Summary
Definitive chemoradiation (CRT) and laryngectomy followed by postoperative radiotherapy (RT) are both considered standard of care options for the management of advanced laryngeal cancer. While organ preservation with chemoradiotherapy is often the preferred up-front approach for appropriately selected candidates, the functional benefits of organ preservation must be carefully balanced against the considerable morbidity of salvage laryngectomy in patients who fail primary chemoradiation. Up-front identification of patients who are likely to require surgical salvage, therefore, is an important aim of any organ preserving approach in order to minimize morbidity while maximizing organ preservation. To this end, a strategy of “chemoselection”, using the primary tumor's response after one cycle of induction chemotherapy as an in vivo method of selecting responders for definitive chemoradiation while reserving primary surgical management for non-responders, has been employed extensively at our institution. The rationale, treatment results, and future directions of this approach are discussed.
Keywords: Larynx preservation, chemoselection, induction chemotherapy, concurrent chemoradiation, salvage laryngectomy
Background
Over the past 40 years, the management of locally advanced head and neck squamous cell carcinoma (SCCA) has evolved from initial approaches with combined primary surgery and RT to modern multimodality approaches using definitive concurrent chemoradiation[1]. In locally advanced SCCA of the glottic and supraglottic larynx, initial approaches in the 1970's and 80's using conventionally fractionated RT alone as a single modality produced unsatisfactory treatment outcomes, with inferior rates of locoregional failure and survival compared with primary surgical approaches [2-6]. For patients with locally advanced lesions not amenable to voice-sparing surgery, total laryngectomy followed by adjuvant RT became the preferred treatment of choice, achieving significant improvements in locoregional control and survival compared with RT alone[3,5]. These gains in local control and survival, however, were achieved at the price of significant functional morbidity from total laryngectomy, the sequelae of which include loss of natural voice, permanent tracheostomy, and alterations in swallowing function that often result in social stigmatization and psychological disturbances [7].
Early Experience with Induction Chemotherapy Response as a Predictive Biomarker
Given the suboptimal outcomes of treatment with primary radiotherapy as a single modality for locally advanced head and neck cancers (HNC), efforts to improve the efficacy of non-operative organ-preserving approaches subsequently turned towards the incorporation of induction chemotherapy (IC) into treatment protocols. Several studies demonstrated that IC delivered prior to locoregional therapy produced high rates of primary tumor response, and that the response to IC correlated closely with freedom from treatment failure [8-10]. In patients treated with definitive RT after IC, Ensley et al. reported that while 97% (41 of 42 patients) of those with a partial-response (PR) to IC subsequently responded to RT, the rate among chemotherapy non-responders was merely 6% (1 of 18 patients) [11].On the basis of such reports, the Northern California Oncology Group conducted a pilot study to assess the feasibility of using the response to induction cisplatin and 5-fluorouracil (PF) to select patients for non-surgical management [12]. Thirty patients with locally advanced head and neck cancer received 3 cycles of PF; those with a complete response (CR) at the primary site were assigned to definitive RT, whereas those with less than a CR underwent surgical resection and postoperative RT. Twelve patients with CR received RT with 2-year relapse-free survival rate of 60% and overall survival of 70%, supporting the feasibility of omitting surgery in patients who respond to IC. Single-institution studies subsequently demonstrated similar rates of organ preservation when patients with a PR or better to induction chemotherapy (defined as a 50% or greater decrease in the sum of the product of the diameters of each measurable lesion) were treated with definitive RT [13,14].
Chemoradiotherapy as an Alternative to Laryngectomy
The viability of organ-preservation as an alternative to definitive surgery was established by the Veterans Affairs Larynx Cancer Study Group (VALCSG) in a landmark phase III randomized trial that tested whether the response to IC could be used to select patients for larynx preservation with definitive RT without compromising survival compared to the standard of surgery followed by RT [15]. Three-hundred thirty two patients with non-metastatic stage III or IV SCCA of the glottic or supraglottic larynx (excluding T1N1 tumors) technically resectable only by laryngectomy were randomized to laryngectomy and postoperative RT (surgery + RT) or sequential induction PF × 3 cycles followed by definitive RT for chemotherapy responders (IC + RT). Those in the IC + RT arm who failed to achieve a PR in the primary tumor after 2 cycles of PF underwent laryngectomy and postoperative RT. By this approach, 82% of patients were able to receive definitive RT, with 64% larynx preservation at 2 years in patients randomized to IC + RT. Local recurrences were more common in the IC + RT arm (12% vs. 2%), while distant failures were more frequent in surgery + RT patients (11% vs. 17%). Of note, patients with T4 tumors in the chemoradiotherapy arm were less likely than those with T1-3 tumors to achieve a PR or CR to induction chemotherapy, and were twice as likely to require salvage laryngectomy (56% vs. 28%) [16]. Overall survival at 2 years, however, was equivalent in both arms at 68%. Thus, the strategy of induction chemotherapy to select patients for organ-preservation with definitive RT was established as anon-surgical alternative for patients with locally advanced larynx cancer.
In a parallel study to the VALCSG, the European Organization for Research and Treatment of Cancer (EORTC) similarly demonstrated the equivalence of organ preservation by sequential chemoradiotherapy and a primary surgical approach in patients in hypopharynx cancer [17]. EORTC 24891 randomized 202 patients with locally advanced SCCA of the hypopharynx to either primary surgical management with total laryngectomy and partial pharyngectomy with postoperative RT (surgery + RT) or induction PF followed by definitive RT (IC + RT) for chemotherapy responders who achieved a CR at the primary site. Those with less than a CR after 3 cycles underwent immediate laryngectomy and postoperative RT. Using these more stringent standards compared with the VALCSG, 54% received definitive RT after IC, with larynx preservation achieved in 42% at 3 years and 35% at 5 years. On long-term follow-up of the IC + RT arm, 60% of patients remaining alive at 5 years and 63% of those alive at 10 years had retained a functional larynx [18]. Overall survival was statistically equivalent between treatment arms (3 year OS: 43% for surgery + RT vs. 57% for IC + RT; median survival 25 months vs. 44 months, respectively). These findings therefore validated those of the VALCSG: larynx preservation could be achieved without compromising survival by the use of sequential chemoradiation.
With larynx-preservation established as an alternative to primary surgery, the Radiation Therapy Oncology Group (RTOG) embarked upon a phase 3 study to determine the optimal non-operative treatment strategy in locally advanced larynx cancer. RTOG 91-11 randomized 547 patients to sequential chemoradiotherapy (IC + RT), concurrent chemoradiotherapy (C+RT), or RT alone [19]. The primary endpoint was preservation of the larynx. Due to the lower rate of response to induction chemotherapy and high rate of salvage laryngectomy among patients with T4 tumors in the VALCSG study, “large volume” T4 tumors with either penetration through the thyroid cartilage or with > 1 cm of base of tongue invasion were specifically excluded. Sequential chemoradiotherapy was performed as in the VALCSG study, with only patients who achieved a PR or CR after 2 cycles of PF going on to definitive RT after a 3rd cycle. Concurrent chemoradiation involved single-agent cisplatin beginning on day 1 of RT. Radiotherapy in all arms consisted of 70 Gy in conventional fractionation over 7 weeks to all gross disease and comprehensive nodal radiation of at least 50 Gy to the neck. The initially reported results demonstrated that concurrent chemoradiation resulted in superior 2-year locoregional control (IC + RT 64% vs. C + RT 80% vs. RT alone 58%) and larynx preservation (75% vs. 88% vs. 70% at 2 years) compared to either sequential chemoradiation or RT alone [19]; the benefit of concurrent chemoradiation for these endpoints was maintained on long-term follow-up (locoregional control 55% vs. 68% vs. 51% at 5 years; larynx preservation 71% vs. 84% vs. 66% at 5 years, respectively) [20]. Sequential chemoradiation was not superior to RT alone for either of these endpoints, and no differences in overall survival were demonstrated between the 3 arms (although beyond 5 years, overall survival non-significantly favored the induction chemotherapy arm) [20,21]. Concurrent chemoradiotherapy was therefore concluded to be the new standard of care for locally advanced laryngeal cancer without gross thyroid cartilage involvement or extensive base of tongue involvement, with laryngectomy reserved only for salvage after failure of organ-preserving therapy [22].
