Abstract
Among obese individuals, increased sympathetic nervous system activity results in increased renin and aldosterone production, as well as renal tubular sodium reabsorption. This study determined the associations between adipokines and selected measures of the reninangiotensinogen-aldosterone system (RAAS). The sample was 1,970 men and women from the Multi-Ethnic Study of Atherosclerosis who were free of clinical cardiovascular disease at baseline and had blood assayed for adiponectin, leptin, plasma renin activity (PRA) and aldosterone. The mean age was 64.7 years and 50% were female. The mean (SD) PRA and aldosterone were 1.45 (0.56) ng/ml and 150.1 (130.5) pg/ml, respectively. After multivariable adjustment, a 1-SD increment of leptin was associated with a 0.55 ng/ml higher PRA and 8.4 pg/ml higher aldosterone (p < 0.01 for both). Although adiponectin was not significantly associated with PRA levels, the same increment in this adipokine was associated with lower aldosterone levels (−5.5 pg/ml, p = 0.01). Notably, the associations between aldosterone and both leptin and adiponectin were not materially changed with additional adjustment for PRA. Exclusion of those taking anti-hypertensive medications modestly attenuated the associations. The associations between leptin and both PRA and aldosterone were not different by gender but were significantly stronger among non-Hispanic Whites and Chinese Americans than African and Hispanic Americans (p < 0.01). The findings suggest that both adiponectin and leptin may relevant to blood pressure regulation via the RAAS, that the associations appear to be robust to anti-hypertension medication use and that the associations are likely different by ethnicity.
Keywords: Adipokines, Renin, Aldosterone, Ethnicity
INTRODUCTION
Previous studies have demonstrated a robust association between greater adipose tissue and higher levels of blood pressure, as well as incident hypertension. Indeed, it is estimated that 70% of hypertension can be attributed to excess adiposity.1-6 Although undoubtedly a multifactorial phenomenon, hypertension in obese individuals has been linked with activation of the renin-angiotensinogen-aldosterone system (RAAS) and plasma volume expansion.7-10 In this regard, higher sympathetic nervous system (SNS) activity, present in obese individuals, results in increased renin release, aldosterone production, and renal tubular sodium reabsorption.11-13
The pathophysiologic linkage between higher levels of adipose tissue and increased sympathetic nervous system activity is not completely understood. Some have hypothesized that cytokines released from adipose tissue, such as adiponectin and/or leptin, may be relevant. Indeed, higher leptin has been associated with higher blood pressure levels, as well as the prevalence of hypertension, independent of other relevant risk factors including obesity.2,6,14 This, combined with findings showing that higher leptin is associated with increased SNS activity7,9, suggest that this adipokine may be important in the regulation of blood pressure. What is unclear is whether adiponectin and leptin are significantly associated with renal and adrenal-specific markers of increased sympathetic nervous system activity; namely, plasma renin activity (PRA) and aldosterone, respectively. Therefore, we tested the hypothesis that adiponectin and leptin would be significantly associated with levels of both PRA and aldosterone, independent of relevant risk factors.
MATERIALS AND METHODS
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of African, Chinese and Hispanic Americans, as well as non-Hispanic Whites. Details about the study design have been published.11 In brief, between July 2000 and August 2002 (visit 1), 6,814 men and women who were 45 to 84 years old and were free of clinically apparent cardiovascular disease (CVD) were recruited from 6 United States communities. Individuals with a history of physician-diagnosed heart attack, angina, heart failure, stroke or TIA, or having undergone an invasive procedure for cardiovascular disease (CABG, angioplasty, valve replacement or pacemaker placement) were excluded from participation. Enrolled participants returned for follow-up clinic examinations on 3 subsequent examinations (visits 2, 3 and 4) at approximately 18 to 24-month intervals. All participants provided written informed consent and the institutional review boards (IRB) at the participating Universities approved the study.
At clinic visits 2 and 3, a random subsample of 1,970 participants from 5 of the 6 MESA field centers enrolled in an ancillary study to determine the presence and extent of calcified atherosclerosis in the abdominal aorta using computed tomography.15 The venous blood samples taken contemporaneously were subsequently analyzed for selected measures of adiposity-associated inflammation, as well as kidney function to include plasma renin activity and aldosterone. The data obtained on this subset of participants comprises the analytic sample for the current study.
Data Collection
At all clinic visits, standardized questionnaires were used to obtain sociodemographic, race/ethnicity and health history information. Cigarette smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumferences were measured using a standard flexible tape measure. Resting blood pressure was measured 3 times in seated participants with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon). The calculation of blood pressure was based on the average of the second and third readings. Hypertension (HTN) was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or current use of an antihypertensive medication.
