate relationship between HDAC1 and AMPKα1 [54]. It was found that AMPKα1 is susceptible to acetylation and deacetylation by the histone acetylase p300 and HDAC1, respectively, at Lys-40, Lys-42, and Lys-80. More importantly, deacetylation increases the phosphorylation and activity of AMPK by enhancing its physical interaction with LKB1. As HDAC1 has been shown to promote tumor cell proliferation and survival [55], this finding implicates AMPK as a downstream effector in HDAC1-mediated oncogenic signaling (see the “The dark side of AMPK” section for discussion).
AMPK DOWNSTREAM TARGETS FOR PHOSPHORYLATION
Once activated, AMPK facilitates energy homeostasis via two distinct mechanisms: direct phosphorylation of cell metabolic enzymes and regulation of gene expression. In response to metabolic stress, AMPK facilitates metabolic reprogramming, in part, by modulating the activities of the aforementioned ACC [2] and HMG-CoA reductase [3], and a host of other metabolizing enzymes associated with glucose and lipid homeostasis via phosphorylation (Table 2). For example, in muscle and/or adipose tissues, AMPK regulates glucose uptake through effects on the RabGAP (Rab GTPase-activating protein) TBC1D1, which has a key role in regulating GLUT4 trafficking [56], and stimulates glycolysis by phosphorylating activation of 6-phosphofructokinase 2 (PFK2) [57]. In adipose tissues, AMPK directly phosphorylates lipases, including hormone-sensitive lipase (HSL) and adipocyte-triglyceride lipase (ATGL), to modulate their activities. In addition, AMPK also phosphorylates a series of signaling effectors and transcription factors, which underlie the unique role of AMPK as a metabolic checkpoint in modulating energy metabolism through a broad range of cellular responses, including insulin signaling, mTORC1 signaling, transcriptional regulation of gene expression, protein acetylation/deacetylation, nuclear transport of cytoplasmic proteins, and angiogenesis (Table 2).
Table 2.
Reported AMPK substrates and their roles in regulating cellular metabolism and functions
| AMPK substrate | Functional consequence | |
|---|---|---|
| Glucose metabolism (glucose uptake↑; glycolysis↑; glycogen biosynthesis↓) | ||
| PFK2 | PFK2 is a bifunctional enzyme central to glycolytic flux. AMPK-mediated PFK-2 activation via phosphorylation at Ser-466 is involved in the stimulation of glycolysis in heart cells during ischemia [59] or in neuronal cells in response to nitric oxide [57]. In cancer cells, PFKBP3, the major form of PFK2, is targeted by AMPK for phosphorylation to increase glycolysis [60]. | |
| TCB1D1 | TCB1D1 functions as a regulator of fuel homeostasis by regulating GLUT4 translocation, and is activated by AMPK through multi-site phosphorylation as part of the insulin- and contraction-stimulated signaling [56]. | |
| Glycogen synthase (GYS) | Phosphorylation of GYS1/2 by AMPK led to enzyme inactivation by attenuating the affinity for its substrates, UDP-Glc and Glc-6-P [61]. | |
| Lipid metabolism (fatty acid oxidation↑; fatty acid and cholesterol biosynthesis↓) | ||
| ACC | AMPK phosphorylates and inactivates ACC [2], the key enzyme governing the rate-limiting step of fatty acid biosynthesis. | |
| HMG-CoA reductase | AMPK phosphorylates and inactivates HMG-CoA reductase [3], leading to the inhibition of cholesterol biosynthesis. | |
| HSL | HSL facilitates triacylglycerol degradation by hydrolyzing diacylglycerols to monoacylglycerols in adipose and skeletal muscles. AMPK phosphorylates HSL at Ser-565, thereby preventing β-adrenergic agonist-induced HSL activation by blocking protein kinase A-mediated Ser-660 phosphorylation [62]. | |
| ATGL (aka, desnutrin /iPLA2ζ) | ATGL is phosphorylated and activated by AMPK to increase lipolysis in brown adipose tissues, which stimulates fatty acid oxidation and UCP-1 induction for thermogenesis [63]. | |
| Phospholipase D1 (PLD1) | AMPK activates PLD1 through phosphorylation at Ser-505, and this AMPK-induced PLD1 activation is required for increased glucose uptake in muscle cells under glucose deprivation conditions [64]. | |
| Nucleoside metabolism | ||
| Nucleoside diphosphate kinase (NDPK) | NDPK maintains pools of nucleoside and deoxynucleoside triphosphates for processes central to energy utilization; for example, DNA synthesis and translation [65]. During nutritional stress, AMPK switches off NDPK through phosphorylation at Ser-120, thereby conserving energy [66]. | |
| Insulin signaling | ||
| Insulin receptor substrate-1 (IRS-1) | IRS-1 represents the most upstream component of the insulin-signaling cascade. AMPK phosphorylates IRS-1 at Ser-789 in cell-free assays, as well as in mouse C2C12 myotubes, leading to increases in insulin-stimulated IRS-1-associated phosphatidylinositol 3-kinase activity [67]. | |
| mTORC1 signaling (cell growth and protein biosynthesis ↓) | ||
| Tuberous sclerosis protein 2 (TSC2) Raptor |
AMPK negatively regulates mTORC1 activity by targeting two key proteins for phosphorylation, the TSC2 tumor suppressor and the mTORC1 scaffold subunits raptor [68]. In light of the pivotal role of mTORC1 in regulating cell growth, cell cycle progression, autophagy, and macromolecule biosynthesis [69], suppression of mTORC1 signaling represents a major mechanism by which AMPK activators inhibit cancer cell proliferation. | |
| Autophagy ↑ | ||
| ULK1 | ULK1 (hATG1), a mammalian ortholog of the yeast protein kinase Atg1, plays a crucial role in autophagy and mitochondrial homeostasis. Evidence suggests that AMPK triggers autophagy through two ULK1-dependent mechanisms [29, 70]: (a) AMPK overrides the suppressive effect of mTORC1 on ULK1, and (b) AMPK directly phosphorylates and activates ULK1. | |
| Activity of transcription factors that regulate cell metabolism | ||
| Forkhead box type O3a (Foxo3a) | Foxo3a is a transcriptional factor with known tumor suppressive activity [71]. It was reported that AMPK phosphorylates Foxo3a, leading to the activation of Foxo3a transcriptional activity without affecting Foxo3a subcellular localization [72]. This AMPK-Foxo3a signaling axis promotes the transcription of target genes involved in cell metabolism, cell cycle arrest, cell death, autophagy, and stress resistance [73]. | |