Despite the success of chemoradiation in preserving the larynx without compromising survival, a significant proportion of patients still ultimately require salvage laryngectomy due to local failure or laryngo-esophageal dysfunction after RT. In RTOG 91-11, for example, the 5-year rate of salvage laryngectomy was 16% in the concurrent chemoradiation arm, compared with 31% in the RT alone arm and 28% % in the induction chemotherapy arm (including those patients assigned per-protocol to immediate laryngectomy after achieving less than a PR to 2 cycles of IC) [23]. Over 50% of patients experienced postoperative complications, including pharyngocutaneous fistula formation in 30%, 25%, and 15% of patients in the concurrent CRT, sequential CRT, and RT alone arms, respectively [23]. The increased incidence of wound and systemic complications after salvage laryngectomy, as well as the detrimental impact of salvage laryngectomy on quality of life, are well documented [24-26]. Given the significant morbidity of surgical salvage and the lack of a proven survival benefit for any larynx preserving therapy compared with up-front surgery, avoidance of salvage laryngectomy is therefore among the most important aims of patient selection for organ-preservation. To this end, identifying patients with radiation sensitive tumors has the potential to improve the therapeutic ratio of organ preservation by minimizing exposure of those patient unlikely to respond to the toxicities of chemoradiation, while at the same time allowing patients with tumors traditionally considered unsuitable for organ preservation (i.e. T4 tumors with major thyroid cartilage or base of tongue involvement) to maximize the potential for a non-surgical cure.
Rationale for Single Cycle Chemoselection Prior to Concurrent Chemoradiotherapy
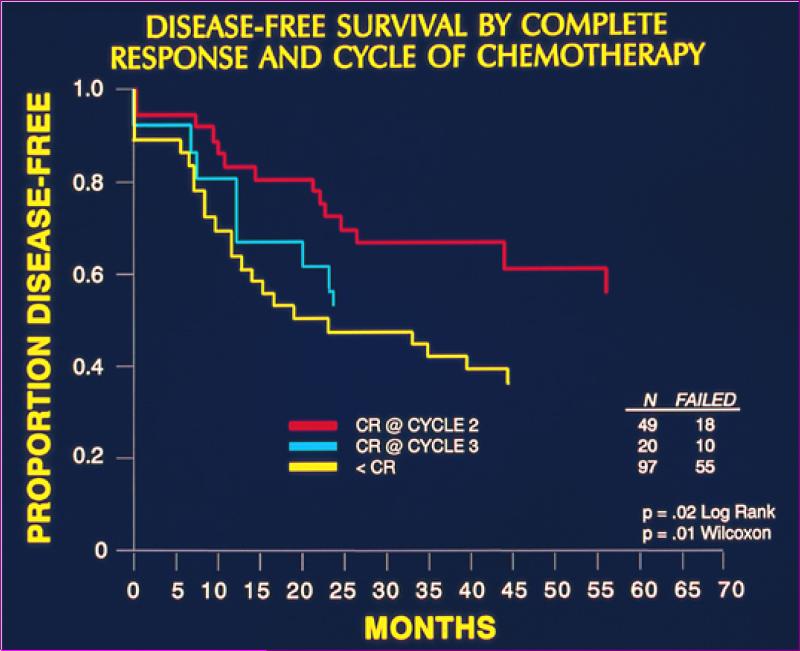
As previously noted, early studies of induction chemotherapy demonstrated that the response of the primary tumor to chemotherapy was highly predictive of the tumor's sensitivity to subsequent radiotherapy and was prognostic for treatment failure [11,13,27]. Similar findings were observed in the VALCSG study, in which patients who required 3 cycles of IC for a CR were more likely to undergo salvage laryngectomy than those who achieved a CR after only 2 cycles (86% vs. 70%) [16]. The rapidity and completeness of the response to IC was similarly predictive of disease-free survival, with superior outcomes observed in patients achieving CR after 2 cycles than those achieving CR or <CR only after 3 cycles (log rank P=0.02) (figure 1) (personal communication from G. Wolf). An early study of induction chemotherapy followed by accelerated hyperfractionated radiation therapy from the University of Michigan demonstrated that the response after a single cycle of chemotherapy was closely associated with the response after 3 cycles [28]. In this study, 18 out of 20 patients with a PR after a single cycle of PF achieved a major response (CR or downstaging to T1) after 3 cycles which qualified them for organ preservation, whereas of only 1 of 4 patients with no response to the first cycle achieved a major response. A PR to 1 cycle of PF, therefore, had a positive predictive value of 90% and negative predictive value of 75% for the endpoint of selecting patients for organ preservation (as determined by a major response after 3 cycles). As a full course of induction PF is associated with significant toxicity that prevents a substantial proportion of patients from receiving definitive organ-preserving therapy, and is associated with inferior locoregional control and larynx preservation compared to concurrent chemoradiation, it was concluded that the primary role of induction chemotherapy in any organ preservation treatment paradigm is to serve as an in vivo assay to identify patients most likely to be cured by chemoradiation [19,28-30].
Figure 1.
Disease-Free Survival by Response to Induction Chemotherapy in the Veterans Affairs Larynx Trial [15]. Patients with a complete response (CR) after 2 cycles of induction PF achieved superior disease-free survival compared to those who requires 3 cycles for a CR or who did not achieve a CR.
Results of Chemoselection for Locally Advanced Larynx Cancer
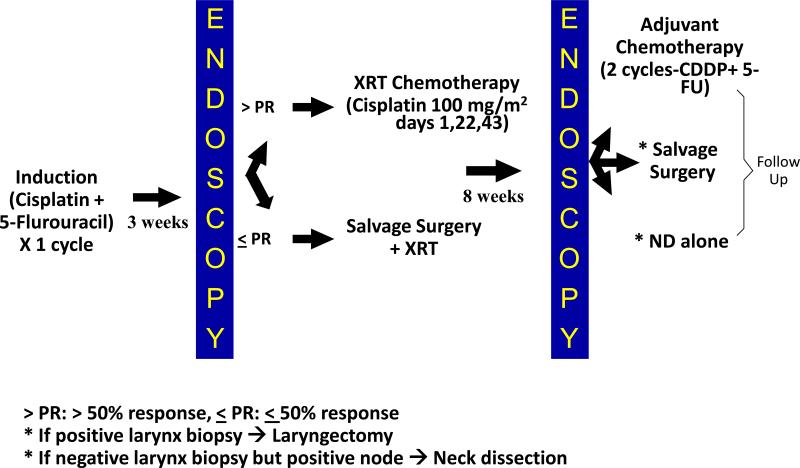
To test the concept of “chemoselection”, the University of Michigan undertook a Phase II protocol (UMCC 9520) to determine whether the response to a single cycle of induction chemotherapy could be used to identify patients for larynx preservation by concurrent chemoradiotherapy (Figure 2) [31]. Ninety seven patients with advanced laryngeal cancer (46% stage III, 54% stage IV) who were surgical candidates for total laryngectomy were enrolled and staged with direct laryngoscopy and contrast-enhanced computed tomography (CT). Twenty three percent of patients had SCCA of the glottis and 77% of the supraglottis, which are associated with poorer survival rates. Following one cycle of induction PF, all candidates underwent endoscopy to assess tumor response, classified as either PR (> 50% reduction of bidimensional production) or stable disease (≤ 50% regression). Those achieving a PR continued on to definitive chemoradiotherapy, consisting of daily fractionated RT to 70 Gy in 2 Gy per fraction with concurrent cisplatin 100 mg/m2 every 3 weeks those with stable disease went on to total laryngectomy followed by RT. Eight weeks after completion of chemoradiotherapy, repeat endoscopy was performed and adjuvant chemotherapy or salvage laryngectomy or neck dissection alone was performed as indicated.