Laboratory
At all clinic examinations, blood samples obtained in the sitting position were obtained after a 12-hour fast. Blood was drawn after the participants had been resting in the sitting position for about 1 hour. During this time, they did not perform physical activity. Participants were instructed to take their usual medications before the clinic visit. Processing of the samples began no longer than 30 minutes after the blood draw and the tubes were placed on ice during the lag time. Tubes were centrifuged at 4° C at least 2000 g for 15 minutes or 3000 g for 10 minutes, for a total of 30,000 g-minutes. Once centrifugation was complete, tubes were placed on ice in preparation for pooling and aliquoting. Aliquots were frozen at −70 ° C within 10 minutes of preparation.
The blood samples were assayed for total and HDL cholesterol, triglycerides, glucose and creatinine levels, as well as measures of systemic inflammation (C-reactive protein, fibrinogen, interleukin-6) and insulin concentration.16 Dyslipidemia was defined as a total-cholesterol/HDL-cholesterol ratio > 5.0 or if the participant used medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycemic medication. Estimated glomerular filtration rate (eGFR) was computed using the CKD-Epi equation.14
Stored fasting blood samples obtained at clinic visits 2 and 3 were analyzed to provide serum concentrations of adiponectin, leptin, plasma renin activity and aldosterone. The adipokines (leptin and total adiponectin) were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA). Average analytical coefficients of variation across several control samples for these analytes ranged from 6.0-13.0%. Plasma renin activity was measured using the GammaCoat Plasma Renin Activity 125I radioimmunoassay (RIA) Kit (DiaSorin; Stillwater, MN), while aldosterone was measured using a competition-based radioimmunoassay (ALDOCTK-2; Diasorin, Stillwater, MN). The intra-assay coefficients of variation for PRA ranged from 6.89 to 18.38% and 6.30 to 8.87% for aldosterone, respectively. All assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Statistical Analysis
Among the 1,970 potential participants, there were 81 individuals who were missing values for aldosterone and the covariates, resulting in a final analytic sample of 1,889 participants available for analysis when aldosterone was the outcome. Similarly, there were 169 individuals who were missing values for PRA and the covariates leaving a sample size of 1,801 for these analyses.
Characteristics of the population were described with a mean and standard deviation for continuous variables, while categorical variables were summarized as a count and percentage of the study population. Multivariable linear regression was used to assess the association between adipokines and both PRA and aldosterone (separately). This was done using 1-standard deviation increments of the adipokines, as well as categorizing adiponectin and leptin into quartiles to assess for potential threshold effects and non-linearity in the associations. For all regression analyses, the initial model included adjustments for age, gender and race/ethnicity. Models were subsequently adjusted for body mass index (model 2), diabetes, dyslipidemia, smoking and hypertension medication use (model 3), estimated glomerular filtration rate (model 4) and, for when aldosterone was the outcome, PRA (model 5). To account for the potential confounding due to the effects of central adiposity, we also performed the analyses described above but replaced BMI with values for height and the waist to hip ratio.
Multiplicative interactions between each adipokine and gender, race/ethnicity and body mass index were assessed. A two-tailed p-value < 0.05 was considered statistically significant for all analyses, except interaction terms where a p-value < 0.20 was considered significant. All statistical analyses were conducted using STATA (Version 11; StataCorp, College Station, TX).
RESULTS
The characteristics of the study cohort are provided in Table 1. The mean age of the cohort was 64.7 years and 50% were female. Forty percent were non-Hispanic White, 26% were Hispanic/Latino, 21% were African American and 13% were Chinese American. The mean BMI was 28.2 kg/m2 and 31% had a BMI greater than 30 kg/m2. Most (46%) were never smokers, whereas 43% were former smokers and 11% were current smokers. Over half (54%) of the participants were hypertensive, 40% were classified as dyslipidemic and 14% as having diabetes mellitus. The mean serum creatinine and eGFR were 0.94 mg/dl and 78 mL/min/1.73 m2, respectively.
TABLE 1.
