Abstract
Studies on depression risk emphasize the importance of both cognitive and genetic vulnerability factors. The current study provided the first examination of whether working memory capacity, the BDNF Val66Met polymorphism, and their interaction predicted change in symptoms of depression during the transition to university. Early in the semester, students completed a self-report measure of depressive symptoms and a modified version of the Reading Span task to assess working memory capacity in the presence of both neutral and negative distractors. Whole blood was genotyped for the BDNF Val66Met polymorphism. Students returned at the end of the semester to complete additional self-report questionnaires. Neither working memory capacity nor the BDNF Val66Met polymorphism predicted change in depressive symptoms independently or in interaction with self-reported semester difficulty. The BDNF Val66Met polymorphism, however, moderated the association between working memory capacity and symptom change. Among met carriers, lower working memory capacity in the presence of negative – but not neutral – distractors was associated with increased symptoms of depression over the semester. For the val/val group, working memory capacity did not predict symptom change. Findings directly contribute to biological and cognitive models of depression and highlight the importance of examining gene x cognition interactions when investigating risk for depression.
Keywords: depression, working memory, cognitive control, stress, gene-environment
The transition to university is a time of high stress (Bouteyre, Maurel, & Bernaud, 2007; Stader & Hokanson, 1998). Adolescents are challenged to adjust to increased academic rigor, independence from prior support systems, and greater financial responsibility (Bouteyre et al., 2007; Dwyer & Cummings, 2001; Smyth, Hockemeyer, Heron, Wonderlich, & Pennebaker, 2008). There is considerable variability in how well students adapt to these stressors (Osinsky, Losch, Hennig, Alexander, & MacLeod, 2012). Although more than 40% of students report increased symptoms of depression during the transition to university, other students report no change or even improvement in their mood (Bouteyre et al., 2007; Osinsky et al., 2012). Both cognitive and genetic factors may be associated with individual differences in response to stressors (Beck, 1967, 2008; Gibb, Beevers, & McGeary, 2013; Monroe & Simons, 1991). In particular, individual differences in cognitive control and the met allele of the brain-derived neurotropic factor (BDNF) gene have both been associated with difficulty regulating emotions in response to stress and with increased risk for depression. The current study aimed to test these two variables separately and interactively as contributors to change in symptoms of depression during students’ transition to university.
Increasing evidence points to the importance of cognitive control as a risk factor for depression (Goeleven et al., 2006; Joormann, 2010; Joormann & Gotlib, 2010). Given that working memory has a limited capacity, efficient functioning depends on maintaining task-relevant material while ignoring, or inhibiting, task-irrelevant distractions. Engle and colleagues (e.g., Engle, 2002; Engle, Tuholski, Laughlin, & Conway, 1999) have defined working memory capacity as the ability to maintain task-relevant information in working memory in the presence of distraction (see Conway et al., 2005, for an overview). Working memory capacity (as defined by Engle, 2002) does not directly assess the number of items that can be stored in working memory but assesses the ability to maintain task-relevant information by minimizing interference from task-irrelevant information. Thus, low working memory capacity indicates larger interference from irrelevant material. Low working memory capacity has been associated with difficulty regulating emotional responses to stress (Schmeichel & Demaree, 2010) and has been linked with various types of psychopathology, including depression (Goeleven, De Raedt, Baert, & Koster, 2006; see Joormann, 2010, for a review).
The majority of research on working memory capacity focuses on interference from neutral distractors. Although depression is associated with deficits in general cognitive processes, it is more often characterized by difficulties with mood-congruent content (see Mathews & MacLeod, 2005), as predicted by cognitive models of depression (Beck, 1967, 1976). Depressed individuals show particular difficulty ignoring negative information and disengaging from it (Joormann & Gotlib, 2008). In fact, difficulty controlling the contents of working memory in the presence of negative, compared to neutral, distractors was a better predictor of baseline negative affect and was a better predictor of increases in negative affect in response to stress (Compton, Arnstein, Freedman, Dainer-Best, & Liss, 2011). It might thus be important to examine working memory capacity in the presence of not only neutral but also negative distractors.
Similar to evidence on low working memory capacity, the met allele of the BDNF Val66Met polymorphism has been associated with impaired responses to stress and heightened vulnerability to depression (e.g., Alexander, Osinsky, Schmitz, Mueller, Kuepper, & Hennig, 2010; Classen, Wells, Knopik, McGeary, & Beevers, 2011; Colzato, Van der Does, Willem, Kouwenhoven, Elzinga, & Hommel, 2011; Gatt et al., 2009; Pandey & Dwivedi, 2009; Pei et al., 2012; Shalev et al., 2009; Vinberg, Trajkovska, Bennike, Knorr, Knudsen, & Kessing, 2009; see also a meta-analysis by Hosang, Shiles, Tansay, McGuffin, & Uher, 2014). The genetic variation in exon 11 of the BDNF gene results in an amino-acid substitution from valine to methionine at codon 66 (Val66Met) and influences BDNF protein production. The met allele is typically associated with less BDNF secretion. The BDNF protein is central to neuronal growth, facilitates neuronal plasticity, and promotes adaptive responses to stress (McAllister, 2002).