| cAMP-response element-binding protein (CREB) | Atypical protein kinase C (PKC)ι/λ | AMPK activates aPKCι/λ by increasing its phosphorylation at Thr403 and Thr555, leading to the phosphorylation of the transcriptional coactivator CREB binding protein (CBP) at Ser-436 [74]. This event triggers the dissociation of the CREB-CBP-CRTC2 (CREB-regulated transcription coactivator 2) transcription complex and reduces gluconeogenic enzyme gene expression, including genes encoding peroxisome-proliferator-activated receptor γ coactivator 1α (PGC-1α) and its downstream targets including phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). |
| CREB-regulated transcriptional co-activator 2 (CRTC2) | AMPK attenuates the gluconeogenic program by promoting the phosphorylation of CTRC2, a transcriptional co-activator for CREB, which blocks its nuclear accumulation through cytoplasmic sequestration by binding to 143-3 scaffold proteins [75]. | |
| Glycogen synthase kinase (GSK)3β | AMPK phosphorylates GSK3β at Ser-9, leading to reductions in Ser-129 CREB phosphorylation and its transcriptional activation of PEPCK expression [76]. | |
| PGC-1α | PGC-1α is a master regulator of mitochondrial biogenesis, and has been shown to mediate certain AMPK effects on cell metabolism, particularly in fatty acid oxidation [77]. AMPK binds to and activates PGC-1α in muscle by direct phosphorylation on two critical residues, threonine-177 and serine-538 [78] | |
| Sterol regulatory element binding protein-1c (SREBP-1c) | AMPK phosphorylates SREBP-1c at Ser-372, and thereby suppresses its cleavage and nuclear translocation, and represses the expression of SREBP-1c target genes, including those encoding ACC1 and fatty acid synthase, leading to reduced lipogenesis and lipid accumulation [79]. | |
| Hepatic nuclear factor 4α (HNF4α) | HNF4α is an orphan nuclear receptor that regulates the expression of genes involved in energy metabolism in the liver, intestine, and endocrine pancreas. AMPK phosphorylates HNF4α at Ser-304, thereby repressing its transcriptional activity by reducing the ability of the transcription factor to form homodimers and bind DNA and increasing its degradation rate [80]. | |
| AICAR responsive element binding protein (AREBP) | AREBP is a zinc finger transcription factor. AMPK-mediated phosphorylation of AREBP at Ser-470 results in loss of DNA-binding activity, leading to transcriptional repression of PEPCK [81]. | |
| Testicular nuclear receptor 4 (TR4) | TR4 is a nuclear receptor that might play a role in lipid metabolism. AMPK phosphorylates TR4 at Ser-351 in hepatocytes, leading to the suppression of the target gene stearoyl-CoA desaturase (SCD)1 [82] | |
| Carbohydrate-response-element-binding protein (ChREBP) | The glucose-responsive transcription factor ChREBP binds to the carbohydrate-responsive element of the L-type pyruvate kinase and fatty acid synthase genes, thereby redirecting glucose metabolism toward lipogenesis in hepatocytes [83]. AMPK phosphorylates ChREBP at Ser-568, resulting in the inactivation of its transcriptional activity by reducing DNA binding [84]. | |
| p53 | AMPK activation has been reported to induce phosphorylation of the cell cycle regulator p53 at Ser-15 in primary mouse embryonic fibroblasts (MEFs), which is required for glucose deprivation-induced cell cycle arrest [85]. In human osteosarcoma-derived cells, this glucose starvation-induced AMPK activation, however, facilitates p53 phosphorylation at Ser-46, but not Ser-15 [86]. Alternatively, a recent paper indicates that AMPK enhances the acetylation and stability of p53 by phosphorylating inactivation of the NAD+-dependent class III HDAC Sirt1 in HCC cells [22]. | |
| Histone-modifying enzymes | ||
| Class II HDACs (HDAC4, 5, and 7) | Class II HDACs are targeted by AMPK family kinases for phosphorylation, which lead to cytoplasmic sequestration by 14-3-3 binding [87] (Fig. 1B). In response to glucagon, these HDACs are rapidly dephosphorylated and translocated to the nucleus where they associate with the promoters of genes encoding gluconeogenic enzymes, which stimulates the transcriptional induction of these genes. | |
| Histone acetyl-transferase (HAT) p300 | Phosphorylation of p300 at Ser-89 by AMPK and its related kinases inhibits the HAT activity of p300, which, in turn, decreases the acetylation and activity of ChREBP in mediating lipogenesis [88]. | |
| Sirt1 | Two contradictory mechanisms have been reported for the modulatory effect of AMPK on Sirt1, which might underlie differences between nonmalignant versus cancer cells. In C2C12 myotubes, pharmacological activation of AMPK enhances Sirt1 deacetylase activity indirectly by increasing cellular NAD+ levels [89] (Fig. 1B), while in liver cancer cells, activated AMPK phosphorylated Sirt1 at Thr-344, leading to its inactivation [22]. | |
| Nuclear import of the mRNA-binding protein HuR | ||
| Importin α1 | Importin α1 functions as an adaptor that, in association with importin β, transports cargo proteins, such as HuR, through the nuclear pore complex into the nucleus. AMPK phosphorylates importin α1 at Ser-105, which promotes the shuttling of HuR from the cytoplasm to nucleus [90]. As HuR controls the stability and translation of mRNA, this AMPK-facilitated nuclear sequestration of HuR leads to decreased stability/translation of target mRNAs encoding critical cell cycle regulators such as cyclin A, cyclin B1, and p21 in cancer cells [91]. | |
| Angiogenesis | ||
| Endothelial nitric oxide synthase (eNOS) | AMPK is one of the three kinases, besides Akt and protein kinase A, that phosphorylate eNOS at multiple sites, leading to eNOS activation, in endothelial cells in response to various stimuli [92]. | |
Although the AMPK phosphorylation sites among these substrates do not contain a clearly defined consensus sequence, a recent study utilizing the positional scanning peptide library (PSPL) technique has identified three optimal AMPK motifs: LRRVxSxxNL, MKKSxSxxDV, and IxHRxSxxEI (x is any amino acid and S in bold denotes the phosphoreceptor site), which are found in the phosphorylation sites of many AMPK substrates [58]. This PSPL strategy was validated by the identification of raptor, the WD40 repeat-containing scaffold subunit of the mTORC1 complex, as an AMPK substrate [58].