COHORT CHARACTERISTICS
| Characteristic | Mean (SD)/Median or Frequency (%) |
|---|---|
| Age (years) | 64.7 (9.7)/64.5 |
| Gender (male) | 987 (50) |
| Race | |
| - Non-Hispanic White | 791 (40.0) |
| - Chinese American | 259 (13.1) |
| - African American | 412 (20.9) |
| - Hispanic American | 514 (26.0) |
| Height (cm) | 166.4 (9.9)/166.4 |
| Waist circumference (cm) | 98.3 (14.2)/97 |
| Hip circumference (cm) | 104.4 (10.9)/102.8 |
| BMI (kg/m2) | 28.2 (5.2)/27.4 |
| Waist to Hip Ratio | 0.94 (0.07)/0.94 |
| Total Cholesterol (mg/dl) | 189.6 (35.5)/188 |
| HDL Cholesterol (mg/dl) | 51.5 (15.2)/49 |
| Cholesterol Medication Use | 481 (25.1) |
| Dyslipidemia | 784 (39.9) |
| Glucose (mg/dl) | 98.3 (27.7)/91 |
| Diabetes | 283 (14.4) |
| Cigarette Smoking | |
| - Current | 228 (11.6) |
| - Former | 841 (42.6) |
| - Never | 904 (45.8) |
| Systolic Blood Pressure (mmHg) | 124 (20.8)/121 |
| Diastolic Blood Pressure (mmHg) | 70.8 (9.9)/69.5 |
| Hypertension | 1068 (54) |
| Calibrated Creatinine (mg/dl) | 0.96 (0.27)/0.92 |
| Calibrated eGFR (mL/min/1.73 m2) | 77.6 (17.3)/78.1 |
| Adiponectin (μg/ml) | 20.7 (13.2)/17.4 |
| Leptin (ng/ml) | 20.9 (22.3)/13.5 |
| Renin (ng/ml) | 1.45 (3.3)/0.56 |
| Aldosterone (pg/ml) | 150.1 (86.9)/130.5 |
In men and women, the mean adiponectin levels were 17.0 and 24.5 μg/ml, while the mean values of leptin were 10.8 and 31.1 pg/ml, respectively. The mean values for adiponectin and leptin among the four ethnic groups were as follows: African American: 17.2 μg/ml and 24.1 pg/ml, Chinese American: 24.1 and 8.5, Hispanic Americans: 20.3 and 18.9, non-Hispanic White: 22.9 and 17.4, respectively. The correlations between BMI and adiponectin were modest and somewhat different between men (r = −0.18, p = 0.01) and women (r = −0.25, p = 0.01), while the correlations between BMI leptin were similar and strong for both men (r = 0.63, p = 0.01) and women (r = 0.64, p = <0.01). Correlations between BMI and both adiponectin and leptin in the different ethnic groups were: African Americans: r = −0.19 and 0.58, p < 0.01 for both; Chinese Americans: −0.18 and 0.50, p < 0.01 for both; Hispanic Americans: −0.13 and 0.52, p < 0.01 for both; non-Hispanic White: −0.31 and 0.58 respectively, p < 0.01 for both.
Associations of Adiponectin with Plasma Renin Activity and Aldosterone
With adjustment for age, gender and race/ethnicity, a 1-SD increment of adiponectin was not associated with PRA level (β = 0.01 ng/ml, p = 0.85)[Table 2]. Conversely, after the same adjustment for age, a 1-SD increment in adiponectin was significantly associated with a 6.0 pg/ml lower aldosterone level (p < 0.01). In the fully adjusted model, adiponectin remained significantly associated with lower aldosterone (β = −5.5, p = 0.01) and the addition of PRA minimally changed the association (β = −5.4, p = 0.02). When BMI was replaced by height and the waist to hip ratio, adiponectin remained non-significantly associated with PRA (β = −0.06, p = 0.46), but significantly associated with lower aldosterone (β = −4.7, p = 0.04).
TABLE 2.
MULTIVARIABLE LINEAR REGRESSION OF ADIPONECTIN AND LEPTIN FOR PLASMA RENIN ACTIVITY AND ALDOSTERONE
| PLASMA RENIN ACTIVITY (PRA) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 | Leptin | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
| β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | ||
| Continuous (1-SD)* | 0.01 | 0.85 | 0.09 | 0.32 | 0.07 | 0.41 | 0.04 | 0.60 | Continuous (1-SD) | 0.57 | <0.01 | 0.60 | <0.01 | 0.59 | <0.01 | 0.55 | <0.01 |
| Categorical** | Categorical* | ||||||||||||||||
| - Quartile 2 | −0.29 | 0.20 | −0.23 | 0.32 | −0.13 | 0.55 | −0.13 | 0.57 | - Quartile 2 | 0.27 | 0.23 | 0.22 | 0.34 | 0.01 | 0.95 | −0.02 | 0.92 |
| - Quartile 3 | −0.13 | 0.58 | 0.02 | 0.92 | 0.19 | 0.42 | 0.20 | 0.39 | - Quartile 3 | 0.57 | 0.02 | 0.49 | 0.07 | 0.29 | 0.28 | 0.21 | 0.42 |
| - Quartile 4 | 0.02 | 0.91 | 0.27 | 0.28 | 0.40 | 0.11 | 0.32 | 0.20 | - Quartile 4 | 1.12 | <0.01 | 0.96 | <0.01 | 0.76 | 0.03 | 0.66 | 0.05 |
