Abstract
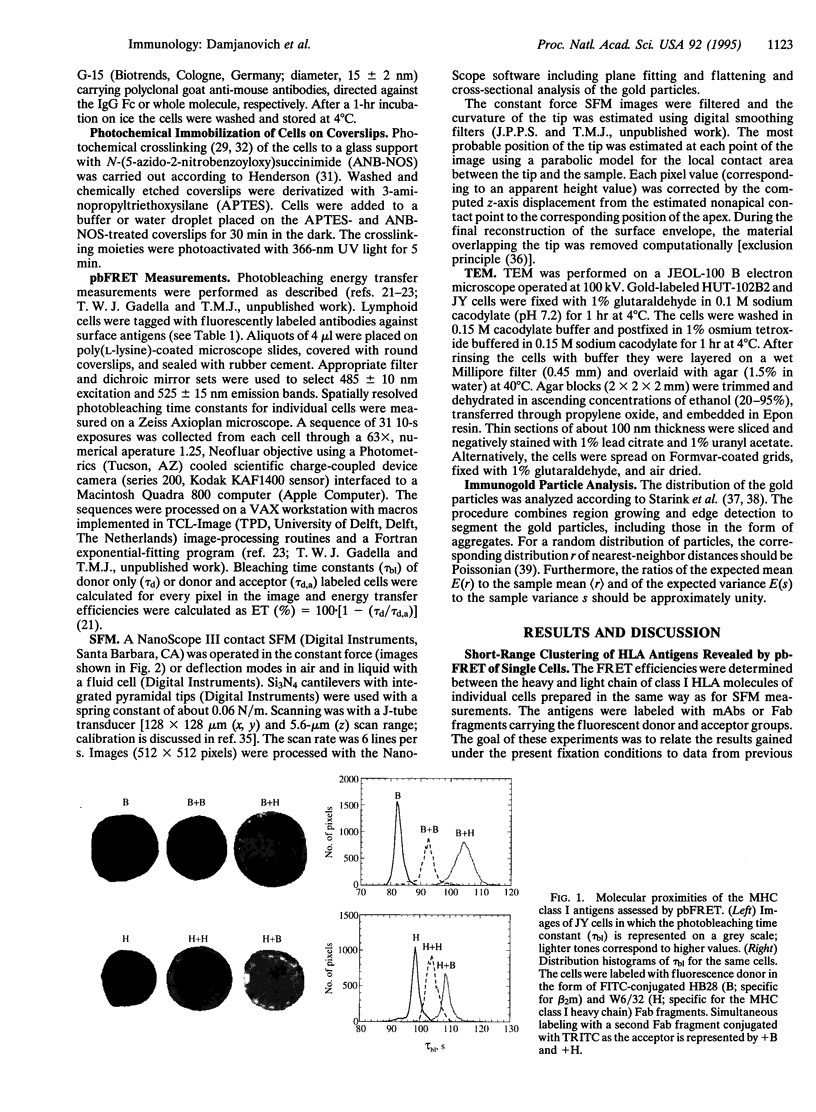
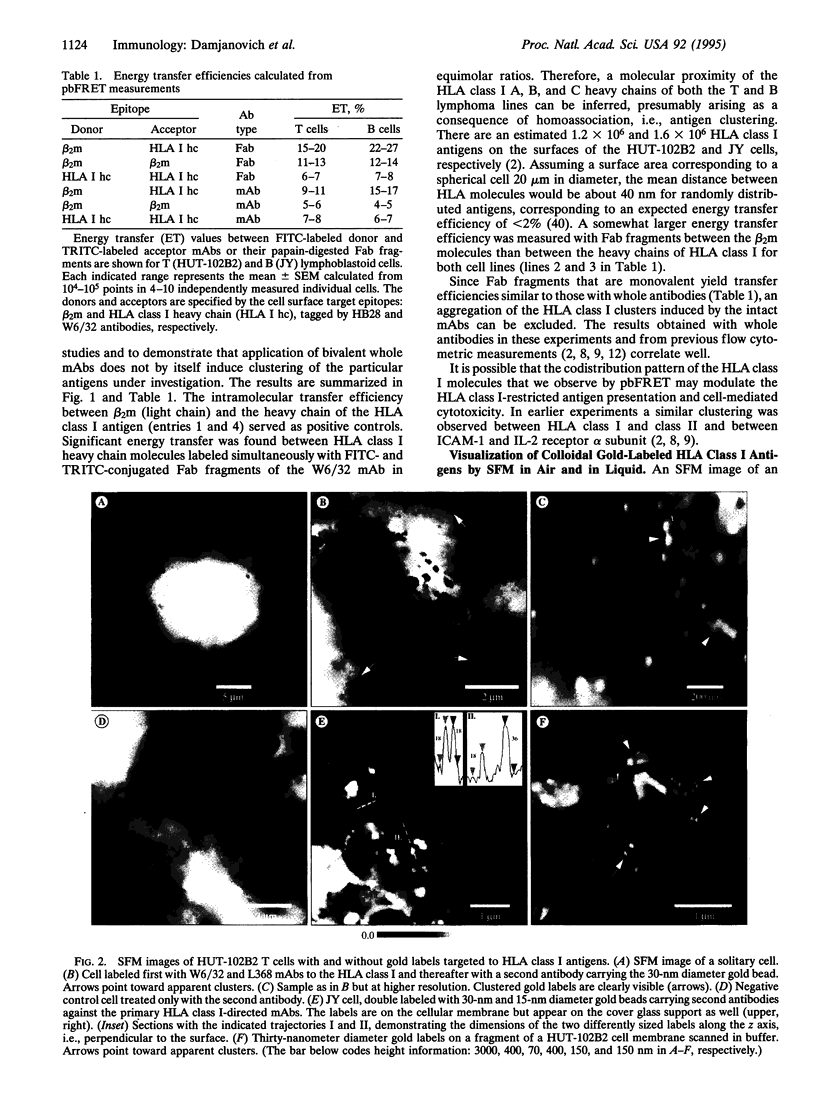
Major histocompatibility complex (MHC) class I antigens in the plasma membranes of human T (HUT-102B2) and B (JY) lymphoma cells were probed by immunochemical reagents using fluorescence, transmission electron, and scanning force microscopies. Fluorescent labels were attached to monoclonal antibodies W6/32 or KE-2 directed against the heavy chain of HLA class I (A, B, C) and L368 or HB28 against the beta 2-microglobulin light chain. The topological distribution in the nanometer range was studied by photobleaching fluorescence resonance energy transfer (pbFRET) on single cells. A nonrandom codistribution pattern of MHC class I molecules was observed over distances of 2-10 nm. A second, nonrandom, and larger-scale topological organization of the MHC class I antigens was detected by indirect immunogold labeling and imaging by transmission electron microscopy (TEM) and scanning force microscopy (SFM). Although some differences in antigen distribution between the B- and T-cell lines were detected by pbFRET, both cell lines exhibited similar clustering patterns by TEM and SFM. Such defined molecular distributions on the surfaces of cells of the immune system may reflect an underlying specialization of membrane lipid domains and fulfill important functional roles in cell-cell contacts and signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Burton J., Goldman C. K., Rao P., Moos M., Waldmann T. A. Association of intercellular adhesion molecule 1 with the multichain high-affinity interleukin 2 receptor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7329–7333. doi: 10.1073/pnas.87.18.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushkin Y., Demaria S., Le J. M., Schwab R. Physical association between the CD8 and HLA class I molecules on the surface of activated human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3985–3989. doi: 10.1073/pnas.85.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Matko J., Rahman N. A., Barisas B. G., Edidin M. Self-association of class I major histocompatibility complex molecules in liposome and cell surface membranes. Biochemistry. 1992 Aug 11;31(31):7182–7189. doi: 10.1021/bi00146a022. [DOI] [PubMed] [Google Scholar]
- Chan S. S., Arndt-Jovin D. J., Jovin T. M. Proximity of lectin receptors on the cell surface measured by fluorescence energy transfer in a flow system. J Histochem Cytochem. 1979 Jan;27(1):56–64. doi: 10.1177/27.1.374620. [DOI] [PubMed] [Google Scholar]
