Abstract
Background
Individuals at clinical high-risk (CHR) who progress to fully psychotic symptoms have been observed to show a steeper rate of cortical gray matter reduction compared with those without symptomatic progression and with healthy controls. Whether such changes reflect processes associated with the pathophysiology of schizophrenia or exposure to antipsychotic drugs is unknown.
Methods
In this multisite study, 274 CHR cases, including 35 who converted to psychosis, and 135 healthy comparison subjects were scanned with MRI at baseline, 12-month follow-up, and/or the point of conversion for those who developed fully psychotic symptoms.
Results
In a traveling subjects sub-study, we observed excellent reliability for measures of cortical thickness and subcortical volumes. Controlling for multiple comparisons throughout the brain, CHR converters showed a steeper rate of gray matter loss in right superior frontal, middle frontal, and medial orbitofrontal cortical regions, as well as a greater rate of expansion of the third ventricle, compared with CHR non-converters and healthy controls. Differential tissue loss was present among cases who had not received antipsychotic medications during the inter-scan interval and was predicted by baseline levels of an aggregate measure of pro-inflammatory cytokines in plasma.
Conclusions
These findings demonstrate that the brain changes are not explained by exposure to antipsychotic drugs, but likely play a role in psychosis pathophysiology. Given that the cortical changes were more pronounced among cases with briefer durations of prodromal symptoms, contributing factors may predominantly play a role in acute-onset forms of psychosis.
Keywords: schizophrenia, psychosis, prodromal, MRI, prefrontal cortex, inflammation
Evidence of progressive loss of gray matter among clinical high-risk (CHR) individuals who convert to psychosis (1–7) suggests that disturbances in neuromaturational processes during the transition from adolescence to early adulthood (8–12) may play a role in psychosis onset. However, a number of questions remain to be answered before such an interpretation would be warranted.
First, this effect may be a secondary phenomenon. Antipsychotic drugs are associated with gray matter decline in animal models (13) and in patients with schizophrenia (14, 15), including first-episode patients (16). Because the follow-up scans for converting CHR cases in all longitudinal MRI studies occurred post-conversion, most of the converters (and relatively fewer of the non-converters) received antipsychotic drug treatment during the inter-scan interval. In the only prior study to examine this question, converters who had not received antipsychotics during the inter-scan interval (n=5) did not differ in rate of tissue loss from converters who did receive antipsychotics prior to the follow-up scan (n=5) (5). However, this comparison was almost certainly underpowered to detect a difference if one exists; in any case, a more conclusive result would emerge from comparing the rate of loss among converters not exposed to antipsychotics during the inter-scan interval with non-converters and controls.
If the accelerated gray matter loss associated with psychosis onset is not a secondary phenomenon, then it could be related to factors that participate in the pathophysiology of schizophrenia and related disorders, such as neuroinflammation (17). Neuroinflammatory markers are elevated in postmortem neural tissue from patients with schizophrenia (18), and these same markers are associated with microglial-mediated synaptic pruning and dendritic retraction in animal models (19), thus providing a potential mechanistic basis for the reduced neuropil seen in patients (20). Although neuroinflammatory processes initiated during prenatal stress exposures could play a role (21), activation of such processes in association with the synaptic pruning characteristic of adolescent brain development represents an influence more proximal to psychosis onset (12, 17, 20, 21). Recently, an elevation in plasma-based markers of inflammation and oxidative stress was found to precede and predict onset of psychosis among CHR cases (22). It remains to be determined whether such markers also predict the acceleration in gray matter loss around the time of psychosis onset.
Given that CHR cases are ascertained at different ages and at various points along the putative trajectory toward overt illness, such variability could obscure different subgroups of future converters with different profiles of change in brain structure over time. In particular, accelerated gray matter decline would be expected especially among cases with shorter durations from onset of prodromal symptoms to conversion (because the underlying pathology among cases with longer durations would likely be relatively more slowly progressing). In addition, although studies of early psychosis patients are generally consistent in showing lower volumes in dorsolateral prefrontal, superior temporal, and parahippocampal cortex (23, 24), prior longitudinal MRI studies are in conflict as to whether the steeper rate of loss in CHR converters is general or specific to these regions (1–7). However, these discrepancies may merely reflect regional differences in measurement reliability and/or between-study differences in statistical power.
In this multisite study, 274 CHR cases, including 35 who converted to psychosis, and 135 demographically comparable healthy comparison subjects were scanned with MRI at baseline and at 12-month follow-up and/or the point of conversion for those who developed fully psychotic symptoms (25). We hypothesized that converters would show steeper rates of gray matter reduction in prefrontal, superior temporal, and parahippocampal regions compared with non-converters and controls and that these effects would be present among cases without exposure to antipsychotic medications during the inter-scan interval. We further hypothesized that the cortical changes would be greatest among cases with a more recent onset of prodromal symptoms and that baseline levels of pro-inflammatory cytokines would predict the rate of gray matter loss especially among converters. We also evaluated statistical power to detect differential change across brain regions by incorporating information on reliability from a traveling subjects substudy.
Methods
Subjects
The study protocol and consent form was reviewed and approved by the Institutional Review Boards at each of the 8 data collection sites (UCLA, Emory, Beth Israel Deaconess Medical Center, Zucker Hillside Hospital, UNC, UCSD, Calgary, Yale). Participants were evaluated using the Structured Interview for Prodromal Syndromes (SIPS) (26) and the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM-IV) (27) at each assessment by trained interviewers who met high reliability standards (ICCs=0.92–0.96) (25). CHR cases met SIPS/SOPS criteria for a psychosis risk syndrome,(26) excluding cases who had ever met DSM-IV criteria for a psychotic disorder. Control participants were excluded if they met criteria for a psychotic disorder, had a first-degree relative with a current or past psychotic disorder, or met prodromal criteria. General exclusions included substance dependence, neurological disorder, or full scale IQ < 70.