| ALDOSTERONEE | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 | Leptin | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
| β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | ||
| Continuous (1-SD)* | −6.0 | <0.01 | −5.7 | 0.01 | −5.1 | 0.02 | −5.5 | 0.01 | Continuous (1-SD) | 8.5 | < 0.01 | 10.7 | <0.01 | 9.3 | <0.01 | 8.4 | <0.01 |
| Categorical** | Categorical* | ||||||||||||||||
| - Quartile 2 | −8.1 | 0.16 | −8.0 | 0.16 | −10.0 | 0.08 | −9.8 | 0.08 | - Quartile 2 | 6.5 | 0.26 | 9.0 | 0.13 | 4.3 | 0.46 | 3.3 | 0.57 |
| - Quartile 3 | −12.8 | 0.03 | −12.7 | 0.03 | −11.2 | 0.06 | −10.7 | 0.08 | - Quartile 3 | 14.3 | 0.02 | 19.2 | <0.01 | 14.2 | 0.03 | 12.2 | 0.07 |
| - Quartile 4 | −23.6 | <0.01 | −23.4 | <0.01 | −21.8 | <0.01 | −22.8 | <0.01 | - Quartile 4 | 27.7 | <0.01 | 37.1 | <0.01 | 29.8 | <0.01 | 27.1 | <0.01 |
β = parameter estimate, Sig = Level of significance (p-value)
Model 1: Age, gender, race/ethnicity; Model 2: Model 1 + body mass index; Model 3: Model 2 + Dyslipidemia + Diabetes + Smoking + HTN Meds; Model 4: Model 3 + creatinine
Standard Deviations: Leptin = 22.3 ng/ml, Adiponectin = 13.2 μg/ml
Quartile Cut-points: Leptin – Q1: < 4.47, Q2: 4.47 – 11.14, Q3: 11.14 – 24.77, Q4: > 24.77; Adiponectin: Q1: < 11.82, Q2: 11.82 – 17.52, Q3: 17.52 -26.32, Q4: > 26.32 Units: PRA (ng/ml), Aldosterone (pg/ml)
The results of multivariable linear regression when adiponectin was considered as a categorical variable and divided into quartiles are provided in Figures 1 & 2. Compared to the lowest quartile and after adjustment for age, gender and race/ethnicity, there were modest, non-significant differences in PRA with each higher quartile of adiponectin (Figure 1), which was not changed after full adjustment. Conversely, after multivariable adjustment, there were step-wise increases in the differences in aldosterone with each higher quartile of adiponectin (Figure 2). More specifically, compared to the 1st quartile, the 2nd, 3rd and 4th quartiles of adiponectin were associated with 9.8 (p = 0.08), 10.7 (p = 0.08) and 22.8 (p < 0.01) pg/ml lower aldosterone levels. Addition of leptin to the models described above, as well as PRA to the models where aldosterone was the outcome, did not materially change the findings.
FIGURE 1. DIFFERENCES IN LEVELS OF PLASMA RENIN ACTIVITY BY QUARTILES OF LEPTIN AND ADIPONECTIN.
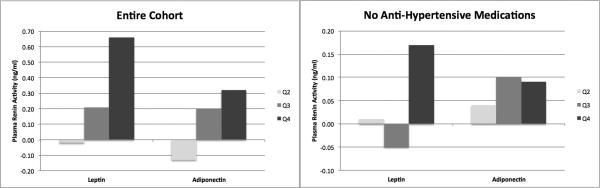
Referent Category = Quartile 1; Q2 = 2nd Quartile, Q3 = 3rd Quartile, Q4 = 4th Quartile Adjusted for age, gender, race/ethnicity, body mass index, diabetes, dyslipidemia, smoking and estimated glomerular filtration rate
FIGURE 2. DIFFERENCES IN LEVELS OF ALDOSTERONE BY QUARTILES OF LEPTIN AND ADIPONECTIN.

Referent Category = Quartile 1; Q2 = 2nd Quartile, Q3 = 3rd Quartile, Q4 = 4th Quartile Adjusted for age, gender, race/ethnicity, body mass index, diabetes, dyslipidemia, smoking and estimated glomerular filtration rate
Associations of Leptin with Plasma Renin Activity and Aldosterone
With adjustment for age, gender and race/ethnicity, a 1-SD increment of leptin was associated with a 0.57 ng/ml higher PRA level (p < 0.01) [Table 2]. Additional adjustments for BMI (β = 0.60, p < 0.01), diabetes, dyslipidemia, smoking and hypertension medication use (β = 0.59, p < 0.01) and estimated glomerular filtration rate (β = 0.55, p < 0.01) did not materially change the magnitude and significance of the association. Similarly, after adjustment for age, gender and race/ethnicity, the same increment in leptin was significantly associated with an 8.5 pg/ml higher aldosterone level (p < 0.01). In the fully adjusted model, leptin remained significantly associated with higher aldosterone (β = 8.4, p < 0.01), which was modestly attenuated when PRA was added (β = 7.2, p < 0.01). When BMI was replaced by height and the waist to hip ratio, leptin remained significantly associated with higher PRA (β = 0.38, p < 0.01), but not associated with higher aldosterone (β = 3.9, p = 0.12).
