Abstract
Abetalipoproteinemia is a rare metabolic disorder that causes disturbed lipid absorption with consequent hypocholesterolaemia and liposoluble avitaminosis. The broad spectrum of presentations includes malabsorption, failure to thrive and acanthocytosis in children, while later in life expected manifestations include coagulopathy, myopathy, retinitis pigmentosa, peripheral neuropathy, hyporeflexia and ataxia. These neurological complications stem from demyelination secondary to vitamin E deficiency. Another complication is reduced fertility in women. In the event of a successful conception, issues arise in vitamin supplementation, the mainstay of treatment of abetalipoproteinemia. The skilful clinician must master the delicate balance between the teratogenic effects on the fetus of over as well as under replacement of vitamins, pregnancy complications such as premature rupture of membranes and eclampsia, and, finally, maternal complications such as corneal ulcers. We describe the management of a patient ranging from pubertal growth to bearing a successful spontaneous pregnancy with an outcome of a completely healthy mother and child.
Background
Abetalipoproteinemia, eponymously Bassen-Kornzweig disease, is a rare metabolic disorder (<1 per million) caused by mutations of the microsomal triglyceride transfer protein gene that follows an autosomal recessive pattern of inheritance. There is complete absence of plasma apolipoprotein (apo) B-containing lipoproteins, namely chylomicrons, marked hypocholesterolaemia, absence of low-density lipoprotein (LDL) cholesterol and low triglyceride concentrations. In addition, increased serum aminotransferases due to hepatic steatosis and fat-soluble vitamin deficiency are found.1
Interestingly, coronary arteries of abetalipoproteinemia patients appear to be free of atherosclerotic plaques. In keeping with this fact, pharmacological inhibition of the microsomal triglyceride transfer protein gene is being studied as a novel method to reduce plasma cholesterol and prevent cardiovascular disease. Therefore, establishing the long-term clinical outcome of patients with an inborn deficiency in microsomal triglyceride transfer protein might provide insight into the possible side effects of the treatment.2 3
Furthermore, following of such cases might grant us guidelines to perfect vitamin supplementation regimes and hence optimise management of abetalipoproteinemia and also familial hypolipoproteinemia patients. It will contribute to avoiding late and devastating complications such as hyporeflexia, reduced proprioception and vibratory sensation, muscle weakness, ataxia, retinitis, as well as promote fertility and prevent fetal and maternal complications.
Case presentation
Our patient, currently a 37-year-old Caucasian woman, had the diagnosis of abetalipoproteinemia confirmed at Great Ormond Street Hospital at age 4. She presented with episodes of diarrhoea, abdominal pain and failure to thrive, and had started to walk slightly later than average.
The diagnosis was established on inspection of a fresh blood film, which showed acanthocytosis in virtually every cell, and biochemical studies that demonstrated a plasma total cholesterol of 1.68 mmol/L with 1.57 mmol in the high-density lipoprotein (HDL) fraction, plasma triglyceride less than 0.1 mmol/L and no betalipoprotein band on agarose electrophoresis. The patient was also subjected to a fat loading test, and after consumption of 30 mg of fat there was no rise in plasma triglyceride and no evidence of chylomicrons at any time. Genetic testing for mutations in microsomal triglyceride transfer protein gene was not carried out as the clinicians at Great Ormond Street deemed the aforementioned results sufficient to establish the diagnosis and the family was not keen to undergo further investigations.
On this occasion the patient was started on oral fat-soluble vitamin supplementation, which comprised a daily regime of 1200 mg vitamin E, 10 mg vitamin K, Ketovite liquid 5 mL and an additional 50 000 units of vitamin A weekly.
She had a brother who manifested the same condition, but sadly died at age 15 from causes unrelated to abetalipoproteinemia. There is no consanguinity in the family.
The patient came under our care in May 2003, when her general practitioner questioned the need for her heavy vitamin E supplementation regime. Generally, she had always been compliant with her therapy. Throughout follow-up there has been no peripheral neuropathy or posterior column involvement, except for some evidence of retinal dystrophy.
She attempted to conceive for 2 years, and was then referred to a fertility clinic and started on clomifene therapy in 2008. It is noteworthy that her partner had continuously demonstrated a low sperm count. The fertility treatment proved unfruitful and was discontinued. They subsequently travelled to Amsterdam for a holiday, where the patient achieved her much desired goal, a spontaneous pregnancy.
At the beginning of pregnancy our patient stood at 165 cm and weighed 59 kg.
It was an uneventful gestation, apart from one episode of vaginal bleeding sustained in 11/2009, and in March 2010 she gave birth to a healthy baby boy. No complications were ever noted in either mother or child.
Investigations
On diagnosis, there was evidence of acanthocytosis, total plasma cholesterol of 1.68 mmol/L and plasma triglyceride levels of 0.1 mmol/L. There was no betalipoprotein band on electrophoresis and no evidence of chylomicrons at any time. The paternal lipid profile was normal.
Vitamin A, D, K and E serum levels, and vitamin E/serum cholesterol ratio were intensively monitored at each clinic appointment. Therapy was titrated to achieve normal levels.