- Damjanovich S., Trón L., Szöllösi J., Zidovetzki R., Vaz W. L., Regateiro F., Arndt-Jovin D. J., Jovin T. M. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5985–5989. doi: 10.1073/pnas.80.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Wei T. Lateral diffusion of H-2 antigens on mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):458–462. doi: 10.1083/jcb.95.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M., Radmacher M., Gaub H. E. Granula motion and membrane spreading during activation of human platelets imaged by atomic force microscopy. Biophys J. 1994 May;66(5):1328–1334. doi: 10.1016/S0006-3495(94)80963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz P. Self-organization of the fluid mosaic of charged channel proteins in membranes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6353–6357. doi: 10.1073/pnas.85.17.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Arndt-Jovin D. J. Luminescence digital imaging microscopy. Annu Rev Biophys Biophys Chem. 1989;18:271–308. doi: 10.1146/annurev.bb.18.060189.001415. [DOI] [PubMed] [Google Scholar]
- Karrasch S., Dolder M., Schabert F., Ramsden J., Engel A. Covalent binding of biological samples to solid supports for scanning probe microscopy in buffer solution. Biophys J. 1993 Dec;65(6):2437–2446. doi: 10.1016/S0006-3495(93)81327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch S., Hegerl R., Hoh J. H., Baumeister W., Engel A. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):836–838. doi: 10.1073/pnas.91.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent U. M., Mao S. Y., Wofsy C., Goldstein B., Ross S., Metzger H. Dynamics of signal transduction after aggregation of cell-surface receptors: studies on the type I receptor for IgE. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3087–3091. doi: 10.1073/pnas.91.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegler T., Szollosi J., Hyun W., Goodenow R. S. Proximity measurements between H-2 antigens and the insulin receptor by fluorescence energy transfer: evidence that a close association does not influence insulin binding. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6755–6759. doi: 10.1073/pnas.88.15.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matko J., Bushkin Y., Wei T., Edidin M. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J Immunol. 1994 Apr 1;152(7):3353–3360. [PubMed] [Google Scholar]
- Matkó J., Szöllösi J., Trón L., Damjanovich S. Luminescence spectroscopic approaches in studying cell surface dynamics. Q Rev Biophys. 1988 Nov;21(4):479–544. doi: 10.1017/s0033583500004637. [DOI] [PubMed] [Google Scholar]
- Mittler R. S., Goldman S. J., Spitalny G. L., Burakoff S. J. T-cell receptor-CD4 physical association in a murine T-cell hybridoma: induction by antigen receptor ligation. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8531–8535. doi: 10.1073/pnas.86.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. S., Rankin B. M., Kiener P. A. Physical associations between CD45 and CD4 or CD8 occur as late activation events in antigen receptor-stimulated human T cells. J Immunol. 1991 Nov 15;147(10):3434–3440. [PubMed] [Google Scholar]