REGULATION OF GENE EXPRESSION
AMPK modulates the expression of a number of genes crucially involved in glucose and fatty acid metabolism through two pathways. First, AMPK facilitates metabolic reprogramming in muscle and adipose tissues, in part, by directly phosphorylating transcription factors or coactivators (Table 2). For example, although AMPK does not directly target CREB for phosphorylation per se, it reduces the transcriptional activity of CREB by phosphorylating its regulatory components, including PKCι/λ, CRTC2, and GSK3β, thereby shutting down glucose production by downregulating the expression of the target genes encoding PEPCK and G6Pase. Moreover, AMPK phosphorylates SREBP1, suppressing its transcriptional induction of lipogenic gene expression in adipose tissues. Other transcription regulators targeted by AMPK include Foxo3a, and the nuclear receptors HNF4α and TR4, the co-activator PGC-1α, the zinc-finger protein AREBP, and the glucose-responsive transcription factor ChREBP, all of which contribute to the effect of AMPK on energy homeostasis.
Second, recent evidence indicates that, HDACs, including the class II HDACs [87] and NAD+-dependent class III HDAC Sirt1 [89], act as a separate set of transcriptional regulators targeted by AMPK. As aforementioned, HDAC1 serves as an upstream regulator of AMPK through the deacetylation of AMPKα1 [54]. Consequently, there exist intricate interactions between AMPK and different HDACs isoforms in regulating cell metabolic functions. Considering the increasing use of HDAC inhibitors in cancer treatment, how these drugs affect energy metabolism in vivo warrants investigation.
FUNCTIONAL INTERPLAY BETWEEN AMPK AND HDACs IN REGULATING GENE EXPRESSION
Through a bioinformatics and proteomics screen for substrates of AMPK family kinases, the class II HDACs (HDAC4, 5, and 7) were identified as direct targets of the AMPK pathway in the liver [87]. In the nucleus of hepatocytes, these class II HDACs activate Foxo family transcription factors (Foxo1 and Foxo3a) by facilitating HDAC3-mediated deacetylation [87], thereby increasing the expression of gluconeogenesis genes, including those encoding PEPCK and G6Pase. Accordingly, phosphorylation of these HDACs by AMPK and its family members results in the cytoplasmic sequestration of these HDACs due to 14-3-3 binding, in a manner similar to that of CRCT2. Consequently, this nuclear exclusion results in the down-regulation of Foxo-dependent target gene expression (Fig. 1B). However, this acetylation-dependent signaling event appears to be liver cell-specific since in other cell types, AMPK is reported to directly phosphorylate and activate Foxo3a, but not Foxo1, to stimulate the expression FOXO-dependent target genes in stress resistance [72]. The dual regulation of Foxo transcription factors via phosphorylation versus acetylation underlies the complicated function of AMPK in metabolic control in different tissues.
Another HDAC reported to be targeted by AMPK in the regulation of metabolic reprogramming is Sirt1, a metabolic regulator that modulates the activity of a host of transcription programs through deacetylation [93]. It was demonstrated in C2C12 skeletal muscle myocytes that AMPK regulates the expression of genes involved in energy metabolism by acting in coordination with Sirt1 [89]. AMPK enhances Sirt1 activity by increasing cellular NAD+ levels, resulting in deacetylation and activation of Sirt1 target proteins, including the transcriptional coactivator PGC-1α and the forkhead transcription factors Foxo1 and Foxo3a (Fig. 1B). AMPK-induced p53 activation promotes cellular survival in response to glucose deprivation, and cells that have undergone a p53-dependent metabolic arrest can rapidly reenter the cell cycle upon glucose restoration [85]. From a mechanistic perspective, the AMPK-Sirt1 metabolic network provides a dual mode of activation of these transcription factors, i.e., phosphorylation and deacetylation, to induce mitochondrial biogenesis and fatty acid oxidation in response to metabolic stresses.
ANTITUMOR EFFECTS OF AMPK
AMPK is well recognized as a target for anticancer drug discovery, of which the proof-of-concept is demonstrated by the ability of pharmacological AMPK activators, such as metformin, the AMP analogue 5-aminoimidazole-4-carboxamide ribose (AICAR), and A-769662 (structures, Fig. 2), to suppress tumorigenesis in various animal models of chemoprevention [27, 94, 95] (please see the “Pharmacological activators of AMPK” section). From a mechanistic perspective, AMPK activation inhibits tumor growth by targeting multiple signaling pathways relevant to tumorigenesis, including cell metabolism, cell cycle progression, cell proliferation, and survival. However, it warrants attention that the mechanisms by which AMPK regulate some of its downstream effectors, such as p53 and Sirt1, might differ between malignant and nonmalignant cells (Table 2). Mechanisms that underlie the tumor-suppressive effects of AMPK activators are summarized as follows.