Considerable evidence supports the BDNF hypothesis of depression, which posits that low levels of the BDNF protein play a central role in depression onset (Dwivedi et al., 2003; Karege, Perret, Bondolfi, Schwald, Bertschy, & Aubry, 2002; Karege, Vaudan, Schwald, Perroud, & La Harpe, 2005; Shimizu et al., 2003). However, evidence for a link between the BDNF polymorphism and depression has been inconsistent. Whereas some studies find the met allele associated with major depressive disorder, particularly in interaction with stress (Carver, Johnson, Joormann, LeMoult, & Cuccaro, 2011; Gatt et al., 2009; Schumacher et al., 2005), other studies find the opposite (Chen et al., 2012), and still others – including a recent meta-analysis – reported no association between the BDNF Val66Met polymorphism and depression (Oswald et al., 2005; Verhagen et al., 2010). Researchers, therefore, have begun to examine associations between BDNF and cognitive risk factors, and particularly strong associations have been found between BDNF and constructs related to working memory capacity. For example, the BDNF met allele has been associated with deficits in working memory and executive functioning (e.g., Egan et al., 2003; Gatt et al., 2007; Rybakowski, Borkowska, Czerski, Skibinska, & Hauser, 2003), reduced grey matter volume in corresponding brain regions such as the hippocampus and prefrontal cortex (e.g., Bueller et al., 2006; Pezawas et al., 2004) and abnormal hippocampal activation during the N-back working memory task (e.g., Egan et al., 2003). Thus, research suggests that BDNF and working memory capacity are important and related risk factors for depression (see reviews by Egan et al., 2013; Joormann 2010).
There are several ways BDNF and working memory capacity may influence depression during times of stress. On one hand, we might anticipate a mediation model, whereby working memory capacity mediates the relation between BDNF, stress, and depression. In this context, we might expect the BDNF met allele to be associated with lower working memory capacity, which in turn, would be associated with increased symptoms of depression during increased stress. On the other hand, other models of depression (Gibb, Beevers, McGeary, 2013) posit a moderation model, whereby BDNF, working memory capacity, and stress interact to influence the pathophysiology of depression. In this view, during times of stress, one might expect the influence of working memory capacity to depend on BDNF genotype: Whereas individuals with the val-val genotype might be protected against other risk factors for depression given associations between the BDNF Val66Met polymorphism and activity-dependent BDNF release in the brain (Egan et al., 2003), met carriers might be susceptible to risk factors that are related to BDNF, such as working memory capacity. Increasing evidence has been found for moderation models of risk (Gibb, Benas, Grassia, & McGeary, 2009; Gibb, Uhrlass, Grassia, & Benas, 2009; Lau, Rijskijk, & Ely, 2006; Osinski, Losch, Hennig, Alexander, & MacLeod, 2012). Moreover, studies that have examined both mediation and moderation models have found support exclusively for the latter (Gibb, Benas et al., 2009; Osinski et al., 2012).
Our goal in the current study was to examine the role of working memory capacity, BDNF, and stress on vulnerability to depression. We focused on BDNF and working memory capacity given evidence that they are important and related risk factors for depression (see reviews by Egan et al., 2013; Joormann 2010). We predicted change in depressive symptoms over the course of a semester using self-reported semester difficulty, BDNF, working memory capacity in the presence of neutral distractors, and working memory capacity in the presence of negative distractors. In line with the BDNF hypothesis of depression, we expected BDNF to interact with stress experienced during the semester such that greater stress would predict greater increases in depressive symptoms for met carriers. Based on cognitive model of depression (Beck, 1967, 1976), we expected working memory capacity with negative distractors – but not neutral ones – to interact with stress experienced during the semester such that greater stress would predict greater increases in depressive symptoms for individuals with low working memory capacity for negative distractors. Moreover, building on gene x cognition x environment models of depression (Gibb et al., 2013) and increasing empirical evidence (Osinski et al., Gibb, Benas et al., 2009; Gibb, Uhrlass et al., 2009), we predicted that working memory capacity for negative distractors would be particularly predictive of increases in depressive symptoms for participants who were met carriers.