Figure 2.
Schema for the Larynx Preservation Chemoselection Protocol Used at the University of Michigan [31,33]
Seventy three patients (75%) achieved at least a PR after one cycle of IC (all had tumor regression ≥ 70%), all of whom went on chemoradiation. Of these, three had residual disease at the second endoscopic evaluation after chemoradiation and underwent TL. Six more patients underwent TL for eventual recurrence at primary site, while one patient underwent TL for severe dysphagia and chondroradionecrosis. Of the 22 (23%) nonresponders to one cycle of induction PF, 19 underwent total laryngectomy per protocol. At a median follow-up was 41.9 months, the rate of larynx preservation for the entire study population was 70% (68 of 97 patients), with successful preservation achieved in 86% (63 of 73 patients) of those selected to receive chemoradiation after a PR to one cycle of induction PF. Laryngectomy-free survival was 63% at 2 years and 61% at 3 years, with disease-free survival of 80% at 2 years and 78% at 3 years. Overall survival was 88% at 2 years and 85% at 3 years. As in prior studies of organ preservation, overall survival did not differ between patients requiring laryngectomy for failure to achieve PR after one cycle of PF and those with successful organ preservation [15]. Despite the inclusion of more T4 and supraglottic tumors in the UM study, the overall survival results compare favorably with the 3-yearrates of 54% in the VALCSG trial sequential chemoradiation arm and 68% in the RTOG 91-11 trial concurrent chemoradiation arm [15,21]. The results of UMCC 9520 therefore support the hypothesis that a single cycle of IC can select a patients with advanced larynx cancer for successful organ preservation by concurrent chemoradiation with approximately 86% accuracy, while sparing the approximately 30% of patients with a poor response to IC from the morbidity of chemoradiation with a high likelihood of subsequent salvage laryngectomy.
Toxicity, feasibility and QOL were also analyzed in this cohort of patients [31]. Although 24 patients received feeding tubes during chemoradiation (5 prophylactically and 19 due to treatment-related toxicity), only 2 retained feeding tubes permanently. No long term tracheostomy tubes were required in those patients without laryngectomy. In addition, a validated voice-related quality of life (V-RQOL) measure was used to help quantify swallowing and voice function [32]. Mean V-RQOL scores were significantly higher in the organ preservation group compared to the salvage laryngectomy group; however, there was no significant difference between early and late salvage laryngectomy groups. Understandability of speech was worse in laryngectomy patients, but there was no significant difference between groups regarding eating in public or normalcy of diet. Larynx intact patients were also more likely to obtain all nutrition orally, although this was not statistically significant.
As previously mentioned, patients with stage T4 tumors with large volume or cartilage invasion were specifically excluded from RTOG 91-11 due to high rates salvage laryngectomy in the VALCSG organ preservation arm. As a result, such patients have been traditionally considered not suitable for organ preservation, and have been typically managed with total laryngectomy and postoperative radiotherapy. To determine whether patients with T4 primary larynx tumors may be spared total laryngectomy using the single cycle chemoselection approach, the University of Michigan group analyzed the outcomes of these patients in two sequential phase II chemoselection trials (UMCC 9520 and 0056) [33]. Thirty-six patients with T4 tumors with radiographic evidence of tumor extension through the thyroid cartilage or tumor extension beyond the larynx, treated with a single-cycle of induction PF followed by selection for subsequent chemoradiation or laryngectomy as described above, were included (Figures 3 and 4). Among this poor prognosis subset, 29 patients (81%) achieved at least a PR, of whom 27 went on to chemoradiation (of the two patients not receiving chemoradiation, 1 died of sepsis unrelated to neutropenia prior to the start of C+RT, while the other received radiation alone due to deterioration in performance status). Four out of these 27 patients had residual disease at the second endoscopic evaluation and underwent TL, while two more underwent TL for eventual recurrence at primary site. Seven patients (19%) had stable disease after one cycle of PF, of whom 6 underwent total laryngectomy. One patient was deemed unresectable and underwent XRT. At median follow-up of 69 months, overall larynx preservation was 66% (24 of 36 patients), with 3-year laryngectomy-free survival of 58% and overall survival of 78%. A comparison of the T4 patients with T3 patients treated with chemoselection during the same period (UMCC 9520 and 0056) showed comparable larynx preservation and survival outcomes [33]. In addition, compared to historical controls, overall survival and laryngectomy free survival appear similar to the superior concurrent chemoradiation arm of RTOG 91-11 [19].
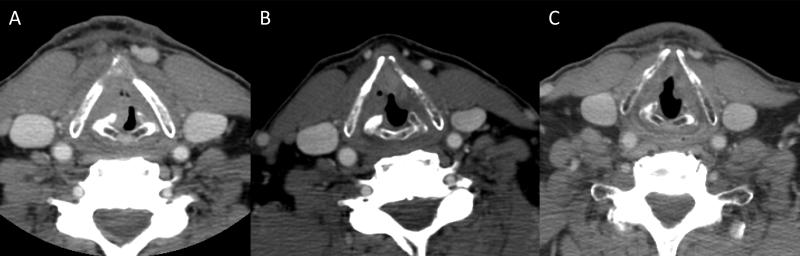
Figure 3.
Contrast enhanced CT images of a 74 year old male with T4 N1 M0 squamous cell carcinoma of the right glottic larynx treated successfully with chemoselection. (A) CT demonstrates tumor invasion through the anterior aspect of the thyroid cartilage at the time of diagnosis. (B) Three weeks after a single induction cycle of cisplatin-fluorouracil, repeat CT scan demonstrated greater than 50% tumor response, and he went on to receive radiotherapy to 70 Gy concurrent with cisplatin 100 mg/m2 every 3 weeks. (C) He remains free of disease at 2 years after completion of chemoradiation.
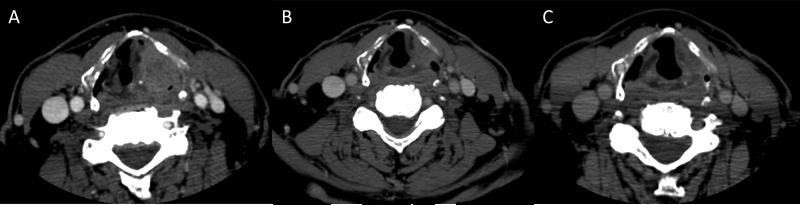
Figure 4.