Subjects included in this report are those with MRI scans at baseline (BL) and at 12-month follow-up (FU) or at the point of conversion to psychosis. Given that nearly all (37/41) of the converters with both BL and FU scans available had converted before the scheduled 12-month FU, few converting subjects had FU scans prior to conversion. To avoid mixing cases whose FU scans occurred pre and post-conversion, the 4 converters whose FU scans were obtained prior to conversion were excluded from the primary analyses (but included in secondary analysis). In total, 35 CHR cases who converted to psychosis, 239 CHR cases who did not convert, and 135 healthy comparison subjects had usable data and were included. These subjects were drawn from the larger pools of subjects (N’s=62 converters, 491 non-converters, and 224 controls) who had been scanned at baseline. Subjects with both BL and FU scans available did not differ from subjects with BL scans only on age, sex, education, parental education or socioeconomic class overall or in any group separately (all p-values > .30).1 Demographic characteristics of the three groups are shown in Table 1. There were no significant differences in age, sex, site of origin, or socioeconomic class by group. Non-converters had lower parental education than converters and controls, who did not differ. Converters and non-converters had a higher rate of substance use disorders than the controls but did not differ from each other. The inter-scan interval was significantly briefer among converters compared to non-converters and controls, who did not differ. Dividing at the median duration from onset of prodromal symptoms to FU scan among converters (26 months, range=2 to 149 months) produced subgroups of 18 cases with short durations and 17 with long durations. For non-converters, applying the same cutoff resulted in 140 cases with short durations and 76 with long durations (information on age at onset of symptoms was not available for the remaining 23 non-converters).
Table 1.
Demographic Characteristics and Mean Rates of Change in Subcortical Regions by Group.
| Characteristic | Converters1 (N=35) | Non-Converters (N=239) | Controls2 (N=135) | Statistic |
|---|---|---|---|---|
| Gender | ||||
| Male | 25 (71%) | 146 (61%) | 73 (54%) | χ2=3.9, p=0.13 |
| Female | 10 (29%) | 93 (39%) | 62 (46%) | |
| Race | ||||
| Caucasian | 19 (54%) | 134 (56%) | 73 (54%) | χ2=0.1, p=0.92 |
| Non-Caucasian | 16 (46%) | 105 (44%) | 62 (46%) | |
| Ethnicity | ||||
| Latino | 6 (17%) | 47 (20%) | 20 (15%) | χ2=1.4, p=0.49 |
| Non-Latino | 29 (83%) | 192 (80%) | 115 (85%) | |
| Substance Abuse Diagnosis | 5 (14%) | 24 (10%) | 4 (3%) | χ2=7.8, p=0.02 |
| Antipsychotics3 | 22 (63%) | 73 (30%) | 0 (0%) | χ2=78.8, p<.0001 |
| Means and SDs | ||||
| Age at Baseline | 18.8 (3.8) | 19.7 (4.2) | 20.5 (4.6) | F=2.7, p=0.06 |
| Parental Education | 6.7 (1.4) | 6.2 (1.4) | 6.8 (1.4) | F=7.7, p=0.0005 |
| Income | 4.7 (1.9) | 4.7 (1.9) | 4.6 (1.8) | F=0.1, p=0.89 |
| Inter-scan Interval | 0.9 (0.5) | 1.0 (0.3) | 1.1 (0.4) | F=7.1, p=0.001 |
| Positive Symptoms BL4 | 13.5 (3.1) | 11.9 (4.1) | 1.1 (1.7) | F=479.6, p<.0001 |
| Positive Symptoms FU4 | 18.3 (4.4) | 7.8 (4.6) | 0.7 (1.4) | F=329.7, p<.0001 |
| Antipsychotic dosage5 | 93.3 (133.7) | 97.5 (122.3) | N/A | |
| Change in Brain Volumes6 | FDR-Corrected P | |||
| Left Thalamus | −0.005 (0.06) | 0.010 (0.06) | 0.010 (0.04) | 0.30 |
| Right Thalamus | 0.011 (0.09) | 0.004 (0.04) | 0.005 (0.04) | 0.69 |
| Left Caudate | 0.015 (0.05) | −0.003 (0.04) | −0.004 (0.03) | 0.20 |
| Right Caudate | 0.010 (0.05) | −0.004 (0.04) | −0.004 (0.03) | 0.21 |
| Left Putamen | 0.014 (0.04) | −0.001 (0.04) | −0.001 (0.04) | 0.20 |
| Right Putamen | 0.006 (0.10) | 0.005 (0.05) | 0.001 (0.03) | 0.69 |
| Left Pallidum | 0.051 (0.19) | 0.001 (0.12) | 0.006 (0.11) | 0.20 |
| Right Palldium | 0.017 (0.10) | 0.003 (0.06) | −0.006 (0.09) | 0.30 |
| Left Hippocampus | −0.013 (0.06) | 0.003 (0.04) | −0.001 (0.04) | 0.62 |
| Right Hippocampus | −0.009 (0.04) | 0.001 (0.03) | −0.002 (0.03) | 0.30 |
| Left Amygdala | 0.022 (0.15) | 0.016 (0.08) | −0.009 (0.07) | 0.20 |
| Right Amygdala | 0.036 (0.13) | 0.016 (0.09) | −0.001 (0.08) | 0.26 |
| Left Accumbens | −0.032 (0.15) | −0.019 (0.10) | −0.008 (0.09) | 0.63 |
| Right Accumbens | −0.028 (0.16) | −0.009 (0.09) | −0.023 (0.07) | 0.36 |
| Left Lateral Ventricle | 0.064 (0.13) | 0.024 (0.17) | 0.011 (0.07) | 0.31 |
| Right Lateral Ventricle | 0.064 (0.15) | 0.024 (0.16) | 0.011 (0.07) | 0.33 |
| Brain Stem | 0.007 (0.04) | 0.009 (0.03) | 0.009 (0.02) | 0.69 |
| Third Ventricle | 0.086 (0.12) | 0.015 (0.10) | 0.011 (0.07) | 0.01 |
| Fourth Ventricle | 0.061 (0.14) | 0.006 (0.12) | 0.002 (0.06) | 0.13 |
A SIPS diagnosis of a psychotic syndrome refers to psychotic symptoms of particular intensity (eg, delusional conviction) and frequency or duration (≥ 1 h/d for ≥ 4 d/wk during the past month) or of particular impact (seriously disorganizing or dangerous), designed to operationalize the threshold for a DSM-IV psychotic disorder diagnosis. DSM-IV diagnoses of converters were as follows: schizophrenia (N=9), schizophreniform disorder (N=9), schizoaffective disorder, depressed type (N=2), bipolar disorder with psychotic features (N=3), psychosis not otherwise specified (N=12).