Method
Participants and Procedure
Undergraduate students participated in exchange for partial credit toward a course requirement. Interested students replied to a posting on the department website early in the semester. They came to the laboratory in groups of about 20 to complete session 1. After providing informed consent, participants completed a measure of working memory capacity and a self-report measure of depressive symptoms (described below). Blood also was drawn for genotyping by a trained phlebotomist. Approximately 2.5 months later, participants returned to the laboratory to complete session 2, during which they completed additional self-report questionnaires. Of the 246 students (160 females) who provided valid information at session 1, 169 returned at the end of the semester, and of those 167 completed all session 2 measures. The sample size was determined according to power calculations, with the aim of detecting a moderate effect size that would be clinically significant (cf. Cohen’s f2 = 0.15) with 80% power, assessed at a 2-tailed alpha of .05. Power calculations were conducted using G*power 3.1.7 based on guidelines provided by Faul, Erdfelder, Buchner, and Lang (2009) for conducting power analyses for interaction terms. Reasons for attrition included students dropping the course or participating in alternative experiments (returning for the second session had not been specified as a precondition for participating in the first). Mean age of the final sample was 18.49 years (SD = 1.69). Participants self-identified as the following ethnicities: 99 non-Hispanic White, 40 Hispanic, 10 Asians, 7 African Americans, 4 Caribbean islanders, and 7 “others.”
Working Memory Capacity
Working memory span tasks are widely used measures of working memory capacity (see Conway et al., 2005, for an overview). We created an affective version of the Reading Span task (RSpan; Kane et al., 2004; Engle et al., 1999) to assess individual differences in working memory capacity in the presence of negative versus neutral distraction. During each trial of the RSpan task, letters were presented one at a time for 1,000ms each. Participants were asked to memorize these letters for a later check of memory. After the presentation of each letter, a sentence appeared, and participants were asked to determine if the sentence was logical. If the sentence was a logical sentence (e.g., “I like to run in the park”), participants selected TRUE; if the sentence was an illogical sentence (e.g., “I like to run in the sky”), participants selected FALSE. The computer progressed to the next letter if participants made a response or if their response time was 1,000ms longer than the person’s average reading time during practice trails. Cumulative sentence accuracy was recorded and displayed to participants. Unanswered sentences were recorded as inaccurate, and participants were informed that average sentence accuracy must be above 85% for data to be valid (as recommended by Conway et al., 2005). Each trial consisted of between 3 and 7 letter-sentence sets. At the end of each trial, 12 letters appeared in a 4×3 matrix on the screen, and participants indicated which of those letters they had been shown in the trial.
The current RSpan task differed from past versions in that half of the 30 trials presented sentences with negative content (e.g., “When I saw the man get shot I felt terrified and helpless.” or “Liz couldn’t stop crying when she found out that she had failed her class.”). The other half of trials presented neutral sentences (e.g., “We like to eat eggs and bacon for breakfast in the morning.” or “The seventh graders had to build a volcano for their science class.”). The outcome measures of interest for this task were the sum of correctly recalled letters on negative-sentence trials (RSpan-Negative) and neutral-sentence trials (RSpan-Neutral) as a proportion of total letters presented (see guidelines by Conway et al., 2005). Lower RSpan scores reflected lower working memory capacity when performing in the presence of negative or neutral distractors, respectively. Stated differently, lower RSpan scores indicated larger interference from irrelevant material, reflecting less cognitive control.
Genotyping
Extraction and genotyping were completed at the Hussman Institute of Human Genomics, University of Miami Miller School of Medicine. Three ng of genomic DNA was extracted from whole blood according to established protocols. Genotyping was done for the GrA (valinermethionine) variation at position 758 of the BDNF coding sequence (rs6265) using Taqman allelic discrimination assays from Applied Biosystems (ABI). Cycling was performed on GeneAmp PCR Systems 9700 thermocyclers using conditions specified by ABI. After endpoint fluorescence was measured on the ABI 7900 HT system, genotype discrimination of results was conducted using ABI’s HT Sequence Detection Systems version 2.3 analyses. As a check of genotyping accuracy, 32 quality control samples were included. Sample call rates were > 99.7%. The BDNF genotype frequencies were as follows: 110 val/val, 47 val/met, and 10 met/met. Genotype frequencies were in Hardy-Weinberg equilibrium, χ2 (N = 167) = 2.51, p > .05. In line with previous studies, carriers of one or more met allele were combined in one group (met carrier) and compared against val/val.
Questionnaires
Depression
To assess depression severity within the past two weeks, participants completed the Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, 1996). The BDI is a 21-item, self-report measure assessing the severity of depressive symptoms. It has high test-retest reliability (r = .93) and good internal consistency (α = .91; Beck et al., 1996), including in student samples (Beck et al., 1988). This measure was completed at the first session early in the semester, and again at the second session later in the semester.