Contrast enhanced CT of a 62 year old male with T4 N2b M0 squamous carcinoma of the left supraglottic larynx treated successfully with chemoselection. (A) Contrast enhanced CT scan at time of diagnosis demonstrates an aggressive appearing left supraglottic mass with erosion through the left lateral aspect of the thyroid cartilage. (B) Three weeks after a single induction cycle of cisplatin-fluorouracil, a greater than 50% tumor response was achieved. (C) CT at 6 months after completion of chemoradiation demonstrated residual left supraglottic tissue fullness attributable to post-radiotherapy change without evidence of residual disease, confirmed on endoscopic examination. The patient remains disease free 2 years after completion of therapy.
Quality of life was analyzed in this patient cohort via the Performance Status Scale for Head and Neck Cancer Patients [33]. Patients with T3 lesions from the UMCC 9520 and UMCC 0056 studies were used for comparison. All 36 patients were evaluated for G-tube dependence and persistent tracheostomy tube. Seventeen percent of T4 patients required G-tubes at 12 months post-treatment, as compared to 4% of the T3 population (p=0.03). There was no difference in persistent tracheostomy between the two cohorts. Understandability of speech was evaluated in 11 T4 patients, of whom 8 had at least 75% understandability (73%), similar to the 71% rate among T3 patients. The results of this study indicate that chemoselection is a potential alternative to total laryngectomy in T4 cases, without compromising QOL.
Chemotherapy Alone for Larynx Cancer
Based on the success of single cycle chemoselection prior to chemoradiation, our institution tested the hypothesis that the response to one cycle of induction PF could be used to select patients with chemosensitive tumors who could be cured by chemotherapy alone, thereby avoiding the morbidity of radiotherapy [34]. Thirty-two patients with stage III (31%) or IV (69%) SCC of the larynx or hypopharynx were enrolled in a single arm phase II study in which those with a clinical and histological CR (by direct laryngoscopy visualization and biopsy) after one cycle of induction PF were assigned to an additional 3 cycles of chemotherapy (PF for cycles 2 and 4, weekly docetaxel × 3 weeks for cycle 3). Those with PR after single cycle induction PF received definitive chemoradiotherapy, whereas those who failed to achieve a PR after the initial single induction cycle proceeded directly to laryngectomy. In both the chemotherapy alone and chemoradiotherapy groups, all patients underwent clinical and histological reassessment by direct laryngoscopy after completion of therapy; those in sustained CR were assigned an additional 4 cycles of adjuvant chemotherapy (single agent weekly docetaxel for cycles 5 and 7, PF for cycles 6 and 8), while those with residual disease underwent salvage laryngectomy. After single cycle induction PF, 4 patients (13%) with a clinical and histology CR proceeded to chemotherapy alone, while 24 (75%) with a PR underwent chemoradiation. Four patients (13%) had less than a 50% PR, of whom 3 underwent laryngectomy (one refused and received chemoradiation). All 4 patients with initial CR who received chemotherapy alone had persistent or progressive neck disease at the end of chemotherapy (one additionally had progressive local disease), and therefore all 4 required salvage therapy. Thus, despite careful selection of a subset of patients with chemosensitive tumors who achieved complete clinical and histological responses to single cycle induction PF, subsequent treatment with chemotherapy alone failed to provide definitive locoregional control in locally advanced larynx and hypopharynx SCC.
Our institutional experience contrasts with that reported by Holsinger et al. using definitive paclitaxel, ifosfamide, and cisplatin (TIP) in 31 patients with predominantly earlier stage SCC of the larynx (61% stage II and 23% stage III) [35]. All patients initially received 3-4 cycles of TIP followed by clinical and histological reassessment; those achieving CR assigned to 3 additional cycles, whereas those with PR underwent conservative laryngeal surgery and those with less than PR underwent salvage surgery with or without adjuvant radiotherapy. Of 30 assessable patients, 11 (37%) achieved a CR and went on to additional chemotherapy, while 19 (63%) PR and underwent conservative surgery. At a median follow-up of 65 months, only 1 of 11 patients (9%) with CR treated with chemotherapy alone experience local recurrence, which was salvaged with conservative surgery and postoperative radiotherapy. Overall larynx preservation for the entire cohort was 83% at 2 years. Although the toxicity of the TIP regimen and need for surgical intervention in the nearly two-thirds of patients who achieved only a PR after 3-4 chemotherapy cycles, the results of this study nonetheless provide proof of principle that the use of chemotherapy alone may achieve successful larynx preservation and long-term control of a subset of patients with SCC of the larynx. Validation of this strategy in larger patient cohorts and identification of predictive biomarkers to select patients who are likely to be cured with chemotherapy alone, however, remain necessary before such a strategy can be widely adopted.
Intensification of Induction Chemotherapy for Larynx Preservation
While chemoselection as employed at our institution utilizes a single cycle of induction PF to select patients for definitive chemoradiation, advocates of induction chemotherapy have proposed that a full course of intensified induction chemotherapy using docetaxel with cisplatin and 5-FU (TPF) may further improve outcomes in locally advanced HNC, and should therefore represent an acceptable standard of care for organ preservation as an alternative to definitive chemoradiation [36]. Whether the addition of TPF to concurrent chemoradiation can indeed improve larynx preservation, and thereby challenge our assertion that the primary role of induction chemotherapy in larynx preservation should be to differentiate responders from non-responders for further management, can be addressed by analyzing the results of several recently reported clinical trials. The benefit of induction TPF over PF in locally advanced HNC was conclusively established in the TAX 323 and 324 studies [29,30]. Despite minor differences in study design and inclusion criteria, both studies demonstrated that the addition of docetaxel to PF improves tumor response rates, locoregional control, progression-free survival, and overall survival. Notably, in both studies, induction chemotherapy was associated with a high rate of failure to receive definitive therapy, ranging from 10-15% in TAX 323 and 21-25% in TAX 324. An unplanned subset analysis of patients on TAX 324 with operable locally advanced larynx and hypopharynx SCC further demonstrated that induction TPF additionally improved laryngectomy-free survival (52% vs. 32% at 3 years, p=0.03) and progression-free survival (48% vs. 32% at 3 years, p=0.03), with a trend toward improvement in overall survival (58% vs. 47% at 3 years, p=0.12) [37]. Similar results were demonstrated by the French Head and Neck Oncology Radiotherapy Group (GORTEC), who randomized 213 patients with stage III or IV SCC of the larynx(46%) or hypopharynx (54%) operable only by total laryngectomy to either induction TPF or PF followed by radiotherapy for complete responders or partial responders who recovered normal larynx mobility [38]. Twenty percent of patients in the TPF group and 16% of those in the PF group received concurrent chemotherapy (either cisplatin, carboplatin, 5-FU, or a two drug combination) with radiotherapy. Overall responses rates after induction chemotherapy favored the TPF arm (80% vs. 59.2%, p=0.002) and translated into improved larynx preservation at 3 years (70.3% vs. 57.5%, p=0.03), although without a statistical improvement in disease-free survival (58% vs. 44% at 3 years, p=0.11) or any improvement overall survival (60% in both arms at 3 years). On the basis of the studies, TPF was thus established as the regimen of choice for induction chemotherapy is planned prior to radiotherapy or chemoradiotherapy in locally advanced head and neck cancer.