The only diagnostic exclusions for control subjects were psychotic disorders. A total of 17 (13%) of the controls had one or more DSM-IV diagnoses (8 with depression, 5 with anxiety disorders, 4 with substance use disorders, 2 with attention deficit disorder, 1 with somatization disorder, and 1 with oppositional-defiant disorder).
In addition to (second-generation) antipsychotics, 10 (29%) of the converters and 104 (44%) of the non-converters received anti-depressant medications during the inter-scan interval. Prescriptions for other types of medicine (stimulants, anticonvulsants, benzodiazapines) occurred infrequently (4 or fewer converters).
Positive symptoms reflect sum of the P1–P5 ratings on the Scale for the Assessment of Prodromal Symptoms.
In chlorpromazine equivalent units (65).
Mean (SD) annualized rates of change (negative values indicate shrinkage, positive values indicate expansion), with False-Discovery Rate (FDR) corrected p-values. Multiplying the values by 100 provides an index of annualized percentage change.
As this was a naturalistic study, subjects were treated in their respective communities according to prevailing standards and the judgment of the treating clinicians, who were often primary care physicians rather than psychiatrists. We collected and coded information on medication prescriptions at baseline, 6- and 12-month follow-up (and at the conversion assessment when relevant). Based on this information, 13 (37%) of the 35 converters and 166 (70%) non-converters had not received antipsychotics during the inter-scan interval.
MRI Scans
Five sites operated Siemens scanners and three sites operated GE scanners, all at 3 Tesla. All Siemens sites used a 12-channel head coil and all GE sites used an 8-channel head coil. Sequence parameters were optimized for each scanner manufacturer, software version and coil configuration according to the ADNI protocol (http://adni.loni.ucla.edu/research/protocols/mri-protocols/). Scans were acquired in the sagittal plane with a 1mm × 1mm in-plane resolution and 1.2mm slice thickness. Siemens scanners used an MPRAGE sequence with a 256 (axial) × 240 (sagittal) × 176 (coronal) mm field of view, TR/TE/TI=2300/2.91/900ms and a 9 degree flip angle, while GE scanners used an IR-SPGR sequence (efgre3d_cs) with a 26cm field of view, TR/TE/TI=7.0/minimum full/400ms and an 8 degree flip angle.
Image Processing, Quality Assurance, and Reliability
All MR images were processed using Freesurfer v5.2 (http://surfer.nmr.mgh.harvard.edu/) at Yale by investigators who had participated in the Freesurfer training course. Surface-based cortical reconstruction was performed to extract thickness measures by calculating the shortest distance from each point on the gray/white boundary to the pial surface at each vertex, along with subcortical volumetric segmentation (28–32). The subcortical segmentation procedure assigns a neuroanatomical label to each voxel of the MRI volume using a probabilistic atlas and a Bayesian classification rule (30). The reconstructed baseline and follow-up scans were further processed using Freesurfer’s longitudinal stream to extract change in thickness and volume estimates (33). This processing stream initializes each time point scan by utilizing an unbiased within-subject template space and average image (34), created by robust, inverse consistent registration (35), which has been shown to significantly increase statistical power for detecting subtle changes over time (33). The Supplementary Material provides details of the quality assurance procedures.
Each of the 8 sites also recruited one healthy subject (4 males, 4 females) who was scanned twice on successive days at every site to permit evaluation of between-site and test-retest reliabilities using intraclass correlations (ICCs). In addition, the ADNI structural phantom was scanned at each site and processed using the AQUAL2 algorithm (36). We have previously reported on phantom-based performance metrics as well as two processing streams applied to the traveling human data: cortical pattern matching and voxel-based morphometry (37). Briefly, the 8 scanners performed within the range of accepted tolerance according to the phantom-based metrics, and all achieved excellent reliabilities for intracranial, brain, gray matter, white matter, and subcortical volumes. Superior reliability was achieved when using surface-based registration (as implemented in Cortical Pattern Matching) compared with standard VBM registration and when applying a global scaling correction. Because Freesurfer incorporates both surface-based registration and global scaling correction but also has a built-in module for voxel-based correction of the Type I error rate (which Cortical Pattern Matching does not), we opted to process the imaging data from the primary study using Freesurfer, and in the Supplementary Material we report the test-retest ICCs for Freesurfer-derived measures of cortical thickness and subcortical and ventricular volumes from the traveling subject substudy.
Blood Analytes
As part of a pilot study, blood samples obtained at the BL assessment for 87 of the subjects with BL and FU MRI scans available (14 converters, 38 non-converters, 35 controls) were processed using the Luminex® Human DiscoveryMap® bead-based multiplex immunoassay. Blood samples were collected in Becton Dickenson P100 tubes containing EDTA and proprietary protein stabilizers along with a mechanical separator and stored at −80°C until analysis. Analytes were transformed to z-scores based on the means and standard deviations of the control group. Although particular cytokines may have differential functions (e.g., pro- vs anti-inflammatory) in different contexts, several included in the Luminex panel (i.e., TNF-α, IL-2, Interferon-γ) are consistent activators of the M1 cytotoxic phenotype of microglia (38–41); these were summed together into an index of pro-inflammatory signaling. Similarly, four analytes most consistently associated with the M2 repair and regeneration phenotype of microglia (i.e., IL-10, GM-CSF, IL-1RN, Chemokine [C-C motif] ligand 2) (38–41) were summed together to form an index of anti-inflammatory signaling.