Semester difficulties
To assess difficulties experienced during the semester, participants completed a 10-item questionnaire in the second session. Questions included, “I am happy overall with my academic performance this semester,” “All in all, my semester has been quite good,” and “My social life has been pretty nonexistent this semester” (reverse coded). Responses were made on a 5-point scale that ranged from 1 (I agree a lot) to 5 (I disagree a lot). Items were coded so that higher scores indicate a more difficult semester, M = 23.56, SD = 7.32, α = .80.
Results
Sample Characteristics
Participants who completed session 2 did not differ from those who did not by ethnicity, Time 1 BDI scores, working memory capacity scores, or BDNF genotype, ps > .05. However, non-returners were older (M = 19.26, SD = 2.62) than returners (M = 18.49; SD = 1.69), t(242) = 2.75, p = .01. In addition, there was a slightly lower percent female in the non-returner versus returner group (55.84% and 68.86% respectively), χ2(1, N = 244) = 3.91, p = .05. We, therefore, tested the effect of age and gender on change in depressive symptoms.
Table 1 shows characteristics of the final sample stratified by BDNF genotype groups. Genotype groups did not significantly differ in age, t(165) = 0.58, p = .57. Groups also did not differ in percent female, χ2(1, N = 167) = .01, p = .93, or ethnic distribution, χ2(5, N = 167) = 10.47, p = .06. Nonetheless, given that BDNF Val66Met allele frequencies have been found to differ across ethnic group, ethnicity was also entered in the model when testing our main hypotheses. Semester difficulty did not significantly differ across genotype groups, t(165) = 0.18, p = .86. Moreover, genotype groups did not significantly differ in RSpan-Negative or RSpan-Neutral, ts(165) < 1, ps > .05.
Table 1.
Participant Characteristics
| Variable | Val-Val (N = 110) | Met-Carrier (N = 57) |
|---|---|---|
| Age, M (SD) | 18.55 (1.62) | 18.39 (1.83) |
| Female, % | 69.09 | 68.42 |
| Non-Hispanic White, % | 54.55 | 68.42 |
| Hispanic, % | 28.18 | 15.79 |
| African American, % | 6.36 | 0.00 |
| Caribbean, % | 2.73 | 1.75 |
| Asian, % | 3.64 | 10.53 |
| Other, % | 4.55 | 3.51 |
| Semester Difficulty | 23.64 (7.23) | 23.42 (7.56) |
| RSpan-Negative | 0.78 (0.12) | 0.79 (0.14) |
| RSpan-Neutral | 0.78 (0.13) | 0.79 (0.13) |
| BDI-Time1, M (SD) | 7.08 (6.49) | 9.05 (7.31) |
| BDI-Time2, M (SD) | 6.22 (6.76) | 8.91 (8.26) |
Note. BDI = Beck Depression Inventory; Time 1 was the beginning of the semester, Time 2 the end of the semester
BDI and BDNF
Genotype groups did not significantly differ in their BDI scores at the start of the semester (Time 1), t(165) = 1.78, p = .08. At the end of the semester (Time 2), however, met-carriers reported higher BDI scores than the val/val group, t(165) = 2.26, p = .03 (see Table 1).
Cross-Semester Changes in BDI Scores
Across all participants, BDI scores did not significantly change across the semester, t(166) = 1.55, p = .12; however, there was high variability in cross-semester change in BDI scores (mean difference BDI-Time2 – BDI-Time1 = −0.62; SD = 5.13). A hierarchical multiple regression analysis was conducted to examine the effect of BDNF genotype and working memory capacity in the presence of negative and neutral distractors on cross-semester changes in BDI scores. Following recommendations by Jacobson and Truax (1991) and in line with Osinsky et al. (2012), we used the Reliable Change Index proposed by Jacobson, Follette, and Revenstorf (1984), which accounts for measurement fluctuations over time. Thus, cross-semester change in BDI was quantified using the following formula:
where Sdiff is the standard error of the difference.
Age, gender, and ethnicity were entered in Block 1. BDNF, RSpan-Negative, RSpan-Neutral, and Semester Difficulty, were entered in Block 2. The interaction of BDNF x Semester Difficulty, RSpan-Negative x Semester Difficulty, as well as RSpan-Neutral x Semester Difficulty were entered in Block 3. The interaction of BDNF x RSpan-Neg as well as BDNF x RSpan-Neut were entered in Block 4. The three-way interaction of BDNF x RSpan-Negative x Semester Difficulty as well as BDNF x RSpan-Neutral x Semester Difficulty were entered in Block 5. Continuous variables were centered. Categorical variables were dummy coded.