The results of multivariable linear regression when leptin was considered as a categorical variable and divided into quartiles are provided in Figures 1 & 2. Compared to the lowest quartile and after adjustment for age, gender and race/ethnicity, there was a step-wise increase in the differences in PRA with each higher quartile of leptin (Figure 1), which was maintained after full adjustment. More specifically, in the final multivariable model, the 2nd, 3rd and 4th quartiles of leptin were associated with 0.0 (p = 0.92), 0.21 (p = 0.42) and 0.66 (p = 0.05) ng/ml higher PRA levels. Likewise, there were step-wise increases in the differences in aldosterone with each higher quartile of leptin (Figure 2). That is, compared to the 1st quartile, the 2nd, 3rd and 4th quartiles of leptin were associated with 3.3 (p = 0.57), 12.2 (0.07) and 27.1 (p < 0.01) pg/ml higher aldosterone levels. Addition of adiponectin to the models described above, as well as PRA to the models where aldosterone was the outcome, did not materially change the findings.
Effect of Antihypertensive Medications
Participants who were on anti-hypertensive medications (n = 1,117) were significantly older (67.6 vs. 62.5 years, p < 0.01) and had significantly higher waist (101.6 vs. 95.7 cm, p < 0.01) and hip circumferences (106.0 vs. 102.9 cm, p < 0.01), body mass index (29.2 vs. 27.3, p < 0.01), fasting glucose (102.6 vs. 95.1 mg/dl, p < 0.01), serum creatinine (1.0 vs. 0.9 mg/dl, p < 0.01), interleukin – 6 (2.6 vs. 2.2 pg/ml, p < 0.01), fibrinogen (446 vs. 427 mg/dl, p < 0.01) and resistin (17.5 vs. 15.5 pg/ml, p < 0.01), but significantly lower total (183.7 vs. 194.3 mg/dl, p < 0.01) and HDL (50.6 vs. 52.0 mg/dl, p < 0.05) cholesterol levels. Those on these medications were also more likely to be African American (p < 0.01), diabetic or a former smoker, as well as being on a cholesterol lowering medication (p < 0.01 for all), but were not significantly different by gender or CRP level.
Among those not taking an anti-hypertensive medication and after adjustment for age, gender and race/ethnicity, a 1-SD increment in adiponectin was not significantly associated with PRA level (−0.07 ng/ml, p = 0.18). This association remained non-significant after full adjustment (-0.03 ng/ml, p = 0.56) and the results were not materially different when adiponectin was categorized into quartiles (Figure 1). On the other hand, a 1-SD increment in adiponectin was associated with significantly lower aldosterone levels (−6.7 pg/ml, p < 0.01), which was modestly changed with full adjustment (−5.3 pg/ml, p = 0.05). Categorizing adiponectin into quartiles revealed a step-wise association with aldosterone (Figure 2).
For leptin, 1-SD increment was associated with significantly higher PRA (0.12 ng/ml, p = 0.03), which was not remarkably changed with full adjustment (0.13 ng/ml, p = 0.06). Analyses utilizing quartiles of leptin revealed that those in the highest quartile (Q4) drove this association, where PRA levels were 0.17 ng/ml higher than those in the first quartile (Figure 1). Conversely, a 1-SD increment in leptin was not significantly associated with higher aldosterone (4.0 pg/ml, p = 0.23). Categorizing leptin into quartiles revealed a step-wise increases in aldosterone (Figure 2). While potentially clinically relevant, none were statistically significantly different from the value for those in the first quartile.
Differences in the Associations by Gender and Race/Ethnicity
Using multiplicative interaction terms, we tested for significant differences in the magnitudes of the associations between both adiponectin and leptin for PRA and aldosterone (separately) by gender and then race/ethnicity. There were no significant interactions between gender and either adiponectin or leptin for PRA or aldosterone. Similarly, there were no significant interactions between race/ethnicity and adiponectin for either PRA or aldosterone. However, there was a significant interaction between race/ethnicity and leptin for PRA (p < 0.01), as well as aldosterone (p < 0.01) [Table 3]. After adjustment for age, gender, BMI, diabetes, dyslipidemia, smoking, hypertension medication use and estimated glomerular filtration rate, a 1-SD increment in leptin was associated with 1.74 ng/ml higher PRA level among non-Hispanic Whites (p < 0.01), 0.95 higher among Chinese Americans (p < 0.01) and 0.15 higher among Hispanic Americans (p = 0.39), but a 0.13 lower PRA among African Americans (p = 0.39). The findings were similar for the associations between leptin and aldosterone. Specifically, a 1-SD increment in leptin was associated with 15.9 (p < 0.01) and 14.9 (p = 0.15) pg/ml higher aldosterone for non-Hispanic Whites and Chinese Americans respectively, while being 6.0 (p = 0.17) and 4.0 (p = 0.45) pg/ml higher among African and Hispanic Americans.