Statistical Analysis
Imaging measures were first transformed to annualized rates of change (ARCH) in each cortical voxel and each subcortical and ventricular region of interest (ROI), where ROIARCH = ((ROIFU − ROIBL)/ROIBL)/Interval and where Interval is the time between BL and FU scans in years. This approach was preferred to repeated-measures ANOVA because the inter-scan interval varied across subjects (but results that were significant based on annualized rate of change were also significant using repeated measures ANOVA). Primary hypothesis testing was applied to measures of cortical thickness in Freesurfer using the False-Discovery Rate (FDR) correction for multiple comparisons at the voxel level. In addition, volumetric measures of the ventricular system and subcortical nuclei were evaluated in separate analyses of variance (ANOVAs), treating hemisphere as a repeated measure where appropriate, and controlling for multiple comparisons by FDR correction. Models included age at BL, gender, site, and group (Converter, Non-Converter, and Control) as predictors.
Secondary analyses were conducted to determine the contributions of antipsychotic drug exposure, duration of prodromal symptoms, and the plasma indices of pro- and anti-inflammatory cytokines to the regional measures showing evidence of differential change over time by group status. In one set of ANOVAs, converters with and without antipsychotic drug exposure during the inter-scan interval were compared with non-converters with and without antipsychotic use and controls. In another set of ANOVAs, converters with short durations of prodromal symptoms with compared to those with longer durations, non-converters with short and long durations, and controls. A third set of analyses evaluated the linear regressions of change in cortical thickness measures on BL levels of pro- and anti-inflammatory cytokines.
Results
Baseline Differences
There were no significant differences between converters, non-converters and controls in terms of cortical thickness or subcortical or ventricular volumes at BL, nor did BL measures vary by duration of prodrome among converters.
Rates of Change
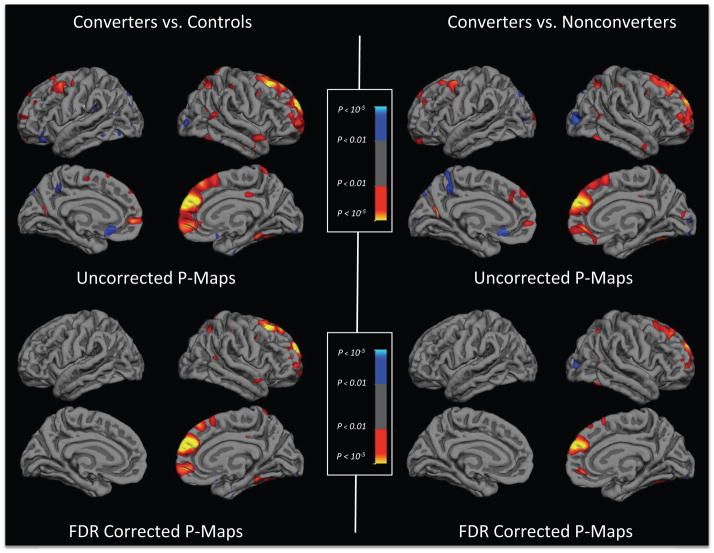
Figure 1 shows statistical brain atlases plotting differences in the mean annualized rates of change in cortical thickness among converting CHR subjects, non-converting CHR subjects, and healthy controls. In the uncorrected maps (upper panels), converters showed steeper rates of reduction in cortical thickness in a large cluster including the left and right superior frontal, middle frontal, and medial orbitofrontal gyri compared with both controls and non-converters, and in smaller clusters in the right superior and inferior parietal cortex, superior temporal gyrus and parahippocampal gyrus, compared with controls. After applying an FDR correction thresholded at p≤0.01 (two-tailed), only the differences in right superior frontal, middle frontal, and medial orbitofrontal regions remained significant for both contrasts (Figure 1, lower panels). There were no significant differences in rates of change between non-converting CHR subjects and controls before or after FDR correction. In parallel analyses of the volumes of subcortical and ventricular ROIs (Table 1), converters showed significantly greater expansion of the third ventricle compared with non-converters and controls, who did not differ from each other.
Figure 1.
Statistical brain atlases plotting differences in the mean annualized rates of change in cortical thickness among converting CHR subjects (n=35), non-converting CHR subjects (n=239), and healthy controls (n=135). Panels on the left show differences between converters and controls and panels on the right show differences between converters and non-converters. In the uncorrected maps (upper panels), compared with both non-converters and controls, converters showed greater thinning (warmer colors) in left and right superior frontal, middle frontal, and medial orbitofrontal gyri and in the right superior and inferior parietal cortex, superior temporal gyrus and parahippocampal gyrus. After applying an FDR correction (p ≤ 0.01; lower panels), only the differences in right superior frontal, middle frontal, and medial orbitofrontal regions remained significant for both contrasts. The small clusters showing greater expansion (cooler colors) in the converters compared with controls in the uncorrected maps did not survive correction for multiple comparisons. There were no differences between non-converters and controls before or after FDR correction.
Given that the reliabilities of thickness measures from most regions of cortex and most subcortical volumes were equivalent to or higher than that in right superior frontal and medial orbitofrontal cortex (ICCs=0.92 and 0.68, respectively; see Supplementary Material), the topography of regions showing differential change over time in converters must primarily reflect differences in the true effect sizes rather than measurement reliability. The effect size observed in right superior frontal and medial orbitofrontal gyrus was quite large (d=−1.0). In fact, there were medium to large effect sizes (d=−0.30 to −0.63) across most of the remaining bilateral prefrontal cortex (including middle and inferior frontal gyri and frontal pole) and bilateral parahippocampal gyrus.