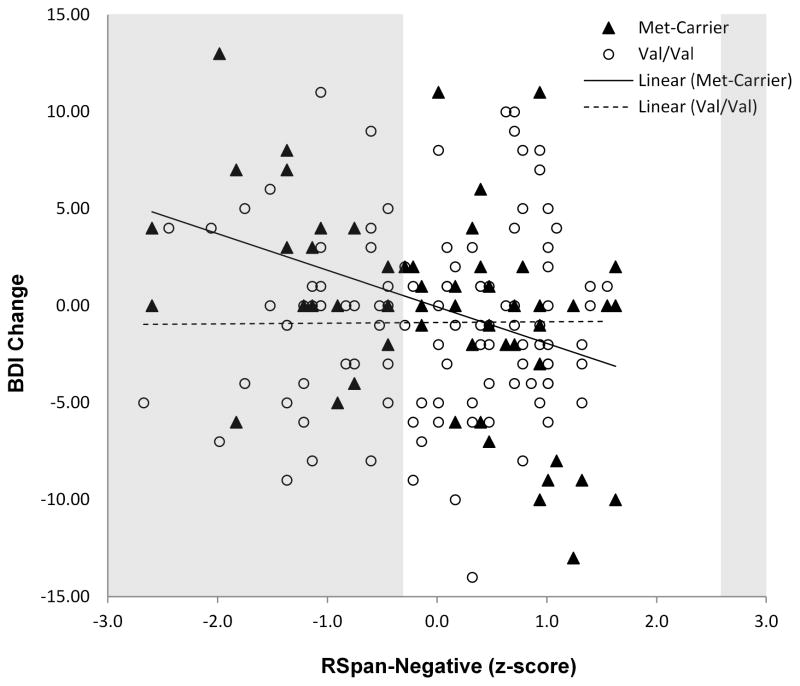
Block 1 did not yield a significant effect, R2 = .04, F(7, 159) < 1, p = .56, f2 = .04. Block 2 accounted for a significant portion of variance, R2change = .07, Fchange(4, 155) = 2.84, p = .03, f2 = .07. Of Block 2 variables, Semester Difficulty was the only variable to significantly predict BDI change, t(155) = 2.43, p = .02, β = .19, rs.part(155) = .19. Higher Semester Difficulty predicted greater increase in BDI scores. Block 3 did not account for a significant portion of variance, R2change = .02, Fchange(3, 152) = 1.02, p = .39, f2 = .02. Block 4 significantly improved the prediction of BDI change, R2change = .04, Fchange(2, 150) = 3.20, p = .04, f2 = .04. The interaction between BDNF and RSpan-Neutral did not significantly contribute to BDI scores, t(150) = 1.06, p = .29, β = .16, rs.part(150) = .08. The interaction between BDNF and RSpan-Negative, however, significantly predicted change in BDI, t(150) = 2.31, p = .02, β = −.36, rs.part(150) = −.17.1 Block 5 did not significantly improve the prediction of BDI change, R2change = .02, Fchange(2, 148) = 1.68, p = .19, f2 = .02. We, therefore, examined the interaction between BDNF and RSpan-Negative without the higher order interaction included in the model. Simple slope analyses determined that lower RSpan-Negative scores were associated with greater increases in BDI scores across the semester among met carriers, t(45) = 2.70, p = .01, β = −.50, rs.part(45) = −.30, but did not have a significant effect among those homozygous for the val allele, t(97) = 1.03, p = .31, β = .15, rs.part(97) = .10 (see Figure 1). Thus, for participants in the met-carrier group, there was a negative association between working memory capacity in the presence of distraction from negative information and change in depressive symptoms over the course of the semester. Moreover, as recommended by Roisman et al. (2012) and using tools developed by Preacher, Curran, and Bauer (2006), we conducted region of significance (RoS) analyses to examine the values of RSpan-Negative for which there was a significant difference between BDNF met carriers and those homozygous for the val allele at α = .05. For RSpan-Negative (centered) scores less than −0.04, met carriers reported greater increase in depressive scores across the semester than those homozygous for the val allele, t(150) = 1.98. In contrast, for RSpan-Negative (centered) scores greater than 0.36, met carriers reported greater decrease in depressive scores across the semester than those homozygous for the val allele, t(150) = 1.98.
Figure 1.
Scatter plot depicting the relation of cross-semester change in BDI symptoms and RSpan-Negative scores (presented as z-scores) for the BDNF Val66Met genotype groups. The shaded area depicts the regions of signifiance.