While the TAX 323/324 and GORTEC experiences demonstrated the superiority of induction TPF over PF for larynx preservation in patients who undergo full course induction chemotherapy, the efficacy of induction TPF when added to definitive chemoradiation in locally advanced HNC remained uncertain until recently. Despite a promising phase II study reported by Paccagnella et al. demonstrating improved radiographic response rates and trends towards improved progression-free and overall survival for the addition of TPF to definitive chemoradiotherapy, the recently reported phase 3 PARADIGM and DeCIDE trials failed to show any improvement in either locoregional control or overall survival in patients in whom induction TPF was added to concurrent chemoradiation [39-41]. The negative results of these studies suggest that induction chemotherapy is unlikely to improve organ preservation in locally advanced larynx cancer compared with concurrent chemoradiation, and should therefore be employed only in single-cycle induction regimens with PF to select patients for larynx preservation.
Biomarkers and Targeted Therapeutics for Advanced Laryngeal Cancer
Interestingly, tumor volume was evaluated radiographically in a subset of patients from UMCC 9520 and no survival difference was noted between the largest and smallest tumors. This indicates that the biology of these tumors, as opposed to cartilage invasion or bulky disease, may be more predictive of response to chemoradiation treatment. To study this, biopsy specimens from UMCC 9520 were prospectively collected and evaluated for relevant biomarkers that might predict outcome in this cohort of patients with advanced larynx cancer.
Tissue microarrays were constructed from 58 pretreatment biopsies, consisting of 40 (69%) from chemotherapy-responders and 18 (31%) from non-responders. TMA's were stained for cyclin D1, CD24, EGFR, MDM2, PCNA, p53, survivin, Bcl-xL, Bcl-2, BAK, rhoC, and NFKB. Pattern of the invasive front was scored according to published criteria : (1) pushing borders; (2) well-formed, infiltrating cords; (3) thin, irregular, infiltrating cords; and (4) small groups and dissociated cells. These results were correlated with overall survival, disease-specific survival, time free from indication of surgery, induction chemotherapy response, and chemoradiation response (Bradford CR et al, submitted).
Evaluation of the chemotherapy response revealed higher levels of BAK significantly associated with poorer response to induction chemotherapy (p=0.0004). High expression of cyclin D1 predicted poorer overall survival (p=0.008) and increased risk of death from disease (p=0.0147). In contrast, high expression CD24 cytoplasmic expression was associated with lower overall risk of death. Additionally, EGFR expression correlated with increased risk of death from disease (p=0.0424). Patterns of growth were highly predictive of response to chemotherapy as well as likelihood of organ preservation. Aggressive growth patterns 3 and 4 responded to induction chemotherapy (13/16, 81%) as compared to tumors with growth patterns 1 and 2 of which 27/39 (69%) of tumors responded. Patients whose tumors showed the least aggressive invasive front (pattern 1: pushing borders) were more likely to have laryngectomy compared to patients with growth patterns 2-4 (p = 0.0557). Patients with thin cords (pattern 3) and single cell (pattern 4) invasive fronts were less likely to present with indication for laryngectomy (hazard ratio 0.6, 95% CI 0.321, 1.014). Finally, twenty-nine pre-treatment TMA's were also compared to tissue from 33 persistent/recurrent tumors. Increased expression of anti-apoptotic protein Bcl-xLwas observed in salvage vs. pretreatment tumor specimens (P-0.0003), suggesting that Bcl-xL may play a role in chemoradioresistance. In addition, Trask et al demonstrate that pre-treatment levels of Bcl-xL may be predictive of treatment response and larynx preservation after chemoradiation in laryngeal squamous cell carcinoma [42]. These differences in tumor biology may provide a biological basis for the heterogeneity of tumor response to induction chemotherapy and chemoradiotherapy in organ preservation protocols.
In an effort to minimize the cumulative toxicity profile of definitive therapy and maximize the antitumor effects of conventional platinum-based chemotherapy, use of molecular targeted agents has been proposed. To this end, the University of Michigan is currently accruing untreated stage 3 and 4 larynx cancer patients in a trial evaluating the use of a Bcl-xL inhibitor (AT101) in a chemoselection protocol. After enrollment, patients are randomized to receive docetaxel and carboplatin (TP) either with or without concurrent AT101 (40 mg twice daily on days 1 and 2). Endoscopic evaluation is performed after one cycle of chemotherapy to assess tumor response as in our prior chemoselection studies, with those who achieving CR proceeding directly to definitive CRT, while those with PR or less than PR receive a second cycle of TP, all with AT101. Repeat assessment of tumor response is performed and those with CR or PR proceed to CRT. Those with less than PR after 2 cycles of chemotherapy proceed to total laryngectomy with postoperative (chemo)radiotherapy as clinically indicated. Such mechanism-based targeted therapy may allow for more precise in vivo identification of patients most likely to be complete responders to CRT, and differentiate them from those more likely to benefit from early definitive laryngectomy.
Chemoselection in Oropharynx and Oral Cavity Cancer
The success of single cycle chemotherapy as an induction selection agent in laryngeal squamous cell carcinoma (UMCC 9520 and 0056) stimulated further hypotheses as to the role of chemoselection in other head and neck cancer subsites. To this end, the University of Michigan designed a phase II clinical trial to examine the role of induction chemotherapy in oropharyngeal and oral cavity squamous cell carcinoma (UMCC 9921). The oropharyngeal and oral cavity arms of this study had vastly different outcomes in response to induction as well as survival, and thus are discussed separately.
Sixty six patients with previously untreated, locally advanced (26% stage III, 74% stage IV), pathologically confirmed squamous cell carcinoma of the oropharynx and who were candidates for surgical resection were enrolled onto the oropharyngeal arm of UMCC 9921 [43]. Disease subsites were 53% base of tongue, 41% tonsillar pillar and fossa, and 6% other oropharyngeal subsites. Induction chemotherapy consisted of cisplatin 100mg/m2 (or carboplatin AUC 6 on day 1 for patients with cisplatin contraindications) and 5-fluorouracil 1000mg/m2/d for days 1-5 by continuous infusion. Tumor assessment was performed at three weeks under general anesthesia in the operating room, and patients with CR or PR received definitive chemoradiotherapy with either concurrent cisplatin or carboplatin (AUC 6). Patients with less than PR (i.e. <50% response) underwent surgery followed by radiation.
The response rate to single cycle induction chemotherapy was 82% (54/66), slightly higher than the laryngeal response rate in UMCC 9520 (75%) [31]. Of 11 nonresponders (17%), 9 underwent surgery, 1 refused surgery, and 1 patient was not a surgical candidate. One patient died of chemotherapy-related toxicity prior to initiation of chemoradiation. Of the 54 patients with a PR or CR, 3 (6%) required salvage after definitive chemoradiation and 3 (6%) other patients developed local recurrence but refused surgery. The overall successful organ preservation rate in this cohort was 73% (48/66).The four-year overall survival and disease specific survival for the entire cohort was 70.4% and 75.8% with a median follow-up of 64 months. Female sex, higher T class, lower N class, current smoking status, and base of tongue subsite were all factors associated with worse disease specific survival.