A mask applied to the FDR-corrected p-maps was used to isolate the prefrontal regions showing significantly greater thinning in converters compared with non-converters and controls; the annualized rates of change for this measure and third ventricle volume were carried forward for use in subsequent analyses.
Antipsychotic Medications and Timing of Follow-Up Scan
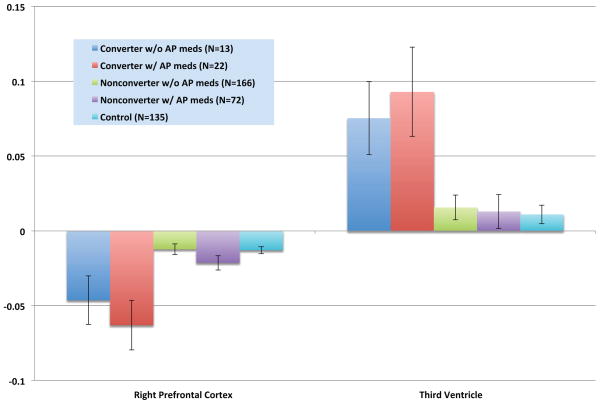
Converters without any anti-psychotic drug exposure during the inter-scan interval showed significantly greater reduction in right prefrontal cortex thickness and significantly greater expansion of the third ventricle compared with non-converters with and without such exposure and with healthy controls, but did not differ from converters with anti-psychotic drug exposures on these measures (Figure 2). On average, the follow-up scan for converters occurred 141 days (SD = 117 days) after conversion. Neither this interval, nor the duration or dosage of antipsychotic medications during the inter-scan interval, was significantly correlated with the rate of prefrontal cortical thinning or third ventricle expansion (Table 2).
Figure 2.
Mean annualized rates of change in right prefrontal cortex thickness and third ventricle volume by anti-psychotic (AP) drug exposure during the inter-scan interval. Group differences were significant for both variables (F=7.46, p=0.000008 for right prefrontal cortex; F=3.74, p=0.005 for third ventricle). Converters with and without AP medications showed significantly steeper reduction in right prefrontal thickness and significantly greater expansion of the third ventricle compared with non-converters with and without AP medications and controls.
Table 2.
Correlations of annualized rates of change in right prefrontal cortex thickness and third ventricle volume with temporal factors.
| Overall Sample | Converters Only | |||||
|---|---|---|---|---|---|---|
| Variable | N | Right PFC | 3rd Ventricle | N | Right PFC | 3rd Ventricle |
| Age at BL scan | 409 | 0.01 | 0.01 | 35 | −0.16 | 0.22 |
| Inter-scan interval | 409 | 0.12§ | −0.09¶ | 35 | 0.46§ | −0.46§ |
| Duration of prodrome1 | 251 | 0.13¶ | 0.04 | 35 | 0.36¶ | −0.31 |
| Duration of AP medications1 | 273 | −0.03 | −0.03 | 35 | 0.11 | −0.20 |
| Dosage of AP medications1 | 273 | −0.01 | −0.05 | 35 | 0.07 | −0.11 |
| Interval from conversion to scan | N/A | N/A | N/A | 35 | 0.15 | −0.23 |
Clinical High-Risk subjects only;
p<0.01;
p<0.05.
Duration of Prodrome
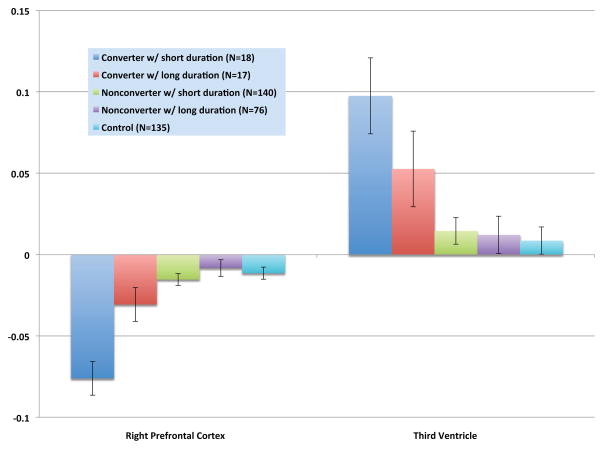
CHR cases with a shorter duration of prodromal symptoms showed a significantly steeper reduction in right prefrontal thickness compared with long-duration converters, short- and long-duration non-converters, and controls; they also showed significantly greater third ventricle expansion compared with all of the other groups except for long-duration converters (Figure 3). Long-duration converters showed a steeper rate of prefrontal thinning and third ventricular expansion compared with long-duration non-converters and controls, but did not differ from short-duration non-converters on these measures.
Figure 3.
Mean annualized rates of change in right prefrontal cortex thickness and third ventricle volume by duration of prodromal symptoms. Group differences were significant for both variables (F=9.68, p=0.0000002 for right prefrontal cortex; F=3.87, p=0.004 for third ventricle). Converters with short durations showed significantly steeper reduction in right prefrontal thickness compared with long-duration converters, short- and long-duration non-converters, and controls; they also showed significantly greater third ventricle expansion compared with all of the groups except for long-duration converters. Converters with long durations showed significantly steeper reduction in right prefrontal thickness and significantly greater expansion of the third ventricle compared with long-duration non-converters and controls.
Markers of Inflammation
Although the three groups did not differ significantly from each other in mean levels of pro-inflammatory (F=0.95, p=0.39) or anti-inflammatory (F=0.98, p=0.38) cytokines at BL, the rate of prefrontal cortical thinning was significantly associated with higher levels of pro-inflammatory markers in the sample overall, and this inverse correlation was significantly greater among converters than among non-converters and controls (Table 3). Pro-inflammatory cytokines were not associated with third ventricular expansion, and anti-inflammatory cytokines were not associated with rate of change in either of the MRI measures.