TABLE 3.
DIFFERENCES IN THE ASSOCIATIONS BETWEEN LEPTIN AND BOTH PLASMA RENIN ACTIVITY AND ALDOSTERONE BY ETHNIC GROUP
| Race/Ethnicity | Sample Size (N) | RENIN | ALDOSTERONE | ||
|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | ||
| Non-Hispanic White | 699 | 1.74 | <0.01 | 15.9 | <0.01 |
| Chinese American | 233 | 0.95 | <0.01 | 14.9 | 0.15 |
| African American | 325 | −0.13 | 0.39 | 6.0 | 0.17 |
| Hispanic American | 499 | 0.15 | 0.39 | 4.0 | 0.45 |
Adjusted for age, gender, race/ethnicity, body mass index, diabetes, dyslipidemia, smoking and hypertension medication use, estimated glomerular filtration rate
* Per SD increment in Leptin (22.3 ng/ml) and Adiponectin (13.2 μg/ml) within each racial group
Effect Modification by Body Mass Index
The mean levels of leptin and adiponectin were significantly different by BMI group. For BMI values of 18.5 – 25, 25 – 30, 30 – 35 and > 35 kg/m2, mean concentrations of adiponectin were 24.9, 19.8, 18.1 and 16.0 μg/ml, while mean leptin concentrations were 8.9, 17.1, 34.5 and 57.6 ng/ml, respectively.
There were no significant multiplicative interactions between either adiponectin or leptin with BMI group for PRA. Conversely, there were significant interactions between adiponectin (p = 0.17) and leptin (p < 0.01) with BMI group for aldosterone (Figure 3). In multivariable linear regression analyses, a 1-SD increment in adiponectin was associated with 2.5 pg/ml higher aldosterone among those with a BMI between 18.5 and 25, while for those with BMI values between 25 and 30, 30 and 35 and greater than 35, the same increment of adiponectin was associated with −3.2, −9.5 and −23.4 pg/ml, respectively, lower aldosterone concentrations. On the other hand, a 1-SD increment of leptin was associated with 8.2, 9.5, 2.9 and 1.3 pg/ml, respectively, higher aldosterone concentrations. When those on anti-hypertensive therapy were removed from the analysis, the results were similar.
FIGURE 3. ASSOCIATIONS BETWEEN ALDOSTERONE AND BOTH ADIPONECTIN AND LEPTIN BY BODY MASS INDEX GROUP.
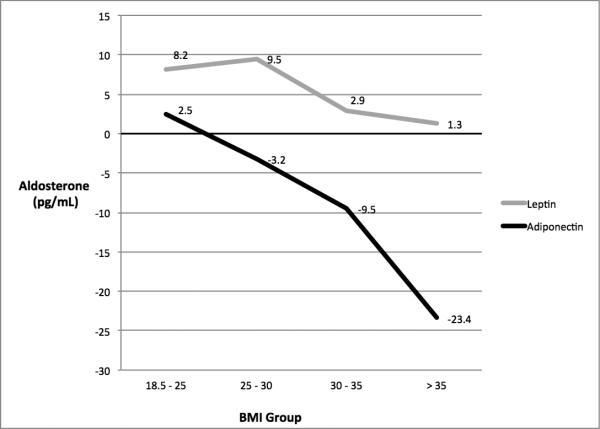
Adjusted for age, gender, race/ethnicity, body mass index, diabetes, dyslipidemia, smoking, estimated glomerular filtration rate and plasma renin activity *Per SD increment in Leptin (22.3 ng/ml) and Adiponectin (13.2 μg/ml) within each BMI strata
DISCUSSION
In this study of a large, multi-ethnic population-based cohort from multiple sites across the United States, higher leptin concentrations were associated with significantly higher levels of both PRA and aldosterone while higher adiponectin concentrations were significantly associated with lower levels of aldosterone only. For both adipokines, the significance of the associations was independent of relevant covariates to include measures of total and central adiposity. Notably, the association between both adipokines and aldosterone remained significant after additional adjustment for PRA. Moreover, among those not taking any anti-hypertensive medications, the magnitudes of the associations between adiponectin and leptin and both PRA and aldosterone were attenuated but similar in pattern to those for the entire cohort. Additionally, there was a significant interaction between leptin and race/ethnicity for both PRA and aldosterone, whereby, compared to African- and Hispanic Americans, the magnitudes of these associations were significantly larger among non-Hispanic Whites and Chinese Americans. Finally, the associations between both adipokines and aldosterone were significantly different by BMI group. Taken together, the findings from this study suggest that both adiponectin and leptin may be related to blood pressure regulation via the RAAS, that the associations appear to be robust to anti-hypertension medication use and that the associations are likely different by ethnicity and BMI group.