Pretreatment biopsies of the tumor were used to identify molecular factors that could also predict survival [44]. High HPV titers, positive p16 status and low epidermal growth factor receptor (EGFR) expression conferred an improved prognosis, while high EGFR expression and low HPV titers conferred a poor prognosis. The combination of low p53 and high Bcl-xL expression was also associated with a poor overall survival and disease specific survival. Smoking was also found to be predictive when categorized as never, past and current smoking status and was thus identified as another way to stratify patients into risk groups.
Toxicities to treatment and chemotherapy compliance were also presented in the study [43]. The single treatment-related mortality from chemotherapy was due to febrile neutropenia in a patient who was later discovered to have dihydropyrimidine dehydrogenase deficiency. In addition, 5 patients were hospitalized for febrile neutropenia, 6 were hospitalized for dehydration and mucositis, and 8 required intravenous fluids as outpatients. Only 2 patients remained G-tube dependent following treatment, and both were surgical salvages after chemoradiotherapy. There were 38/53 (72%) of patients who were able to receive all three cycles of chemotherapy during radiation.
While the overall and disease specific survival are comparable to other treatment paradigms in oropharyngeal cancer, one notable difference in the oropharyngeal cancer chemo-selection protocol outcomes is that oropharyngeal non-responders experienced significantly worse overall survival than responders, which contrasts with results of laryngeal cancer chemoselection protocols such as the VALCSG, in which chemotherapy non-responders who underwent immediate laryngectomy did not have worse outcomes compared with responders who underwent sequential chemoradiotherapy [15]. For example, while survival of 73% was observed in the UMCC 9520 larynx cancer chemoselection experience for patients who required early surgical salvage, the comparable figure for the oropharynx cohort was only 36% [31,43]. Similarly, for those who underwent late surgical salvage, survival was 100% in the larynx cohort compared to 0% in the oropharynx cohort. Thus, while chemoselection was demonstrated to be feasible with excellent outcomes for chemotherapy responders, the poor outcomes after definitive surgical therapy for non-responders suggests that these alternative means to identify such patients need to be developed, with escalation of therapy, possibly in the form of early surgery followed by chemoradiotherapy, potentially warranted. This study was also the first to examine whether a combination of molecular markers could be used identify responders and non-responders to treatment [44].
The oral cavity arm of UMCC 9921 was closed early due to pre-identified stopping rules [45]. After accrual of 19 patients, the oral cavity cohort did not meet at least 40% of patients to achieve organ preservation after definitive chemoradiation, and thus was felt not to be feasible in this patient population. Nineteen patients were enrolled and treated with induction chemotherapy. After induction chemotherapy, only 58% (10/19) had a greater than 50% response, while 42% (9/19) were considered non-responders based on a less than 50% response. The overall survival and disease specific survival of this cohort at 5-years was 32% and 46%, respectively, which was well below the standard for similar patients treated with surgery. The conclusions of this phase II trial was that chemoselection is not feasible in patients with oral cavity squamous cell carcinoma. It is interesting that of the 10 patients who had a greater than 50% response after induction chemotherapy in this trial, only 3/10 patients responded to chemoradiation and had no evidence of disease for five years. This suggests that differences in tumor biology in these patients, unknown to us at this time, may be further elucidated to help select patients that may respond to organ preservation protocols in the future.
Expert Commentary
The promising functional, oncological, and quality of life outcomes from the University of Michigan chemoselection experience suggests that the in-vivo response to a single cycle of induction chemotherapy can accurately select patients with locally advanced larynx cancer for definitive concurrent chemoradiotherapy with a high likelihood of successful organ preservation. Importantly, this strategy limits the morbidity of salvage laryngectomy to only 10% of patients selected for chemoradiation, despite the inclusion of patients with large volume T4 tumors with extension through the thyroid cartilage that have traditionally been excluded from larynx preservation strategies [19,31,33]. Whether patients with large volume T4 tumors are best served by attempted larynx preservation, perhaps via an approach such as chemoselection that avoids subjecting potential non-responders to a full course of chemoradiation before surgical salvage, rather than primary laryngectomy remains an area of ongoing debate, which will only be resolved by a future randomized trial focused on this particular patient subset.
Five-Year View
Several additional issues regarding the future of chemoselection in larynx preservation bear further consideration. Whether the efficacy of larynx preservation by chemoselection may be improved by use of additional intensified chemotherapy in slow-responders or non-responders remains to be established, and is the subject of the current UMCC 2010.010 study, in which patients with less than CR after one cycle of docetaxel and platinum (TP) receive a second cycle of TP along with anti-apoptosis inhibitor AT101 prior to either concurrent chemoradiation for complete and partial responders or laryngectomy for non-responders. Identification of novel biomarkers that predict tumor responsiveness to chemotherapy and radiotherapy, as well as the development of targeted therapies that improve response rates in otherwise chemo- and radio-resistant tumors, additionally hold the potential to further improve patient selection for larynx preservation by either the current standard of chemoradiation or promising future therapeutic options, such as chemotherapy alone regimens [35]. Until such future approaches mature and their promise is fulfilled, chemoselection should retain an important role in optimizing the selection of advanced larynx cancer patients for organ preservation.
Key Issues.
Definitive concurrent chemoradiation is the standard of care for organ preservation in patients with locally advanced squamous cell carcinoma of the larynx without large-volume T4 disease or tumor extension through the thyroid cartilage and with acceptable larynx function to warrant consideration of larynx preservation
The functional benefits of organ preservation must be balanced against the considerable morbidity of salvage laryngectomy in those patients who experience local failure after primary chemoradiation
A strategy of “chemoselection”, in which the response of the primary tumor after one cycle of induction cisplatin and 5-fluorouracil serves as an in vivo test to identify patients most likely to achieve cure with definitive chemoradiation, has been used extensively at the University of Michigan to select patients for organ preservation, with high rates of successful
Chemoselection has been extended to patients with extensive T4 larynx cancers not typically considered for organ preservation, with similar rates of successful larynx preservation and survival achieved compared to patients with T3 tumors.
Chemoselection does not appear to be a viable strategy for patients with squamous cell carcinoma of the oral cavity, for whom definitive surgery followed by adjuvant (chemo)radiation appears to yield superior outcomes. The role of chemoselection in the management of patients with locally advanced oropharyngeal cancer, for whom both definitive chemoradiation and primary surgery with adjuvant (chemo)radiation are both considered acceptable standards of care, remains to be more clearly defined.
Table 1.