Discussion
This study examined whether self-reported semester difficulty, BDNF, working memory capacity, and their interaction predicted change in symptoms of depression during the transition to university, a period of heightened stress (Fisher & Hood, 1987). In line with past research (Clarke, MacLeod, & Shirazee, 2008; Osinsky et al., 2012), there were substantial individual differences in cross-semester change in symptoms of depression. Neither BDNF nor working memory capacity independently predicted more than a nominal change in depressive symptoms. In addition, neither BDNF nor working memory capacity interacted with self-reported semester difficulty to predict more than a nominal change in depressive symptoms (less than 2%). BDNF genotype, however, moderated the association between working memory capacity and change in symptoms of depression. Specifically, for met-carriers (i.e., individuals with what has been labeled the at-risk BDNF genotype), lower working memory capacity in the presence of negative – but not neutral – distractors was associated with increased symptoms of depression over time. In fact, working memory capacity in the presence of negative distractors predicted approximately 9% of the variance in depressive symptom change in this group. For those with the val/val genotype, however, working memory capacity predicted 1% of symptom change.
Findings from this study highlight the importance of including the BDNF Val66Met polymorphism in etiological models of depression. Although our findings do not indicate a direct link between BDNF and depressive symptoms, the importance of BDNF was via its interaction with working memory capacity in the presence of negative distractors. This is the first study to examine the interaction between BDNF and working memory capacity as predictors of change in depressive symptoms; however, our finding is in line with other studies demonstrating that the interaction between genetic and cognitive factors predicts increases in depressive symptoms during times of stress in both children (Gibb, Benas et al., 2009; Gibb, Uhrlass et al. & Benas, 2009) and young adults (Osinsky et al., 2012). Osinsky and colleagues, for example examined whether the interaction between the serotonin transporter gene (5-HTTLPR) and attentional biases for negative information predicted students’ emotional change across their first university semester. Similar to the current study, a gene by cognition interaction was found. Attention biases to negative information predicted increased depressive symptoms across the semester only for those with the at-risk genotype.
Interestingly, neither BDNF nor working memory capacity interacted with semester difficulty to predict a substantial portion of change in BDI scores, with the effect size of f2 = .02 being in the “small” range (Cohen, 1988). Thus, although the interaction of both vulnerability factors predicted change in depressive symptoms during the transition to university, the vulnerability factors did not interact with our measure of stress to predict a significant amount of change in depressive symptoms. It is possible that the presence of stressful experiences is less important than are individual differences in the way stressful periods are processed, indexed via information processing measures such as the ability to inhibit negative irrelevant information. This possibility is consistent with the observed interaction between BDNF and working memory capacity in the presence of negative distractors. It is important to interpret nonsignificant interactions with caution, however, given that our measure of stress was ad hoc, capturing only perceived stress from academic and social domains without assessing important domains such as family or financial stress, and given that it did not include objective ratings of severity that have been shown to be of importance in gene x environment interactions (Karg, Burmeister, Shedden, & Sen, 2011). Our ability to consider stress effects may also have been limited by the relatively restricted variability in the amount of stress participants reported. The current study differed from past research that found gene x stress or even gene x cognition x stress interactions (e.g., Chen et al., 2012; Gibb, Uhrlass, et al., 2009) in that all of our participants were experiencing an objectively stressful transition period (Bouteyre et al., 2007; Stader & Hokanson, 1998). It is also important to note that only a small change in depressive symptoms occurred across the semester. Although there was significant variability in depressive symptom change, the overall magnitude of change may have been insufficient to have been influenced by stress. This may also explain why we found that the interaction between BDNF, working memory capacity, and semester difficulty exerted only a small effect on symptom change. However, this study was underpowered for such an interaction, and the null results should be interpreted with caution. Future research should consider using stronger measures of stress to test these possible explanations.
The current study offers initial insight into how BDNF might increase risk for depression. One possibility is that the BDNF met allele increases depression risk not based simply on whether people experience stressors but how they process them. In the current study, we found that BDNF interacted not with stress but with individual differences in the way negative material is processed. The same amount of stress may be quickly forgotten by some (those with high working memory capacity in the presence of negative distractors) yet may remain in the forefront of others’ memory (those with low working memory capacity in the presence of negative distractors). Thus, rather than focusing exclusively on whether participants experience stress, future research might focus on individual differences in cognitive factors that influence how stress is processed (cf. Lazarus & Folkman, 1984).
It is also important to highlight that the met-allele served as a vulnerability factor for participants who could not easily inhibit negative information and yet – at the extreme – a protective factor for those who could. This pattern of findings suggests a differential susceptibility pattern (Roisman, Newman, Fraley, Haltigan, Groh, & Haydon, 2012), and it might explain inconsistencies in past research examining BDNF, stress, and depression. Some past studies found a relation between the BDNF met allele and increased stress reactivity or increased risk for depression in interaction with stress (see the recent meta-analysis by Hosang et al., 2014). Others, however, have found the opposite (e.g. Alexander et al, 2010; Chen et al, 2012). As opposed to diathesis-stress models that only explain increased risk, differential susceptibility patterns offer insight into both risk and resilience (Roisman et al., 2012).