In all forms of hypertension, there is a shift in renal pressure natriuresis to a higher blood pressure level resulting in sodium retention and expanded plasma volume.17,18 This change in sodium reabsorption may be due to the direct effects of SNS activity on the proximal and distal renal tubules, stimulation of the RAAS or both. 19 In this regard, a major determinant of SNS activity is the amount of excess adipose tissue.10,20 Since leptin is derived principally from this tissue type, higher levels of adiposity can result in chronically higher leptin levels. Also, leptin has been shown to stimulate the region of the hypothalamus that regulates SNS outflow to the periphery.8,21 According to the model just described, the higher levels of adiposity associated with obesity could result in a chronic increase SNS activity due to the hypothalamic stimulatory effects of leptin and that may be manifest by higher levels of both renin and aldosterone. Our results are consistent with this paradigm.
Despite the highest prevalence of hypertension of any racial/ethnic group, 22 African Americans are known to have lower levels of renin, 23 which has led to the hypothesis that the RAAS does not play a significant causative role in hypertension in this group. More specifically, increased sympathetic tone among African Americans may not result in the same elevations in RAAS markers as among other ethnic groups. As such, blood pressure elevation may be attributable to overload and an increased sensitivity to sodium ingestion that both lowers RAAS activity and causes hypertension. 24 The findings of our study are consistent with this paradigm. That is, despite leptin levels that were higher than those for the other 3 ethnic groups, the association between leptin and renin was null and not significant among African Americans while being significant for non-Hispanic Whites and Chinese Americans.
An interesting finding from our study is that the association between leptin and aldosterone was significantly different by BMI group. Specifically, the magnitude of the association between leptin and aldosterone decreased when BMI was in the obese range and suggests that the influence of leptin on aldosterone levels may be attenuated at higher levels of adiposity. This is consistent with the hypothesized concept of leptin resistance25, which is similar to that for insulin whereby, despite higher levels of leptin, the effects are diminished. In this regard, previous studies in animal models demonstrate attenuation of the metabolic, but not autonomic nervous system, effects of leptin with higher levels of obesity.3,5,26 More specifically, the effects of leptin on free fatty acid uptake and oxidation in skeletal muscle are diminished while stimulation of the sympathetic nervous system remains seemingly unchanged. Moreover, reports on the association between leptin and left ventricular mass show an inverse association among those with a BMI with the normal to overweight range27-29, but a positive association in those who are obese.26
Contrasted with the associations between leptin and both PRA and aldosterone, higher adiponectin levels were only significantly [and inversely] associated with aldosterone, which remained after adjustment for both leptin and PRA. Like leptin, adiponectin is secreted primarily by adipose tissue, but, unlike leptin, increasing adiposity typically results in lower levels of adiponectin.30 Of note, previous studies have shown that aldosterone can reduce adiponectin either directly or by increasing TNF-α, which also decreases levels of adiponectin.30,31 Moreover, Li and colleagues have shown that aldosterone and TNF-alpha act synergistically to lower adiponectin levels.4,32 Since increasing SNS stimulation results in higher levels of angiotensinogen and angiotensin II33, and this results in higher aldosterone levels, it is plausible that the SNS stimulation present in those with obesity-associated hypertension will result in lower adiponectin levels due to higher levels of aldosterone. If true, rather than stimulating the RAAS [as is hypothesized for leptin], it would seem that higher adiponectin is the result of upregulation of downstream RAAS components.
Perhaps unexpectedly, the associations between both adiponectin and leptin with aldosterone were independent of PRA. This suggests that there may be an alternative pathway that does not include renin and that results in higher levels of aldosterone. In this respect, it has been demonstrated that, in addition to expressing adiponectin and leptin, adipose tissue can release angiotensinogen and angiotensin II, both of which are stimulates of aldosterone release.34 As such, one would expect that, with increasing adiposity, increases in aldosterone could parallel to the appropriate changes for adiponectin and leptin and be due to stimulation by angiotensinogen and/or angiotensin II.
Strengths of this study include a large, multi-ethnic cohort that allowed for comparison of associations across gender, ethnicity, body mass index and anti-hypertension medication use, as well as very reproducible assays of both adiposity-associated inflammation and selected markers of the renin-angiotensinogen-aldosterone system affording the opportunity to test the study aims. The primary limitation is the cross-sectional study design that precludes elimination of reverse causality as a potential source of error in the interpretation of the results. As such, longitudinal studies of these measures seem warranted.