Selected Phase III Studies of Larynx Preservation Utilizing a Chemoselection Strategy
| Study | N | Patient Population | Arms | Primary Tumor Response to Chemotherapy | % Larynx Preservation | Overall Survival |
|---|---|---|---|---|---|---|
| VA Larynx Trial [15,46] | 332 | Larynx SCCA Operable Stage III/IV |
1.Surgery + RT 2.PF → RT (if CR or PR after 2 cycles) |
85% (CR or PR) | 66% (2y) | 68% (2y) 68% (2y) P=0.98 |
| EORTC 24891 [17,18] | 202 | Hypopharynx SCCA Operable T2-T4, N0 – N3 (excluding N2c) |
1.Surgery + RT 2.PF → RT (if CR after 2 or 3 cycles) |
86% (54% CR, 32% PR) | 42% (3y), 35% (5y) | 43% (3y), 33% (5y) 57% (3y), 38% (5y) P=0.002 for non-inferiority |
| GETTEC [47,48] | 68 | Larynx SCCA Operable T3, N0-N2b |
l.Surgery + RT 2. PF → RT (if ≥ 80% primary tumor regression after 3 cycles) |
39% with ≥ 80% regression | 42% (median 8 y) | 84% (2y) 69% (2y) P=0.006 |
| RTOG 91-11 [19,20] | 547 | Larynx SCCA Operable Stage III/IV, (excluding T1 and large-volume T4) |
l.PF → RT (if CR or PR after 2 cycles) 2.Concurrent CRT* 3. RT alone |
85% (CR or PR) | 71% (5y) 84% (5y) 66% (5y) P<0.01 (for arm 2 vs. arms 1 and 3) |
59% (5y) 55% (5y) 54% (5y) P=NS |
| TAX 324 (Operable larynx &hypopharynx subset) [37] | 123 | Larynx or hypopharynx SCCA Operable Stage III/IV |
l.TPF → CRT^ 2.PF → CRT^ (CRT only if response to IC) |
N.R. N.R. |
52% (3y LFS) 32% (3y LFS) P=0.03 |
58% (3y) 47% (3y) P=0.12 |
| GORTEC[38] | 213 | Larynx or hypopharynx SCCA Stage III/IV |
l.TPF → RT# 2. PF → RT# (RT only if CR or PR to IC) |
80% (42% CR, 38% PR) 59% (30% CR, 29% PR) P=0.002 |
70% (3y) 58% (3y) P=0.03 |
60% (3y) 60% (3y) P=0.57 |
CR = Complete response; CRT = chemoradiotherapy; IC = induction chemotherapy; LFS = laryngectomy-free survival; N.R. = not reported; NS = not significant; PF = cisplatin + 5-fluorouracil; PR = Partial Response; RT = radiation therapy; SCCA = squamous cell carcinoma
Cisplatin 100 mg/m2 every 3 weeks
Carboplatin AUC 1.5 weekly
Concurrent chemotherapy (cisplatin, carboplatin, or 5-fluorouracil, either alone or in combination) given with RT to 15-20% of patients
Table 2.
University of Michigan Protocols Involving Chemoselection with One Cycle of Induction Cisplatin^ and Fluorouracil (PF) to Select Patients for Organ Preservation
| Study | N | Median F/U | Patient Population | Definitive Therapy after Induction PF × 1c | Primary Tumor Response to Induction PF × 1c | % Organ Preservation | DFS | OS |
|---|---|---|---|---|---|---|---|---|
| UMCC 9520 [31] | 97 | 42 mo | Larynx SCCA; Operable stage III/IV |
(a)If PR* → CRT* → adjuvant PF × 2c (b)If < PR* → TL + PORT |
PR = 75% | 70% (all pts) 86% (CRT subset with PR after PF × 1c) |
78% (3y) | 85% (3y) |
| UMCC 0056 [34] | 32 | 45 mo | Larynx & hypopharynx SCCA; Operable stage III/IV |
(a)If CR* → alternating cycles PF & docetaxel (total 8c, including induction PF × 1c) (b)If PR* → CRT* → adjuvant PF × 2c (c)If < PR* → TL + PORT |
CR = 13% (4 pts) PR = 75% (24 pts) < PR = 13% (4 pts) |
78% (all pts) | 78% (3y - all pts) 0% (chemo alone for CR pts) |
68% (3y - all pts) 25% (chemo alone after CR pts) |
| UMCC 9921 Oropharyn × [43] | 66 | 64 mo | Oropharynx SCCA; Operable stage III/IV |
(a)If PR* → CRT → adjuvant paclitaxel × 2c (b)If < PR → Salvage surgery + PORT |
PR = 82% | 73% (all pts) | 76% (4y) | 70.4% (4y) |
| UMCC 9921 Oral Cavity [45] | 19 | 78 mo | Oral Cavity SCCA; Operable stage III/IV |
(a)If PR* → CRT → adjuvant paclitaxel × 2c (b)If < PR → Salvage surgery + PORT |
PR = 58% | 16% (all pts) 30% (CRT subset with PR after PF × 1c) |
46% (5y) | 32% (5y) |
Carboplatin (AUC =6) substituted for cisplatin 100 mg/m2 in patients not eligible for cisplatin
Tumor response assessed by direct laryngoscopy with bidirectional tumor measurements and primary tumor biopsies, performed under anesthesia 3 weeks after induction chemotherapy. Complete response was defined as clinical and histological absence of residual tumor. Partial response was defined as >50% reduction in bidimensional product of the primary tumor
Concurrent Chemoradiotherapy consisted of daily fractionated RT to 70 Gy in 35 fractions to the gross tumor volume, with concurrent cisplatin 100 mg/m2 on days 1, 22, and 43 (carboplatin AUC =6 substituted for patients not eligible for cisplatin)
C = chemotherapy cycles; CR = complete response; CRT = concurrent chemoradiotherapy; DFS = disease-free survival; F/U = follow-up; OS = overall survival; PF = cisplatin and fluorouracil; PORT = postoperative radiotherapy; PR = partial response; TL = total laryngectomy; UMCC = University of Michigan Cancer Center
Acknowledgments
Supported by: P50 CA097248
References
- 1.Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29–42. doi: 10.1016/j.critrevonc.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins NV. The treatment of glottic carcinoma: an analysis of 800 cases. Laryngoscope. 1975;85(9):1485–1493. doi: 10.1288/00005537-197509000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Weems DH, Mendenhall WM, Parsons JT, Cassisi NJ, Million RR. Squamous cell carcinoma of the supraglottic larynx treated with surgery and/or radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13(10):1483–1487. doi: 10.1016/0360-3016(87)90315-4. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Carcinoma of the supraglottic larynx: a basis for comparing the results of radiotherapy and surgery. Head Neck. 1990;12(3):204–209. doi: 10.1002/hed.2880120303. [DOI] [PubMed] [Google Scholar]
- 5.Harris HS, Jr., Watson FR, Spratt JS., Jr Carcinoma of the larynx, a retrospective study of 144 cases. Am J Surg. 1969;118(5):676–684. doi: 10.1016/0002-9610(69)90212-8. [DOI] [PubMed] [Google Scholar]
- 6.Skolnik EM, Yee KF, Wheatley MA, Martin LO. Carcinoma of the laryngeal glottis therapy and end results. Laryngoscope. 1975;85(9):1453–1466. doi: 10.1288/00005537-197509000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Strojan P, Zwitter M. Mental disorders after laryngectomy. Onkologie. 2005;28(12):617–618. doi: 10.1159/000089691. [DOI] [PubMed] [Google Scholar]
- 8.Ervin TJ, Clark JR, Weichselbaum RR, et al. An analysis of induction and adjuvant chemotherapy in the multidisciplinary treatment of squamous-cell carcinoma of the head and neck. J Clin Oncol. 1987;5(1):10–20. doi: 10.1200/JCO.1987.5.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Schuller DE, Metch B, Stein DW, Mattox D, Mccracken JD. Preoperative chemotherapy in advanced resectable head and neck cancer: final report of the Southwest Oncology Group. Laryngoscope. 1988;98(11):1205–1211. doi: 10.1288/00005537-198811000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Thyss A, Schneider M, Santini J, et al. Induction chemotherapy with cis-platinum and 5-fluorouracil for squamous cell carcinoma of the head and neck. Br J Cancer. 1986;54(5):755–760. doi: 10.1038/bjc.1986.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–814. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs C, Goffinet DR, Goffinet L, Kohler M, Fee WE. Chemotherapy as a substitute for surgery in the treatment advanced resectable head and neck cancer. A report from the Northern California Oncology Group. Cancer. 1987;60(6):1178–1183. doi: 10.1002/1097-0142(19870915)60:6<1178::aid-cncr2820600604>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Karp DD, Vaughan CW, Carter R, et al. Larynx preservation using induction chemotherapy plus radiation therapy as an alternative to laryngectomy in advanced head and neck cancer. A long-term follow-up report. Am J Clin Oncol. 1991;14(4):273–279. doi: 10.1097/00000421-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Pfister DG, Strong E, Harrison L, et al. Larynx preservation with combined chemotherapy and radiation therapy in advanced but resectable head and neck cancer. J Clin Oncol. 1991;9(5):850–859. doi: 10.1200/JCO.1991.9.5.850. [DOI] [PubMed] [Google Scholar]