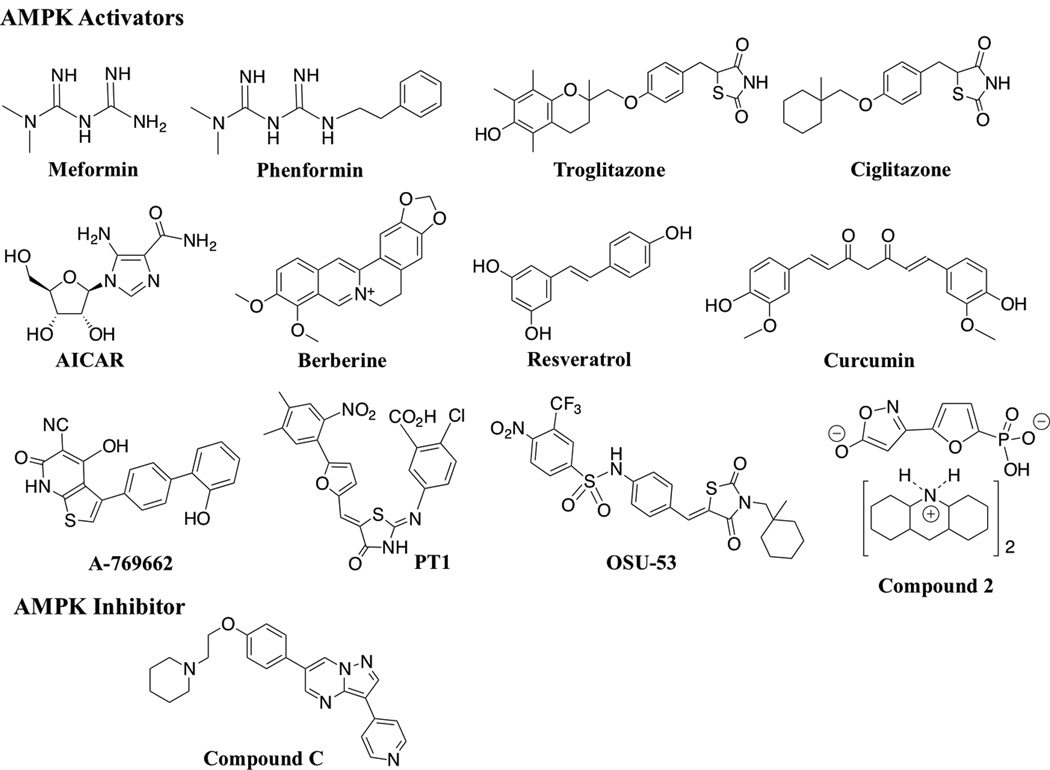
Fig. (2).
Structures of AMPK modulators.
1. Inhibition of lipogenesis
Substantial evidence indicates that AMPK is involved in regulating the adaptive metabolic reprogramming in the course of tumorigenesis [11, 29, 96, 97]. When this metabolic checkpoint is suppressed, as in the setting of LKB1 deficiency [17] or loss of AMPKα2 expression [21], this metabolic reprogramming will proceed unchecked. In the context of tumor development, the ability of AMPK to inhibit fatty acid and cholesterol biosynthesis is noteworthy. AMPK inhibits lipogenesis by targeting the activity/expression of key lipogenic enzymes, including ACC, HMG-CoA reductase, and fatty acid synthase, through two distinct pathways (Table 2). First, AMPK phosphorylates and inactivates ACC and HMG-CoA reductase. Second, AMPK mediates long-term metabolic adaptive effects through the phosphorylation of two transcription factors, SREBP-1c and ChREBP, leading to the inactivation of their transcriptional control of the gene expression of ACC and fatty acid synthase.
2. Downregulation of mTORC1-hypoxia-inducible factor (HIF)-1α signaling
The mTORC1 signaling pathway plays a vital role in the growth and survival of cancer cells by integrating signals from nutrients and growth factors to anabolic processes and cell cycle progression [69, 98]. AMPK negatively regulates mTORC1 through two distinct mechanisms (Table 2). First, AMPK phosphorylates the tumor suppressor TSC2, which blocks mTORC1 activation by suppressing its upstream regulator, the GTPase Rheb [99]. Second, AMPK phosphorylates the mTORC1-positive regulatory subunit raptor, resulting in 14-3-3 binding and the allosteric inactivation of mTORC1 [58].
mTORC1 is activated by mitogenic stimuli acting through the PI3K/Akt and ERK signaling pathways [69, 98], resulting in the phosphorylating activation of proteins involved in the regulation of protein synthesis, including the p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) [100]. Equally important, activation of mTORC1 leads to the translational upregulation of a series of mRNAs encoding pro-growth proteins, among which the HIF-1α transcription factor is most noteworthy [101]. HIF-1α overexpression is a frequent feature of many tumors, which acts as a key regulator of the molecular hypoxic response by shifting metabolism toward anaerobic glycolysis [102]. In addition, HIF-1α promotes angiogenesis by inducing the gene expression of vascular endothelial growth factor (VEGF) and other pro-angiogenic and vascular growth factors [102]. Thus, as a negative regulator of mTORC1 activity, AMPK can also play a role in the modulation of HIF-1α expression, Indeed, the LKB1-deficient mouse model of Peutz-Jeghers syndrome is characterized by dysregulated mTORC1 signaling and an associated upregulation of HIF-1α, indicating that AMPK represents a key effector of LKB1 in the suppression of HIF-1α [101].
3. Inactivation of Akt signaling
Evidence indicates that AMPK cross-communicates with Akt in a mutually antagonistic manner. Akt, when activated, negatively regulates AMPK, in part, by decreasing the cellular AMP/ATP ratio [52, 53]. Conversely, activation of AMPK by adiponectin or the AMPK allosteric activator OSU-53 facilitates Akt dephosphorylation by stimulating protein phosphatase 2A activity [103, 104]. As Akt constitutes an important pathway that governs many aspects of cellular function, including cell cycle progression, cell proliferation, survival, and cell metabolism [105], the suppressive effect of AMPK on Akt signaling contributes to the ability of AMPK activators to suppress tumor growth.
4. Upregulation of the tumor suppressor Foxo3a
AMPK activation has been shown to activate Foxo3a in response to metabolic stress although the exact underlying mechanism remains undefined [73]. While evidence indicates that AMPK plays an important role in regulating the nuclear localization of Foxo3a [73], a recent study shows that AMPK phosphorylates Foxo3a in vivo at six different sites, leading to the activation of Foxo3a transcriptional activity without affecting its subcellular localization [72]. Foxo3a functions as a tumor suppressor in a variety of cancers by targeting genes involved not only in cell metabolism, but also in apoptosis regulation, including Fas ligand, TRAIL, and Bcl-2 family members (Bim, bNIP3, and Bcl-xL) [71]. Thus, the ability of AMPK activators to induce apoptosis in cancer cells can, in part, be attributed to their ability to restore the activity of Foxo3a, the inactivation of which can represent a crucial step to tumorigenesis.