Table 3.
Correlations of annualized rates of change in right prefrontal cortex thickness and third ventricle volume with serum inflammatory indices by outcome group.
| Region | Group | N | Pro-inflammatory cytokines* | Anti-inflammatory cytokines |
|---|---|---|---|---|
| Right PFC | Control | 35 | 0.12 | −0.05 |
| Non-Converter | 38 | −0.32¶ | −0.02 | |
| Converter | 14 | −0.65§ | 0.38 | |
| Overall | 87 | −0.37§ | 0.06 | |
| 3rd Ventricle | Control | 35 | −0.18 | −0.10 |
| Non-Converter | 38 | 0.09 | 0.06 | |
| Converter | 14 | −0.15 | −0.41 | |
| Overall | 87 | −0.02 | −0.10 |
p<0.01;
p<0.05;
the relationship between pro-inflammatory cytokines and right PFC contraction was significantly greater in converters than in non-converters and controls (F=10.1, df=3,83, p=0.00001).
Discussion
Individuals at clinical risk who develop psychosis show a steeper rate of gray matter loss in right superior frontal, middle frontal, and medial orbitofrontal cortex, as well as a greater rate of expansion of the third ventricle, compared with at risk individuals who do not convert and healthy comparison subjects. Importantly, these effects were present among cases who had not received antipsychotic medications during the inter-scan interval, demonstrating that the differential tissue loss associated with onset of psychosis is not explained by exposure to antipsychotic drugs. This finding represents a critical advance, given that antipsychotics clearly complicate the interpretation of progressive brain changes among patients in the first-episode of psychosis (13–16). A similar result was observed among unmedicated familial high-risk subjects who showed increasing symptom severity (42). It is thus possible that progressive gray matter reduction is in some way linked to the pathophysiology of onset of psychosis.
Prior studies of CHR cases have reported steeper reductions in prefrontal, superior temporal, and parahippocampal regions among converters compared with non-converters (1–7). The sample sizes in those studies were significantly smaller than in the present one, and none employed a voxel-based correction for multiple comparisons. Because the traveling subjects substudy revealed comparably high test-retest reliability across most cortical and subcortical regions, the topographical pattern of differential change in this study primarily reflects regional variation in effect size, which was sufficiently high in right superior and medial prefrontal regions and in the third ventricle to survive rigorous control for multiple comparisons throughout the brain. This study thus strongly confirms prior evidence of differential tissue loss in prefrontal cortex among CHR converters to psychosis, with a topography highly similar to that in the only prior study using surface-based anatomical comparison (2). The primary advantage of the voxel-based FDR-corrected threshold employed here is that it protects against false positive findings given the number of tests conducted and distribution of observed p-values. Thus, we can have high confidence that the effects that were significant are true positives. Adoption of this conservative threshold might also lead to some false negative results. In fact, there were medium to large effect sizes across most of the remaining bilateral prefrontal cortex (including left and right middle and inferior frontal gyri and frontal pole) and bilateral parahippocampal gyrus, as well as in some circumscribed regions of temporal and parietal cortex, overlapping the regions showing differential change in prior studies (1–7).
Higher levels of a plasma-based aggregate index of pro-inflammatory cytokines at baseline were strongly predictive of steeper rates of gray matter reduction in right prefrontal cortex among CHR cases who converted to psychosis. Given that peripheral cytokines can affect brain function (43), and because the cytokines included in the pro-inflammatory index are potent activators of the M1 cytotoxic phenotype of microglia that result in synaptic pruning and dendritic retraction (38–41), it is plausible to hypothesize a mechanistic link between neuroinflammation (i.e., microglial activation) and progressive gray matter loss in individuals who develop psychosis, a hypothesis that should be tested using more direct indicators of neuroinflammatory processes in CHR subjects.
The steeper rate of decline in right prefrontal cortex and expansion of the third ventricle were more pronounced among converters with shorter durations than those with longer durations, but the long-duration converters also showed differential change compared with non-converters and controls. This pattern suggests that a steeper rate of tissue loss in these regions, or the factors promoting it, may help to trigger an earlier onset of psychosis. Because there were no significant differences between converters, non-converters and controls in cortical thickness or subcortical volumes at the BL assessment controlling for multiple comparisons, this finding suggests that the reductions in gray matter emerge around the time of onset of psychosis, rather than earlier, among CHR cases. Some but not all prior studies have detected anatomical differences at baseline between CHR individuals who do and do not later convert, despite the use of much smaller sample sizes than that in this study, by focusing on a small number of regions of interest or by otherwise employing a less conservative statistical threshold (1, 3, 4, 44–62). Nevertheless, it must be kept in mind that the CHR criteria are sensitive primarily to acute onset forms of psychosis and may therefore underrepresent cases with more insidious onsets, who might manifest loss of gray matter volume at earlier assessment points due to early life risk exposures, such as fetal hypoxia (63). Thus, the extent to which future converters manifest anatomical deficits at baseline could also potentially reflect the relative proportions of converters with insidious vs. acute onsets in the sample under study.
The question arises as to whether gray matter reduction per se is a causal factor in onset of psychosis. Given that the distributions of cortical gray matter volumes among patients with schizophrenia and healthy controls overlap by 50–75%, it does not appear that there is a critical threshold of cortical thickness (in any one region or combination of regions) below which psychosis develops. Nevertheless, there is likely to be a threshold of integrated synaptic activity and regional functional connectivity that does discriminate those in a psychotic state from others. Gray matter reduction is perhaps best conceptualized as a marker of a set of processes that contributes to reductions in integrated synaptic activity and functional connectivity, which in turn might represent a proximal sufficient mechanism of psychosis onset. Although there are likely mechanisms that disrupt synaptic activity and functional connectivity in psychosis-relevant networks without affecting cortical volume, gray matter reduction is nevertheless an observable and reliably measured phenomenon that appears to play a role in a significant proportion of cases and thereby provides insight into the network of brain regions likely to participate in psychosis onset in general.