- Neagu C., van der Werf K. O., Putman C. A., Kraan Y. M., de Grooth B. G., van Hulst N. F., Greve J. Analysis of immunolabeled cells by atomic force microscopy, optical microscopy, and flow cytometry. J Struct Biol. 1994 Jan-Feb;112(1):32–40. doi: 10.1006/jsbi.1994.1004. [DOI] [PubMed] [Google Scholar]
- Pietrasanta L. I., Schaper A., Jovin T. M. Imaging subcellular structures of rat mammary carcinoma cells by scanning force microscopy. J Cell Sci. 1994 Sep;107(Pt 9):2427–2437. doi: 10.1242/jcs.107.9.2427. [DOI] [PubMed] [Google Scholar]
- Szabà G., Jr, Pine P. S., Weaver J. L., Kasari M., Aszalos A. Epitope mapping by photobleaching fluorescence resonance energy transfer measurements using a laser scanning microscope system. Biophys J. 1992 Mar;61(3):661–670. doi: 10.1016/S0006-3495(92)81871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllösi J., Damjanovich S., Goldman C. K., Fulwyler M. J., Aszalos A. A., Goldstein G., Rao P., Talle M. A., Waldmann T. A. Flow cytometric resonance energy transfer measurements support the association of a 95-kDa peptide termed T27 with the 55-kDa Tac peptide. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7246–7250. doi: 10.1073/pnas.84.20.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trón L., Szöllósi J., Damjanovich S., Helliwell S. H., Arndt-Jovin D. J., Jovin T. M. Flow cytometric measurement of fluorescence resonance energy transfer on cell surfaces. Quantitative evaluation of the transfer efficiency on a cell-by-cell basis. Biophys J. 1984 May;45(5):939–946. doi: 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesenka J., Manne S., Giberson R., Marsh T., Henderson E. Colloidal gold particles as an incompressible atomic force microscope imaging standard for assessing the compressibility of biomolecules. Biophys J. 1993 Sep;65(3):992–997. doi: 10.1016/S0006-3495(93)81171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The interleukin-2 receptor. J Biol Chem. 1991 Feb 15;266(5):2681–2684. [PubMed] [Google Scholar]
- Young R. M., Arnette J. K., Roess D. A., Barisas B. G. Quantitation of fluorescence energy transfer between cell surface proteins via fluorescence donor photobleaching kinetics. Biophys J. 1994 Aug;67(2):881–888. doi: 10.1016/S0006-3495(94)80549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Hera A., Müller U., Olsson C., Isaaz S., Tunnacliffe A. Structure of the T cell antigen receptor (TCR): two CD3 epsilon subunits in a functional TCR/CD3 complex. J Exp Med. 1991 Jan 1;173(1):7–17. doi: 10.1084/jem.173.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]