5. Upregulation of the tumor suppressor p53
Although AMPK has been reported to activate p53 by phosphorylation at Ser-15 in MEFs [85] or Ser-46 in osteosarcoma cells [86], a recent report links AMPK-induced p53 activation with the phosphorylating inactivation of Sirt1 in HCC cells [22]. This AMPK-induced p53 activation plays an integral role in the effect of glucose deprivation or pharmacological activation of AMPK on cell cycle and apoptosis in cancer cells by transcriptional activation of p53 target genes, including those encoding p21 and Bax [22, 86].
THE DARK SIDE OF AMPK: AMPK’S PRO-TUMORIGENIC EFFECTS?
Despite recent advances in understanding the role of AMPK as a metabolic tumor suppressor, emerging evidence indicates that under certain cellular contexts or certain stages of tumorigenesis, AMPK activation might act as a protective response to metabolic stresses, such as hypoxia, acidosis, and nutrient deprivation, to provide survival advantage to tumor cells. For instance, AMPK might have a dual function in regulating the proliferation of prostate cancer cells. While inactivation of AMPK through siRNA-mediated knockdown promotes growth in the C4-2 cell line, an androgen-independent clone of the LNCaP line [106], it inhibited the proliferation of the androgen-sensitive LNCaP and androgen-independent CWR22Rv1 cells [31]. This discrepancy in cellular responses to AMPK inhibition might be attributable to differences in certain genetic traits among these cell lines. In addition, evidence supports a role of AMPK activation as a strategy that tumor cells use to adapt to sub-optimal growth conditions to promote cell survival as detailed below.
1. Constitutive tolerance to nutrient deprivation or low-oxygen environment
Pancreatic cancer cells were reported to be resistant to glucose starvation, in contrast to HCC cells, most of which died within 48 h of exposure to glucose-deficient conditions [30]. This tolerance to glucose deprivation in pancreatic cancer cells was attributable to high expression levels of the AMPK α1 and α2 catalytic subunit as siRNA-mediated knockdown of AMPK diminished their ability to withstand glucose starvation [30]. Moreover, an in vivo study using a mouse fibrosarcoma xenograft tumor model indicated that AMPK is activated in authentic hypoxic tumor microenvironments and that this activity occurs in regions of hypoxia [107]. Based on such findings, the activation of AMPK in nutrient-deprived or hypoxic tumor microenvironments might represent a mechanism by which tumor cells can enter a protective state of quiescence.
2. Molecular adaptation to energy stress by shift in NADPH homeostasis
In response to energy stress, such as glucose deprivation or exposure to 2-deoxyglucose, AMPK inactivates ACC to direct fatty acid metabolism from biosynthesis to oxidation. A recent report demonstrates that through ACC inhibition, AMPK decreases NADPH consumption in fatty acid biosynthesis and increases NADPH generation by means of fatty acid oxidation, and that the knockdown of ACC can compensate for AMPK activation and facilitates anchorage-independent growth and solid tumor formation in vivo [108]. Moreover, as glucose deprivation can lead to ROS production during tumorigenesis, it was demonstrated that stimulation of fatty acid oxidation through AMPK represents a plausible mechanism for the survival of cells in the absence of glucose [109].
3. Adaptation to low pH
It was reported that under long-term low pH exposure, AMPK is activated in tumor cells, resulting in upregulation of PFKFB3, as well as an increase in glucose consumption, and that this AMPK-mediated change in glycolytic flux is sufficient to induce an oxygen-sparing phenotype [33].
4. Stimulation of aerobic glycolysis and anabolic synthesis downstream of androgen receptor (AR)
A recent genomic analysis identified CAMKK2 as a metabolic master regulator downstream of the AR in both androgen-dependent LNCaP and castration-resistant VCaP prostate cancer cells [110]. Specifically, in response to androgen stimulation, CAMKK2 transcript and protein levels were upregulated, leading to increased AMPK phosphorylation, but without altering phospho-mTOR levels. Equally important, this AR-CAMKK2-AMPK signaling pathway led to increases in glucose uptake, glycolysis, anabolic biosynthesis, and cell proliferation in these cells.
5. Regulation of HIF-1α target gene expression
Although AMPK acts as a downstream effector of LKB1 in suppressing HIF-1α expression through downregulation of mTORC1 signaling [101], it has also been reported that AMPK activation plays a critical role in HIF-1α transcriptional activity and its target gene expression, including those encoding GLUT-1 and VEGF, under hypoxic conditions in DU-145 prostate cancer cells [111]. Moreover, activation of AMPK in response to glucose deprivation or pharmacological activators enhances the mRNA stability of VEGF in glucose-deprived DU-145 cells [112] and C2C12 myotubes through a p38-dependent mechanism [113]. From a mechanistic perspective, AMPK and HIF-1α might act in a concerted manner during the general adaptive response of tumor cells to hypoxia or glucose starvation.
6. A downstream effector of c-Src/PKC signaling
c-SRC encodes a nonreceptor tyrosine kinase that, when activated, is involved in cellular proliferation, survival, migration, and angiogenesis [114]. It is proposed that the transforming ability of SRC is linked to its ability to activate key signaling molecules in these pathways, rather than through direct activity. A recent report indicates that in certain cancer cell lines, including L-HF1, BP-HF-1, OVCAR3, and A431, AMPK is activated by c-Src through a PKCα/LKB1 signaling pathway independently of the cellular AMP/ATP ratio [32]. This cSrc-mediated regulation of AMPK, however, is cell type-specific as it was not found in HeLa and MCF-7 cells.