It is important to note that in this study, as well as in all prior longitudinal studies of CHR cases (1–7), the follow-up scans for converters occurred after the point of conversion. Although the change in gray matter volume was not correlated with the interval between onset of psychosis and the second scan, it is not yet known whether significant change occurs prior to onset of psychosis. It is, however, reassuring that the absolute magnitude of change in the right prefrontal ROI among the four converters who had FU scans before the point of conversion (i.e., mean±SD=−0.11±0.08mm) was comparable to that among cases whose FU scans occurred post-conversion (−0.12±0.11mm) and was greater in magnitude compared with non-converters (−0.05±0.13mm) and controls (−0.05±0.10mm). When these four cases were included with those who had FU scans post-conversion, converters continued to show significantly greater contraction of right prefrontal cortex (F[2,401]=12.66, p=0.000004) compared with non-converters and controls. Given that in this study and in a prior large study of prodromal youth at these same sites (64), more than 70% of the conversions had occurred by 10-months, future studies are encouraged to time MRI assessments at 2-month intervals from the baseline to ensure at least 2 assessment points prior to conversion for the majority of cases.
In conclusion, CHR cases who convert to psychosis show a steeper rate of thinning in prefrontal cortex, an effect that is independent of exposure to antipsychotic drugs and is predicted by plasma analytes indicative of an inflammatory process. Future work is encouraged to confirm a neuroinflammatory signature in the regions showing gray matter loss and to determine whether inflammation precedes and predicts the gray matter loss or is a consequence of it.
Supplementary Material
Acknowledgments
This work was supported by a collaborative U01 award from the National Institute of Mental Health at the National Institutes of Health (MH081902 to TDC; MH081857 to BAC; MH081988 to EW; MH081928 to LJS; MH082004 to DP; MH082022 to KC; MH081984 to JA; MH066160 to SWW) and NIMH P50 MH066286 (CEB), NIMH P50 MH080272, and the Commonwealth of Massachusetts (SCDMH82101008006, LIS).
Additional NAPLS personnel involved in this study are listed separately below by site.
UCLA: Katherine Karlsgodt, Dylan Gee, Jennifer Forsyth, Peter Bachman, Jamie Zinberg, Sandra De Silva, Angela Andaya, Cristina Roman, Shauna McManus, Sarah Marvin, Miguel Villodas, Nichol Ferng, Anna Xu, Wendy Lau
Emory University: Hanan Trotman, Xiaoping Hu, Lei Zhou, Stephan Hamann, Arthur Ryan, Erica Duncan, Joy Brasfield, Arthur Ryan, Anna Pless, Ben Perlow, Dan Shapiro, Sandy Goulding, Carrie Holtzman, Allison MacDonald, Arthur Ryan, Erin Jones, David Lui, Ben Perlow
Harvard University/Beth Israel Deaconess Medical Center: Kristen Woodberry, Anthony Giuliano, Michelle Friedman-Yakoobian, William Stone, Heidi Thermenos, Margaret Niznikiewicz, Robert McCarley, Linda Tucker, Corin Pilo, Maryan Picard, Benjamin Brent, Ann Cousins, Raquelle Mesholam-Gately, Andrea Gnong Granato, Janine Rodenhiser-Hill, Joanne Wojcik, Lauren Gibson, Richard Juelich, Danbee Kim, Grace Min, Rachel Serur, Beril Yaffe
Zucker Hillside Hospital: Andrea Auther, Danielle McLaughlin, Doreen Olvet, Peter Kingsley, Ricardo Carrion, Gary Brucato, Ruth Olsen, Tricia Taylor, Rita Barsky, Stephanie Snyder, Miranda Farabaugh, Jeremy Chang, Kristin Candan
University of North Carolina-Chapel Hill: David Penn, Ayse Belger, Franc Donkers, Andrea Pelletier, Karen Graham, Bryan Landaas, Andrea Pelletier, Ellen Rothman, Jennifer Nieri, Bryan Landaas, Katie Lansing, Andrea Pelletier
University of California, San Diego: Robert Heaton, Greg Brown, Heline Mirzakhanian, Greg Light, Tracy Alderman, Isabel Domingues, Nasra Haroun, Steve Reding, Jason Nunag, Daniel Roman, Clara Robles
University of Calgary: Jim Kennedy, Richard Frayne, Brad Goodyear, Thomas Raedler, Neelan Pillay, Jacque Stowkowy, Jack Addington, Lu Lui, Danijela Piskulic, Lisa McGregor, Angie Kumar, Catherine Marshall, Nora MacQuarrie, Kendra Smith, Emma Fitton, Erin Falukozi, Mark Colijn, Aaron Peterson, Sona Sandu, Lianne Legere
Yale University: Keith A. Hawkins, Maolin Qiu, R. Todd Constable, Godfrey D. Pearlson, Jason K. Johannesen, Handan Gunduz-Bruce, John R. Saksa, Barbara C. Walsh, Nicole Popp-Santamauro, Joshua Kenney, Brenton Roman, Andrew Carlquist
Footnotes
Subjects who were not scanned had a slightly lower level of education (11.1 vs 11.9 years) and greater proportion of females (49% vs 42%) compared with those who were scanned, but did not differ on other demographic characteristics.