In conclusion, adiponectin and leptin are significantly associated with markers of the renin-angiotensin-aldosterone system and these measures appear to be robust across anti-hypertension medication use, as well as being different by ethnicity and body mass index groups. The findings for leptin suggest that this adipokine may contribute to higher levels of both renin and aldosterone via activation of the sympathetic nervous system, while lower adiponectin may be due to higher levels of aldosterone that is a result of the increasing adiposity of obesity. Confirmation of these claims by longitudinal studies is recommended.
SUMMARY TABLE.
What is Known About the Topic:
- Previous studies have demonstrated a robust association between greater adipose tissue and higher levels of blood pressure, as well as incident hypertension.
- Although undoubtedly a multifactorial phenomenon, hypertension in obese individuals has been linked with activation of the renin-angiotensinogen-aldosterone system (RAAS) and plasma volume expansion.
- In this regard, higher sympathetic nervous system (SNS) activity, present in obese individuals, results in increased renin release, aldosterone production, and renal tubular sodium reabsorption.
- However, the pathophysiologic links between excess adiposity and higher blood pressure are incompletely understood.
What this Study Adds:
- The findings of this study indicate that both leptin and adiponectin are significantly associated with markers of the renin-angiotensinogen-aldosterone system.
- In the case of adiponectin, the associations are independent of plasma renin activity levels.
- These associations are not materially different among those not taking blood pressure medications and there are differences by ethnicity, as well as levels of adiposity.
ACKOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Research Support:
This research was supported by grants R01-HL-088451 and R01-DK-080015, as well as contracts N01-HC-95159 through N01-HC-95169 from the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
The authors have no financial or other material conflicts of interest to report.
REFERENCES
- 1.Ahima RS. Central actions of adipocyte hormones. Trends in endocrinology and metabolism: TEM. 2005;16:307–313. doi: 10.1016/j.tem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Barba G, et al. Plasma leptin and blood pressure in men: graded association independent of body mass and fat pattern. Obes Res. 2003;11:160–166. doi: 10.1038/oby.2003.25. [DOI] [PubMed] [Google Scholar]
- 3.Jones DW, Kim JS, Andrew ME, Kim SJ, Hong YP. Body mass index and blood pressure in Korean men and women: the Korean National Blood Pressure Survey. J Hypertens. 1994;12:1433–1437. doi: 10.1097/00004872-199412000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr. Pharm. Des. 2004;10:3621–3637. doi: 10.2174/1381612043382855. [DOI] [PubMed] [Google Scholar]
- 5.Jones DW. Body weight and blood pressure. Effects of weight reduction on hypertension. Am J Hypertens. 1996;9:50s–54s. doi: 10.1016/0895-7061(96)00183-5. [DOI] [PubMed] [Google Scholar]
- 6.Bełtowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 7.Hausberg M, et al. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens. 2002;20:1633–1641. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Rahmouni K, Correia MLG, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 9.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montani J-P, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26(Suppl 2):S28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. Am J Hypertens. 1989;2:125S–132S. doi: 10.1093/ajh/2.3.125s. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Louis K. Dahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesity. Hypertension. 1994;23:381–394. doi: 10.1161/01.hyp.23.3.381. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Criqui MH, et al. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S–55S. [PubMed] [Google Scholar]
- 19.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA: The Journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 21.Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes Relat Metab Disord. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 22.Go AS, et al. Heart Disease and Stroke Statistics—2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure differences between blacks and whites. Am J Hypertens. 1999;12:555–562. doi: 10.1016/s0895-7061(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 24.Richardson SI, Freedman BI, Ellison DH, Rodriguez CJ. Salt sensitivity: a review with a focus on non-Hispanic blacks and Hispanics. J Am Soc Hypertens. 2013;7:170–179. doi: 10.1016/j.jash.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correia ML, de G, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13:215–223. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39:486–490. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 27.Pladevall M, et al. The association between leptin and left ventricular hypertrophy: a population–based cross-sectional study. J Hypertens. 2006;24:1447–1473. doi: 10.1097/00004872-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Lieb W, et al. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–605. doi: 10.1016/j.amjcard.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allison MA, et al. Relation of Leptin to Left Ventricular Hypertrophy (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2013;112:726–730. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 31.Flynn C, Bakris GL. Interaction between Adiponectin and Aldosterone. Cardiorenal Med. 2011;1:96–101. doi: 10.1159/000327023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, et al. Aldosterone perturbs adiponectin and PAI-1 expression and secretion in 3T3-L1 adipocytes. Horm. Metab. Res. 2011;43:464–469. doi: 10.1055/s-0031-1277226. [DOI] [PubMed] [Google Scholar]
- 33.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23:1170–1178. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 34.Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Reviews in endocrine & metabolic disorders. 2013;14:49–58. doi: 10.1007/s11154-012-9230-8. [DOI] [PubMed] [Google Scholar]