The current study was limited in its examination of changes in depressive symptoms versus the onset of a depressive episode. Future research should examine whether the interaction between BDNF and working memory capacity predicts who goes on to develop an episode of depression. A second limitation of the current study is that a sizable portion of participants did not return for session 2. One reason for high attrition rates was that session 1 was conducted in the early weeks of the semester, prior to the date when students were allowed to drop the course. In addition, students who completed session 1 were able to complete their course credit through participation in alternative experiments. An additional limitation is that the current study focused on undergraduate students, and it is possible that these findings do not generalize to more representative or impaired populations. Moreover, we should note that the negative sentences used in the current study are not depression specific and instead express negative affect in general. It will be important that future research examine the interaction between BDNF and cognitive control in the presence of depression-specific distractors.
Despite these limitations, findings from the current study might provide insight into the inconsistent results linking BDNF and depression. Our findings also highlight the importance of examining gene by cognitive bias interactions in etiological models of depression. Although the BDNF Val66Met polymorphism may not independently be a consistent predictor of changes in depressive symptoms, the importance of focusing on BDNF is highlighted by the fact that it confers risk in interaction with individual differences in cognitive control when processing negative information. Future research might also examine whether the interaction between BDNF and other executive functions or memory biases might also have similar effects, thereby enhancing our understanding of this debilitating disorder. Future research might also examine gene by gene by cognitive bias interactions. In particular, a sizable literature indicates that the short allele of the serotonin transporter gene (5-HTTLPR) increases risk for depression in interaction with stressful life events (e.g., Karg et al., 2011). More recent work also provides evidence that the interaction between 5-HTTLPR and BDNF increases risk for depression in combination with childhood adversity (e.g., Krishnan & Taylor, 2009). In addition, catechol-o-methyltransferase (COMT) has received increasing attention in recent years. In fact, Nagel and colleagues (Nagel et al., 2008) reported that BDNF met-carriers performed significantly worse on executive functioning tasks if they were also COMT val-carriers. Future work might consider incorporating both of these genes to test more complex etiological models of depression.
Acknowledgments
This work was made possible by seed funds from a University of Miami Distinguished Professorship.
Footnotes
The regression predicting BDI-Time 2 with BDI-Time 1 as a covariate in Block 1 yielded parallel findings, and the BDNF and RSpan-Negative interaction remained significant, p = .04. Similarly, separate regressions for RSpan-Neutral and RSpan-Negative yielded parallel results: The interaction between BDNF and RSpan-Neutral remained nonsignificant, p = .28, and the interaction between BDNF and RSpan-Negative remained significant, p = .02. The BDNF x RSpan-Negative and BDNF x RSpan-Neutral interactions were not moderated by ethnicity (coded with 5 dummy variables and non-Hispanic White as the reference group), gender (dummy coded with female as the reference group), or age (centered), ps > .05.
References
- Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 2010;35(6):949–953. doi: 10.1016/j.psyneuen.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression; Clinical, Experimental and Theoretical Aspects. New York: Hoebner Medical Division, Harper & Row; 1967. [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. Oxford, England: International Universities Press; 1976. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. http://dx.doi.org/10.1016/0272-7358(88)90050-5. [Google Scholar]
- Bouteyre E, Maurel M, Bernaud JL. Daily hassles and depressive symptoms among first year psychology students in France: The role of coping and social support. Stress and Health: Journal of the International Society for the Investigation of Stress. 2007;23:93–99. doi: 10.1002/smi.1125. [DOI] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met Allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59(9):812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J, LeMoult J, Cuccaro ML. Childhood adversity interacts separately with 5-HTTLPR and BDNF to predict lifetime depression diagnosis. Journal of Affective Disorders. 2011;132:89–93. doi: 10.1016/j.jad.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Clarke P, MacLeod C, Shirazee N. Prepared for the worst: Readiness to acquire threat bias and susceptibility to elevate trait anxiety. Emotion. 2008;8:47–57. doi: 10.1037/1528-3542.8.1.47. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes, Brain and Behavior. 2011;10(7):740–746. doi: 10.1111/j.1601-183X.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Van der Does V, Willem AJ, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36(10):1562–1569. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A. Cognitive control in the intertrial interval: evidence from EEG alpha power. Psychophysiology. 2011;48(5):583–590. doi: 10.1111/j.1469-8986.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/BF03196772. [DOI] [PubMed] [Google Scholar]
- Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues in Clinical Neurosciences. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of General Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Dwyer AL, Cummings AL. Stress, self-efficacy, social support, and coping strategies in university students. Canadian Journal of Counselling. 2001;35(3):208–220. [Google Scholar]
- Egan M, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. http://dx.doi.org/10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037/0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Fisher S, Hood B. The stress of the transition to university: a longitudinal study of psychological disturbance, absent-mindedness and vulnerability to homesickness. British Journal of Psychology. 1987;78(4):425–441. doi: 10.1111/j.2044-8295.1987.tb02260.x. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular psychiatry. 2009;14(7):681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition & Emotion. 2012;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Urhlass DJ, Grassia M, Benas JS, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: An integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2009;118:734–745. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Baert S, Koster EH. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93(1–3):149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hosang GM, Shiles C, Tansay KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Medicine. 2014;12:7. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, Follette WC, Revenstorf D. Psychotherapy outcome research: Methods for reporting variability and evaluating clinical significance. Behavior Therapy. 1984;15:336–352. http://dx.doi.org/10.1016/S0005-7894(84)80002-7. [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Joormann J. Cognitive inhibition and emotional regulation in depression. Current Directions in Psychological Science. 2010;19:161–166. doi: 10.1177/0963721410370293. [DOI] [Google Scholar]
- Joormann J, Gotlib IH. Updating contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133(2):189. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Research. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. http://dx.doi.org/10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Molecular Brain Research. 2005;136(1):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KRR, Taylor WD. Neurobiological pathways that link gene and environment: early life stress disorder. Molecular Psychiatry. 2009;14(7):648–649. doi: 10.1038/mp.2009.27. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- McAllister A. Spatially restricted actions of BDNF. Neuron. 2002;36:549–550. doi: 10.1016/S0896-6273(02)01063-2. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, Von Oertzen T, Sander T, Villringer A, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience. 2008;2:1–8. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinsky R, Lösch A, Hennig J, Alexander N, MacLeod C. Attentional bias to negative information and 5-HTTLPR genotype interactively predict students’ emotional reactivity to first university semester. Emotion. 2012;12(3):460. doi: 10.1037/a0026674. [DOI] [PubMed] [Google Scholar]
- Oswald P, Del-Favero J, Massat I, Souery D, Claes S, Van Broeckhoven C, Mendlewicz J. No implication of brain-derived neurotrophic factor (BDNF) gene in unipolar affective disorder: Evidence from Belgian first and replication patient–control studies. European Neuropsychopharmacology. 2005;15(5):491–495. doi: 10.1016/j.euroneuro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y. Peripheral Biological Markers for Mood Disorders. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. 2009;3:121–149. doi: 10.1007/978-1-4020-9838-3_9. [DOI] [Google Scholar]
- Pei Y, Smith AK, Wang Y, Pan Y, Yang J, Chen Q, Ma X. The brain-derived neurotrophic-factor (BDNF) val66met polymorphism is associated with geriatric depression: A meta-analysis. American Journal of Medical Genetics. 2012;159:560–566. doi: 10.1002/ajmg.b.32062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibińska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disorders. 2003;5(6):468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Demaree HA. Working memory capacity and spontaneous emotion regulation: High capacity predicts self-enhancement in response to negative feedback. Emotion. 2010;10:739–744. doi: 10.1037/a0019355. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Cichon S. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biological Psychiatry. 2005;58(4):307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34(3):382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biological Psychiatry. 2003;54(1):70–75. doi: 10.1016/S0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Hockemeyer JR, Heron KE, Wonderlich SA, Pennebaker JW. Prevalence, type, disclosure, and severity of adverse life events in college students. Journal of American College Health. 2008;57(1):69–76. doi: 10.3200/JACH.57.1.69-76. [DOI] [PubMed] [Google Scholar]
- Stader SR, Hokanson JE. Psychosocial antecedents of depressive symptoms: An evaluation using daily experiences methodology. Journal of Abnormal Psychology. 1998;107:17–26. doi: 10.1037/0021-843X.107.1.17. [DOI] [PubMed] [Google Scholar]
- Verhagen M, Van Der Meij A, van Deurzen PAM, Janzing JGE, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Molecular Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Trajkovska V, Bennike B, Knorr U, Knudsen GM, Kessing LV. The BDNF Val66Met polymorphism: relation to familiar risk of affective disorder, BDNF levels and salivary cortisol. Psychoneuroendocrinology. 2009;34(9):1380–1389. doi: 10.1016/j.psyneuen.2009.04.014. [DOI] [PubMed] [Google Scholar]