7. Involvement in MYC-induced metabolic homeostasis in tumor cells
A recent report demonstrated that, in human cell lines with deregulated MYC expression, the oncogenic levels of MYC establish a dependence on AMPK-related kinase 5, an upstream kinase of AMPK, for maintaining metabolic homeostasis and for cell survival through elevated energy consumption and addiction to mitochondrial glutaminolysis [115].
AMPK AND AUTOPHAGY
Autophagy is a cellular process to degrade long-lived or malfunctioning proteins and obsolete or damaged organelles [116, 117], thereby serving to maintain cellular homeostasis and to help cells survive stressful conditions. In response to ATP depletion or glucose starvation, activated AMPK stimulates autophagy, not only through inactivation of mTORC1, but also directly through phosphorylation of ULK1 (Table 2). The ability of AMPK to induce autophagy is in line with the general concept that tumor suppressors positively regulate autophagy, whereas oncogenic products usually inhibit autophagy (reviews: [116–120]). From a physiological perspective, autophagy plays an essential role in maintaining cellular homeostasis and protecting cells from stressful conditions. Nevertheless, in the context of tumorigenesis and therapeutic response, autophagy is a double-edge sword, i.e., although autophagy can contribute to cell death in tumor cells, it can also allow prolonged survival, generating dormant tumor cells that have the capacity to resume growth when conditions are more favorable. For example, it was reported that metformin inhibits melanoma development through autophagy and apoptosis mechanisms [121]. In contrast, our recent study indicates that co-treatment of a novel direct activator of AMPK, OSU-53 (discussed below), with the autophagy inhibitor chloroquine increased its in vivo efficacy in suppressing MDA-MB-231 xenograft tumor growth [104], indicative of autophagy playing a protective role. Thus, whether AMPK activator-induced autophagy contributes to cell death or represents a protective mechanism warrants further investigation.
AMPK MODULATORS AS CANCER THERAPEUTIC AGENTS
1. AMPK activators
Based on the above discussions, activation of AMPK might represent a therapeutically relevant strategy for cancer treatment in light of its tumor suppressive effects on cell metabolism and oncogenic signaling. This premise is supported by recent preclinical findings that the AMPK activators metformin, the AMP analogue AICAR, and A-769662 exhibited in vivo efficacy in blocking carcinogen-induced tumorigenesis and/or suppressing tumor growth in different animal models [27, 94, 95]. Consequently, the past few years have witnessed an increasing interest in developing novel small-molecule AMPK activators. As many pharmacological activators of AMPK, including the biguanides metformin [13] and phenformin [38], the thiazolidinedione peroxisome proliferator-activated receptor (PPAR)γ agonist troglitazone and ciglitazone [122], AICAR [123], the natural products berberine [124], resveratrol [125], and curcumin [126], and the direct activators A-769662 [127] and PT-1 [128] (structures, Fig. 2), have been discussed in details in several recent reviews [10, 129–131], we focus here on two of the most recently reported activators, OSU-53 [132] and compound 2 [5-(5-hydroxy-isoxazol-3-yl)-furan-2-phosphnic acid] [133] (structures, Fig. 2).
OSU-53
In light of the unique ability of thiazolidinediones to mediate PPARγ-independent AMPK activation, we conducted a screening of an in-house, thiazolidinedione-based focused compound library to identify novel AMPK activators, which netted OSU-53 [132]. OSU-53, though devoid of PPARγ activity, exhibits high potency in stimulating recombinant AMPK kinase activity (EC50, 0.3 µM) and in suppressing the viability and clonogenic growth of MDA-MB-231 and MDA-MB-468 cells with equal potency (IC50: MTT, 5 µM; colony formation, 2 µM), despite the lack of LKB1 expression in MDA-MB-231 cells [104, 132]. Computer modeling analysis suggests that OSU-53 activates AMPK by binding to the auto-inhibitory domain of the α-subunit. Beyond the AMPK-mediated suppressive effect on mTOR signaling, OSU-53 also targeted multiple AMPK downstream pathways. Among these, the protein phosphatase 2A-dependent dephosphorylation of Akt is noteworthy because it circumvents the feedback activation of Akt that results from mTOR inhibition. OSU-53 also modulated energy homeostasis by suppressing fatty acid biosynthesis and shifting the metabolism to oxidation by upregulating the expression of key regulators of mitochondrial biogenesis, such as PGC1α and the transcription factor nuclear respiratory factor 1. Moreover, OSU-53 inhibited hypoxia-induced epithelial-mesenchymal transition in association with the silencing of HIF-1α and the E-cadherin repressor Snail. Equally important, OSU-53 is orally bioavailable as daily oral administration of OSU-53 was effective in suppressing MDA-MB-231 xenograft tumor growth.
Compound 2
Compound 2 is an AMP mimetic with high potency in stimulating recombinant AMPK kinase activity (EC50, 6 nM versus 6 µM for AMP) [133]. Because the highly anionic nature of compound 2 represents an impediment to membrane permeability, the authors developed esterase-sensitive prodrugs of compound 2 to enhance intracellular delivery, which exhibit sub-µM potencies in inhibiting de novo lipogenesis in rat hepatocytes.
2. AMPK inhibitors
Relative to AMPK activators, progress in the development of AMPK inhibitors is very limited. To date, there is only one AMPK inhibitor available, compound C (structure, Fig. 2), for testing the effects of pharmacological inhibition of AMPK in various cell-based assays. Compound C is a cell-permeable, ATP-competing inhibitor of AMPK [13]. However, compound C exhibits “off-target” mechanisms, including the inhibition of a number of other kinases with high potencies [134] and inhibition of the adenosine transporter [135]. The suppressive effect of compound C on the adenosine receptor is noteworthy because this receptor is responsible for the uptake of AICAR into cells, suggesting that the effect of this inhibitor on the pharmacological activity of AICAR might be, in part, due to its ability to block the cell entry of AICAR [135].