Urea, creatinine and electrolytes were frequently checked, including calcium.
Liver function tests were performed routinely since there is an association between abetalipoproteinemia and fatty liver.
Throughout follow-up, the patient's liver function tests have been normal in all parameters of bilirubin, international normalised ratio (INR), albumin, aspartate aminotransferase and alkaline phosphatase, but her alanine transaminase has been raised to 100, 110, 79 and 62 iu/L in May 2008, April 2009, October 2012 and December 2012, respectively.
On liver ultrasound scan there was no evidence of steatosis.
Thyroid function was also monitored, since the patient suffers from hypothyroidism. It is interesting to note that a case of subclinical hypothyroidism associated with abetalipoproteinemia has been previously described in the literature.4
As part of the infertility screening, the patient's progesterone levels were measured at mid-luteal phase and registered 22.1 nmol/L, which is within the normal range of 9–110 nmol/L.
Her retinae showed sparse fine granulations peripherally, arguably compatible with early stages of retinitis pigmentosa (figure 1). There was no bone spicule-type pigment clumping. These did not evolve. There were no visual field defects on perimetry at any stage.
Figure 1.
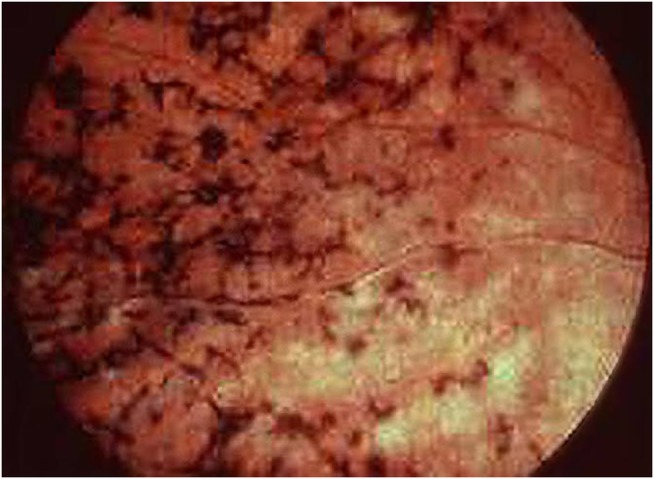
Retinitis pigmentosa.
Throughout follow-up the patient was monitored for neurological deficits through clinical examination of cranial nerve function, limb motor power, tone, coordination, reflexes, sensation and proprioception. No deficits were ever noted.
As of May 2014 her total cholesterol was 1.0 mmol/L, tryglicerides 0.1 mmol/L and HDL 1.0 mmol/L, with no LDL detectable.
Differential diagnosis
The presentation of the patient would be compatible with either abetalipoproteinemia and familial homozygous or compound heterozygous hypobetalipoproteinemia, but the fact that both of her parents’ lipid profiles are normal excludes the latter, since they follow an autosomal dominant inheritance pattern.
Treatment
After diagnosis as a child, the patient was started on a low-fat diet, based mostly on medium chain fatty acids, to control steatorrhoea and prevent failure to thrive.
She was also started on vitamin E supplements 1200 mg daily and vitamin A 2500 IU, vitamin D 400 IU, choline chloride 150 mg and cyanocobalamin 12.5 µg daily in the form of multivitamin liquid formulation. She received, additionally, vitamin A 50 000 units weekly and vitamin K 10 mg weekly. The team decided to treat her orally as opposed to intravenously as they believed this would make the regime less painstaking.
In her childhood, the patient attained satisfactory weight and height centile, and her diet was liberalised with respect to lipid consumption. Once past the early growth spurt, medium chain fatty acid preparations were discontinued, since there is evidence that medium chain fatty acids compete with long chain fatty acids in the portal vein transport system for serum albumin binding sites and that long-term medium chain fatty acid administration in abetalipoproteinemia patients can provoke a micronodular liver cirrhosis.
Throughout follow-up vitamin E was dosed according to the vitamin E/serum cholesterol ratio, since vitamin E serum levels are not a reliable indicator of adequate vitamin E supplementation due to a short half-life.
When the patient presented to us she was, as mentioned above, receiving 1200 mg of vitamin E daily. This proved insufficient to maintain an adequate vitamin E/serum cholesterol ratio and increments in the dose of vitamin E were undertaken from the time of presentation until pregnancy, and the desired ratio was attained by the current dosage, as demonstrated in table 1. When the patient started attempts at conception, vitamin E supplementation was increased from 3000 mg daily to 4000 mg.
Table 1.