- 15**.Wolf G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer: The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [The Veterans Affairs Larynx Cancer Study Group trial is a seminal phase 3 study that established the viability of larynx preservation with sequential chemotherapy and radiotherapy as an alternative to primary surgery with total laryngectomy.] [DOI] [PubMed] [Google Scholar]
- 16.Bradford CR, Wolf GT, Carey TE, et al. Predictive markers for response to chemotherapy, organ preservation, and survival in patients with advanced laryngeal carcinoma. Otolaryngol Head Neck Surg. 1999;121(5):534–538. doi: 10.1016/S0194-5998(99)70052-5. [DOI] [PubMed] [Google Scholar]
- 17**.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–899. doi: 10.1093/jnci/88.13.890. [The EORTC phase 3 study of larynx preservation for locally advanced pyriform sinus cancer confirmed and validated the results of the VALCSG trial.] [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708–2714. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [The RTOG 91-11 study established concurrent chemoradiotherapy as the preferred treatment regimen for larynx preservation, compared to either radiotherapy alone or sequential chemotherapy, for patients with locally advanced larynx cancer (excluding those with large volume T4 disease or invasion through the thyroid cartilage).] [DOI] [PubMed] [Google Scholar]
- 20.Forastiere AA, Maor M, Weber RS, et al. Long-term results of Intergroup RTOG 91-11: A phase III trial to preserve the larynx — induction cisplatin/5-FU and radiation therapy vs. concurrent cisplatin and radiation therapy vs. radiation therapy. J Clin Oncol. 2006;24(Suppl):5517. [Google Scholar]
- 21.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfister DG, Laurie SA, Weinstein GS, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693–3704. doi: 10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 23.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129(1):44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005;103(10):2073–2081. doi: 10.1002/cncr.20974. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin WJ., Jr Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110(3 Pt 2 Suppl 93):1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 26.Sewnaik A, Keereweer S, Al-Mamgani A, et al. High complication risk of salvage surgery after chemoradiation failures. Acta Otolaryngol. 2012;132(1):96–100. doi: 10.3109/00016489.2011.617779. [DOI] [PubMed] [Google Scholar]
- 27.Demard F, Chauvel P, Santini J, Vallicioni J, Thyss A, Schneider M. Response to chemotherapy as justification for modification of the therapeutic strategy for pharyngolaryngeal carcinomas. Head Neck. 1990;12(3):225–231. doi: 10.1002/hed.2880120306. [DOI] [PubMed] [Google Scholar]
- 28.Eisbruch A, Thornton AF, Urba S, et al. Chemotherapy followed by accelerated fractionated radiation for larynx preservation in patients with advanced laryngeal cancer. J Clin Oncol. 1996;14(8):2322–2330. doi: 10.1200/JCO.1996.14.8.2322. [DOI] [PubMed] [Google Scholar]
- 29.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 30.Vermorken JB, Remenar E, Van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 31**.Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24(4):593–598. doi: 10.1200/JCO.2005.01.2047. [This phase 2 study demonstrated the viability of a strategy of using tumor response to a single cycle of induction cisplatin and fluorouracil for selecting patients for organ preservation with concurrent chemoradiotherapy.] [DOI] [PubMed] [Google Scholar]
- 32.Fung K, Lyden TH, Lee J, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63(5):1395–1399. doi: 10.1016/j.ijrobp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33**.Worden FP, Moyer J, Lee JS, et al. Chemoselection as a strategy for organ preservation in patients with T4 laryngeal squamous cell carcinoma with cartilage invasion. Laryngoscope. 2009;119(8):1510–1517. doi: 10.1002/lary.20294. [This retrospective study is the first published report on the successful use of chemoradiation in T4 larynx cancer patients with thyroid cartilage invasion, and demonstrated that excellent functional and survival outcomes can be achieved even in such patients with the use of single cycle induction chemotherapy to select patients for larynx preservation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divi V, Worden FP, Prince ME, et al. Chemotherapy alone for organ preservation in advanced laryngeal cancer. Head Neck. 2010;32(8):1040–1047. doi: 10.1002/hed.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holsinger FC, Kies MS, Diaz EM, Jr., et al. Durable long-term remission with chemotherapy alone for stage II to IV laryngeal cancer. J Clin Oncol. 2009;27(12):1976–1982. doi: 10.1200/JCO.2008.17.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner M. Evolving strategies for combined-modality therapy for locally advanced head and neck cancer. Oncologist. 2007;12(8):967–974. doi: 10.1634/theoncologist.12-8-967. [DOI] [PubMed] [Google Scholar]
- 37.Posner MR, Norris CM, Wirth LJ, et al. Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol. 2009;20(5):921–927. doi: 10.1093/annonc/mdn752. [DOI] [PubMed] [Google Scholar]
- 38.Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101(7):498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 39.Cohen EEW, Karrison T, Kocherginsky M, et al. DeCIDE: A phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2012;30(Suppl) abstr 5500. [Google Scholar]
- 40.Paccagnella A, Mastromauro C, D'amanzo P, Ghi MG. Induction chemotherapy before chemoradiotherapy in locally advanced head and neck cancer: the future? Oncologist. 2010;15(Suppl 3):8–12. doi: 10.1634/theoncologist.2010-S3-08. [DOI] [PubMed] [Google Scholar]
- 41**.Haddad R, O'neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [This phase 3 study demonstrated that the addition of 3 cycles of induction docetaxel, cisplatin, and fluorouracil (TPF) before concurrent chemoradiotherapy did not improve overall survival, progression –free survival, or local or distant control compared to definitive chemoradiotherapy alone.] [DOI] [PubMed] [Google Scholar]
- 42.Trask DK, Wolf GT, Bradford CR, et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112(4):638–644. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinn S, Spector M, Light E, et al. Induction chemotherapy for organ preservation versus surgery for advanced oral cavity squamous cell carcinoma. Induction chemotherapy for organ preservation versus surgery for advanced oral cavity squamous cell carcinoma. 2012 [Google Scholar]
- 46.Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. The Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group. J Clin Oncol. 1994;12(8):1592–1599. doi: 10.1200/JCO.1994.12.8.1592. [DOI] [PubMed] [Google Scholar]
- 47.Richard JM, Sancho-Garnier H, Pessey JJ, et al. Randomized trial of induction chemotherapy in larynx carcinoma. Oral Oncol. 1998;34(3):224–228. doi: 10.1016/s1368-8375(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre JL, Ang KK. Larynx preservation clinical trial design: key issues and recommendations-a consensus panel summary. Int J Radiat Oncol Biol Phys. 2009;73(5):1293–1303. doi: 10.1016/j.ijrobp.2008.10.047. [DOI] [PubMed] [Google Scholar]