SYNPOSIS
As the role of AMPK as a metabolic tumor suppressor is generally recognized, there is a growing interest in the therapeutic exploitation of the AMPK pathway for cancer therapy. However, emerging evidence indicates that tumor cells might also use AMPK activation as a survival strategy to undergo metabolic adaptation in the face of energy or hypoxic stresses. These findings underscore the complexity in the cellular function of AMPK in maintaining energy homeostasis under physiological versus pathological conditions. The metabolic tumor suppressive function of AMPK might be overridden by stress or oncogenic signals in tumor cells, such as low pH and signaling through AR, Src, and MYC. Thus, investigations aimed at defining under which conditions, such as stage(s) of tumorigenesis and cancer progression or presence of certain genetic aberrations, AMPK inhibition might represent a more relevant strategy then AMPK activation for cancer treatment are clearly warranted. Such information will guide the development of more effective AMPK modulators for cancer therapy.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants R01CA112250 and R21CA158807 from the National Cancer Institute
LIST OF ABBREVIATIONS
- ACC
acetyl-CoA carboxylase
- AICAR
5-aminoimidazole-4-carboxamide ribose
- AMPK
adenosine monophosphate-activated protein kinase
- AR
androgen receptor
- AREBP
AICAR responsive element binding protein
- ATGL
adipocyte-triglyceride lipase
- ATM
ataxia telangiectasia mutated
- CAMKK
calmodulin kinase kinase
- CBP
CREB binding protein
- ChREBP
carbohydrate-response-element-binding protein
- CREB
cAMP-response element-binding protein
- CRTC2
CREB-regulated transcription coactivator 2
- eNOS
endothelial nitric oxide synthase
- Foxo3a
forkhead box type O3a
- G6Pase
glucose-6-phosphatase
- GSK3β
Glycogen synthase kinase 3β
- GYS
glycogen synthase
- HAT
histone acetyl-transferase
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HIF-1α
hypoxia-inducible factor
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl-CoA reductase
- HNF4α)
hepatic nuclear factor 4α
- HSL
hormone-sensitive lipase
- IRS-1
insulin receptor substrate-1
- LKB1
liver kinase B1
- MEFs
mouse embryonic fibroblasts
- mTOR
mammalian target of rapamycin
- NDPK
nucleoside diphosphate kinase
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome-proliferator-activated receptor γ coactivator 1α
- PFK2
6-phosphofructokinase 2
- PKC
protein kinase C
- PLD1
phospholipase D1
- PPARγ
peroxisome proliferator-activated receptor γ
- PSPL
positional scanning peptide library
- RabGAP
Rab GTPase-activating protein
- SCD
stearoyl-CoA desaturase
- SREBP-1C
Sterol regulatory element binding protein-1c
- TAK1
TGF-β-activated kinase 1
- TR4
testicular nuclear receptor 4
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- TSC2
tuberous sclerosis protein 2
- VEGF
vascular endothelial growth factor
Footnotes
CONFLICTS OF INTEREST
The authors have declared that no competing interests exist.
REFERENCES
- 1.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248:378–380. [PubMed] [Google Scholar]
- 3.Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem Biophys Res Commun. 1973;54:1362–1369. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Carling D, Sim AT. The AMP-activated protein kinase: a multisubstrate regulator of lipid metabolism. Trends Biochem Sci. 1989;14:20–23. [Google Scholar]
- 5.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 8.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 10.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol. 2011;76:155–164. doi: 10.1101/sqb.2011.76.010819. [DOI] [PubMed] [Google Scholar]
- 12.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 13.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 15.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 19.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 20.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T, Miller DS, Sharpless NE, Bardeesy N, Kwiatkowski DJ, Schorge JO, Wong KK, Castrillon DH. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, Liang H, Liu X, Lee JS, Cho JY, Cheong JH, Kim H, Li M, Downey TJ, Dyer MD, Sun Y, Sun J, Beasley EM, Chung HC, Noh SH, Weinstein JN, Liu CG, Powis G. AMPKalpha modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72:2512–2521. doi: 10.1158/0008-5472.CAN-11-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, Hardie DG, Ng IO, Ching YP. AMPK Promotes p53 Acetylation via Phosphorylation and Inactivation of SIRT1 in Liver Cancer Cells. Cancer Res. 2012;72:4394–4404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- 24.Ollila S, Makela TP. The tumor suppressor kinase LKB1: lessons from mouse models. J Mol Cell Biol. 2011;3:330–340. doi: 10.1093/jmcb/mjr016. [DOI] [PubMed] [Google Scholar]
- 25.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- 31.Park HU, Suy S, Danner M, Dailey V, Zhang Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, Kumar D, Brown ML, Fornace A, Dritschilo A, Collins SP. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–741. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizrachy-Schwartz S, Cohen N, Klein S, Kravchenko-Balasha N, Levitzki A. Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation. J Biol Chem. 2011;286:15268–15277. doi: 10.1074/jbc.M110.211813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza EE, Pocceschi MG, Kong X, Leeper DB, Caro J, Limesand KH, Burd R. Control of Glycolytic Flux by AMP-Activated Protein Kinase in Tumor Cells Adapted to Low pH. Transl Oncol. 2012;5:208–216. doi: 10.1593/tlo.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–443. [PMC free article] [PubMed] [Google Scholar]
- 36.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 43.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 45.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS One. 2008;3:e2009. doi: 10.1371/journal.pone.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 48.Abbott MJ, Edelman AM, Turcotte LP. CaMKK is an upstream signal of AMP-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1724–R1732. doi: 10.1152/ajpregu.00179.2009. [DOI] [PubMed] [Google Scholar]
- 49.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 53.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 54.Lin YY, Kiihl S, Suhail Y, Liu SY, Chou YH, Kuang Z, Lu JY, Khor CN, Lin CL, Bader JS, Irizarry R, Boeke JD. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Senese S, Zaragoza K, Minardi S, Muradore I, Ronzoni S, Passafaro A, Bernard L, Draetta GF, Alcalay M, Seiser C, Chiocca S. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol. 2007;27:4784–4795. doi: 10.1128/MCB.00494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 58.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 60.Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res. 2005;11:5784–5792. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 61.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012;443:193–203. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 62.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 63.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JH, Park JM, Yea K, Kim HW, Suh PG, Ryu SH. Phospholipase D1 mediates AMP-activated protein kinase signaling for glucose uptake. PLoS One. 2010;5:e9600. doi: 10.1371/journal.pone.0009600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel M, Veron M, Theisinger B, Lacombe ML, Seib T, Dooley S, Welter C. A novel serine/threonine-specific protein phosphotransferase activity of Nm23/nucleoside-diphosphate kinase. Eur J Biochem. 1995;234:200–207. doi: 10.1111/j.1432-1033.1995.200_c.x. [DOI] [PubMed] [Google Scholar]