Conflict of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 2.Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pfluger MO, Stieglitz RD, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 7.Walter A, Studerus E, Smieskova R, Kuster P, Aston J, Lang UE, et al. Hippocampal volume in subjects at high risk of psychosis: a longitudinal MRI study. Schizophr Res. 2012;142:217–222. doi: 10.1016/j.schres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Faludi G, Mirnics K. Synaptic changes in the brain of subjects with schizophrenia. Int J Dev Neurosci. 2011;29:305–309. doi: 10.1016/j.ijdevneu.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 10.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 12.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 13.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 16.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res. 2013;147:24–31. doi: 10.1016/j.schres.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M. Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol. 2011;256:219–226. doi: 10.1016/j.taap.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Perkins DO, Jeffries CD, Addington J, Bearden CE, Cardenhead KS, Cannon TD, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: Preliminary results from the NAPLS project. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Des. 2012;18:486–494. doi: 10.2174/138161212799316253. [DOI] [PubMed] [Google Scholar]
- 25.Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, et al. North American Prodrome Longitudinal Study (NAPLS 2) overview and recruitment. Schizophr Res. 2012;142:77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGlashan TH, Walsh BC, Woods SW. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. Oxford: Oxford University Press; 2010. [Google Scholar]
- 27.First M, Spitzer RL, Gibbon M, Williams B, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 32.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 33.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunter JL, Bernstein MA, Borowski BJ, Ward CP, Britson PJ, Felmlee JP, et al. Measurement of MRI scanner performance with the ADNI phantom. Med Phys. 2009;36:2193–2205. doi: 10.1118/1.3116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon TD, Sun F, McEwen SJ, Papademetris X, He G, van Erp TG, et al. Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- 39.Prajeeth CK, Lohr K, Floess S, Zimmermann J, Ulrich R, Gudi V, et al. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, et al. Dynamic structural remodelling of microglia in health and disease: A review of the models, the signals and the mechanisms. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 42.McIntosh AM, Owens DC, Moorhead WJ, Whalley HC, Stanfield AC, Hall J, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Besedovsky HO, del Rey A. Central and peripheral cytokines mediate immune-brain connectivity. Neurochem Res. 2011;36:1–6. doi: 10.1007/s11064-010-0252-x. [DOI] [PubMed] [Google Scholar]
- 44.Dazzan P, Soulsby B, Mechelli A, Wood SJ, Velakoulis D, Phillips LJ, et al. Volumetric Abnormalities Predating the Onset of Schizophrenia and Affective Psychoses: An MRI Study in Subjects at Ultrahigh Risk of Psychosis. Schizophrenia bulletin. 2011 doi: 10.1093/schbul/sbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Hannan KL, Wood SJ, Yung AR, Velakoulis D, Phillips LJ, Soulsby B, et al. Caudate nucleus volume in individuals at ultra-high risk of psychosis: a cross-sectional magnetic resonance imaging study. Psychiatry research. 2010;182:223–230. doi: 10.1016/j.pscychresns.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Iwashiro N, Suga M, Takano Y, Inoue H, Natsubori T, Satomura Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophrenia research. 2012;137:124–131. doi: 10.1016/j.schres.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Jung WH, Jang JH, Byun MS, An SK, Kwon JS. Structural brain alterations in individuals at ultra-high risk for psychosis: a review of magnetic resonance imaging studies and future directions. Journal of Korean medical science. 2010;25:1700–1709. doi: 10.3346/jkms.2010.25.12.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophrenia bulletin. 2011;37:839–849. doi: 10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mechelli A, Riecher-Rossler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Archives of general psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 52.Peters BD, Dingemans PM, Dekker N, Blaas J, Akkerman E, van Amelsvoort TA, et al. White matter connectivity and psychosis in ultra-high-risk subjects: a diffusion tensor fiber tracking study. Psychiatry research. 2010;181:44–50. doi: 10.1016/j.pscychresns.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Phillips LJ, Velakoulis D, Pantelis C, Wood S, Yuen HP, Yung AR, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophrenia research. 2002;58:145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Yucel M, Yung AR, Wood SJ, Phillips LJ, Berger GE, et al. Adhesio interthalamica in individuals at high-risk for developing psychosis and patients with psychotic disorders. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:1708–1714. doi: 10.1016/j.pnpbp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Yung AR, Yucel M, Wood SJ, Phillips LJ, Harding IH, et al. Prevalence of large cavum septi pellucidi in ultra high-risk individuals and patients with psychotic disorders. Schizophrenia research. 2008;105:236–244. doi: 10.1016/j.schres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, et al. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophrenia research. 2008;103:1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 57.Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophrenia research. 2008;102:141–149. doi: 10.1016/j.schres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Witthaus H, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Gallinat J, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry research. 2009;173:163–169. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Witthaus H, Mendes U, Brune M, Ozgurdal S, Bohner G, Gudlowski Y, et al. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. Journal of psychiatry & neuroscience: JPN. 2010;35:33–40. doi: 10.1503/jpn.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood SJ, Kennedy D, Phillips LJ, Seal ML, Yucel M, Nelson B, et al. Hippocampal pathology in individuals at ultra-high risk for psychosis: a multi-modal magnetic resonance study. NeuroImage. 2010;52:62–68. doi: 10.1016/j.neuroimage.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Wood SJ, Yucel M, Velakoulis D, Phillips LJ, Yung AR, Brewer W, et al. Hippocampal and anterior cingulate morphology in subjects at ultra-high-risk for psychosis: the role of family history of psychotic illness. Schizophrenia research. 2005;75:295–301. doi: 10.1016/j.schres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Ziermans TB, Durston S, Sprong M, Nederveen H, van Haren NE, Schnack HG, et al. No evidence for structural brain changes in young adolescents at ultra high risk for psychosis. Schizophrenia research. 2009;112:1–6. doi: 10.1016/j.schres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A. Developmental brain abnormalities in the offspring of schizophrenic mothers. I. Contributions of genetic and perinatal factors. Arch Gen Psychiatry. 1993;50:551–564. doi: 10.1001/archpsyc.1993.01820190053006. [DOI] [PubMed] [Google Scholar]
- 64.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.