- 66.Onyenwoke RU, Forsberg LJ, Liu L, Williams T, Alzate O, Brenman JE. AMPK directly inhibits NDPK through a phosphoserine switch to maintain cellular homeostasis. Mol Biol Cell. 2012;23:381–389. doi: 10.1091/mbc.E11-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5'-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 68.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 73.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9:1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 74.He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 76.Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y, Kurihara H, Asano T. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem. 2008;283:33902–33910. doi: 10.1074/jbc.M802537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 81.Inoue E, Yamauchi J. AMP-activated protein kinase regulates PEPCK gene expression by direct phosphorylation of a novel zinc finger transcription factor. Biochem Biophys Res Commun. 2006;351:793–799. doi: 10.1016/j.bbrc.2006.10.124. [DOI] [PubMed] [Google Scholar]
- 82.Kim E, Liu NC, Yu IC, Lin HY, Lee YF, Sparks JD, Chen LM, Chang C. Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-CoA desaturase 1 gene expression with altered insulin sensitivity. Diabetes. 2011;60:1493–1503. doi: 10.2337/db10-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res. 2006;47:2482–2491. doi: 10.1194/jlr.M600289-JLR200. [DOI] [PubMed] [Google Scholar]
- 84.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 85.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 86.Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283:3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 87.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Yang X, Kawai T, Lopez de Silanes I, Mazan-Mamczarz K, Chen P, Chook YM, Quensel C, Kohler M, Gorospe M. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR. J Biol Chem. 2004;279:48376–48388. doi: 10.1074/jbc.M409014200. [DOI] [PubMed] [Google Scholar]
- 91.Wang W, Fan J, Yang X, Furer-Galban S, Lopez de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bir SC, Xiong Y, Kevil CG, Luo J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res. 2012;95:7–18. doi: 10.1093/cvr/cvs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, Radu CG, Cloughesy TF, Mischel PS. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A. 2009;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 96.Zadra G, Priolo C, Patnaik A, Loda M. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin Cancer Res. 2010;16:3322–3328. doi: 10.1158/1078-0432.CCR-09-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer Prev Res (Phila) 2009;2:301–309. doi: 10.1158/1940-6207.CAPR-08-0166. [DOI] [PubMed] [Google Scholar]
- 98.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, Vera DR, Shaw RJ. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 103.Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, Cho DH, Lim JS, Kim KI, Yang Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 104.Lee KH, Hsu EC, Guh JH, Yang HC, Wang D, Kulp SK, Shapiro CL, Chen CS. Targeting energy metabolic and oncogenic signaling pathways in triple-negative breast cancer by a novel adenosine monophosphate-activated protein kinase (AMPK) activator. J Biol Chem. 2011;286:39247–39258. doi: 10.1074/jbc.M111.264598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 106.Zhou J, Huang W, Tao R, Ibaragi S, Lan F, Ido Y, Wu X, Alekseyev YO, Lenburg ME, Hu GF, Luo Z. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009;28:1993–2002. doi: 10.1038/onc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 110.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N, Bon H, Zecchini V, Smith DM, Denicola GM, Mathews N, Osborne M, Hadfield J, Macarthur S, Adryan B, Lyons SK, Brindle KM, Griffiths J, Gleave ME, Rennie PS, Neal DE, Mills IG. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 112.Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem. 2005;280:9963–9972. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- 113.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 114.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR, Muthalagu N, Rycak L, Rudalska R, Moll R, Kempa S, Zender L, Eilers M, Murphy DJ. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 116.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.White EJ, Martin V, Liu JL, Klein SR, Piya S, Gomez-Manzano C, Fueyo J, Jiang H. Autophagy regulation in cancer development and therapy. Am J Cancer Res. 2011;1:362–372. [PMC free article] [PubMed] [Google Scholar]
- 118.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. Int J Cancer. 2009;125:991–995. doi: 10.1002/ijc.24500. [DOI] [PubMed] [Google Scholar]
- 120.Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol. 2010;22:241–245. doi: 10.1016/j.ceb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, Tartare-Deckert S, Bahadoran P, Auberger P, Ballotti R, Rocchi S. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 123.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 124.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 125.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 126.Fujiwara H, Hosokawa M, Zhou X, Fujimoto S, Fukuda K, Toyoda K, Nishi Y, Fujita Y, Yamada K, Yamada Y, Seino Y, Inagaki N. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res Clin Pract. 2008;80:185–191. doi: 10.1016/j.diabres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 127.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, Li J. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou G, Sebhat IK, Zhang BB. AMPK activators--potential therapeutics for metabolic and other diseases. Acta Physiol (Oxf) 2009;196:175–190. doi: 10.1111/j.1748-1716.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 130.Gruzman A, Babai G, Sasson S. Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a New Target for Antidiabetic Drugs: A Review on Metabolic, Pharmacological and Chemical Considerations. Rev Diabet Stud. 2009;6:13–36. doi: 10.1900/RDS.2009.6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Guh JH, Chang WL, Yang J, Lee SL, Wei S, Wang D, Kulp SK, Chen CS. Development of novel adenosine monophosphate-activated protein kinase activators. J Med Chem. 2010;53:2552–2561. doi: 10.1021/jm901773d. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Gómez-Galeno JE, Dang Q, Nguyen TH, Boyer SH, Grote MP, Sun Z, Chen MH, Craigo WA, van Poelje PD, MacKenna DA, Cable EE, Rolzin PA, Finn PD, Chi B, Linemeyer DL, Hecker SJ, Erion MD. A Potent and Selective AMPK Activator That Inhibits de Novo Lipogenesis. ACS Med Chem Lett. 2010;1 doi: 10.1021/ml100143q. 478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531:189–192. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]