Increments in vitamin E dosage titrated to vitamin E/serum cholesterol levels
| Vitamin E/serum cholesterol ratio (reference range >2.2) | Vitamin E dose | |
|---|---|---|
| May 2003 | 1.5 | Increased to 2000 mg |
| October 2003 | 1.7 | Increased to 2500 mg |
| March 2004 | 2 | Increased to 3000 mg |
| September 2004 | 2.4 | Maintained |
| Vitamin E dose maintained since ratio >2.2 | ||
| June 2008 (attempting pregnancy) |
2.3 | Increased to 4000 mg |
| Vitamin E dose maintained since ratio >2.2 | ||
| July 2009 (pregnant patient) |
4.2 | Maintained |
| October 2009 (pregnant patient) |
4 | Maintained |
| February 2010 (pregnant patient) |
4 | Maintained |
The titration of the remaining vitamin dosages was performed while aiming to keep vitamin serum levels within normal range. Vitamin K was titrated against INR while other vitamins were titrated according to reference ranges (table 2).
Table 2.
Titration of liposoluble vitamins during pregnancy, according to plasma levels and INR
| Vitamin A Plasma level (µg/dL) |
Vitamin A Dose change |
Vitamin D Plasma level (ng/mL) |
Vitamin D Dose change |
Vitamin K INR |
Vitamin K Dose change |
|
|---|---|---|---|---|---|---|
| July 2009 (pregnant patient) |
63 | Maintained at 5000 IU | 65 | 800 IU changed to 400 IU | 1.0 | Maintained at 10 mg |
| October 2009 (pregnant patient) |
60 | Maintained at 5000 IU | 45 | Maintained at 400 IU | 0.9 | Maintained at 10 mg |
| February 2010 (pregnant patient) |
60 | Maintained at 5000 IU | 50 | Maintained at 400 IU | 1.1 | Maintained at 10 mg |
INR, international normalised ratio.
As of February 2014 the patient's daily therapeutic regime consists of the doses included in table 3.
Table 3.
Daily medication
| Vitamin | Dose | Serum levels monitored: reference ranges |
|---|---|---|
| A | 5000 IU daily | 30–65 µg/dL |
| D | 400 IU daily | 8–60 ng/mL |
| E | 4 g daily | Normal vitamin E/cholesterol ratio >2.2 |
| K | 10 mg daily | 0.2–3.2 ng/mL (INR also monitored) |
INR, international normalised ratio.
Outcome and follow-up
As mentioned above, liposoluble vitamin levels were intensively monitored with frequent neurological examination for deficits. On pregnancy there was active vigilance for gestational complications associated with abetalipoproteinemia. Apart from one episode of vaginal bleeding, the pregnancy was uneventful, and mother and child remain healthy. The patient is currently alive and well and has not manifested abnormal neurological findings. It is noteworthy that the patient's son does not suffer from abetalipoproteinemia and is a healthy young boy who had normal plasmatic liposoluble vitamin levels at birth, as illustrated in table 4.
Table 4.
Plasmatic liposoluble vitamin levels of the newborn
| Vitamin A | 28 µg/dL |
| Vitamin D | 17.9 ng/mL |
| Vitamin E | 2.38 mg/L |
| Prothrombin time (correlates with vitamin K plasma levels) |
12 s |
Discussion
Since one of the core features of Bassen-Kornzweig syndrome is a chronic low level of serum cholesterol, it is expected that in female patients, pregnenolone, and subsequently progesterone production, will be decreased. In keeping with this fact, women frequently face fertility problems since the concentrations of progesterone produced by the corpus luteum might be insufficient to maintain adequate uterine proliferation and the placenta might not produce enough levels to harbour a successful pregnancy.5
This has seldom been discussed in the literature, with published reports of pregnancies in only three patients.
In one of the instances, the study revolved around the hormonal profile of a young Greek woman.6 The patient was diagnosed at age 31, severely underweight, and treated with lipid restriction and vitamin supplementation on a daily regime of total amount of fat not greater than 20 g mostly in the form of medium chain fatty acids, liposoluble vitamin supplements and, additionally, 600 mg of vitamin E.
Her hormonal profile was evaluated once more at age 36 and it was not significantly changed (apart from a rise of 17-OH-progesterone from 0.1 to 0.2 ng/L). Within 2 years of therapy her anthropometric and serum parameters improved, with a rise from vitamin E levels of 0 to 0.36 mg% and she became pregnant.
Even though the authors believe there is no influence of dietary changes and vitamin changes on the hormonal profile of the patient, the fact that she was previously infertile might point to the need for further investigation, or at the very least highlights the importance of improving the patient's nutritional profile and body mass index if she is considering conception.
Nevertheless, a significant path of investigation will likely be the possible benefits of progesterone supplementation in infertile abetalipoproteinemia patients. It is likely that progesterone is the culprit hormone as opposed to oestrogen, since there is evidence that required oestrogen levels are lower than required progesterone levels, and therefore even low serum LDL suffices for production of the former.5
According to the authors, the patient chose to terminate pregnancy due to the uncertainty of the influence of maternal disease on fetal outcomes and due to social circumstances.
It is noteworthy that this young woman later developed an array of neurological complications, arguably due to the late diagnosis and implementation of therapy.
Another report describes two pregnancies in a woman with abetalipoproteinemia describing contrasting outcomes of subtherapeutic versus normal vitamin levels.7
The patient presented as a 26-year-old primigravida, having been diagnosed at age 18 months with abetalipoproteinemia. She was non-compliant with therapy from ages 7 to 22. At the time of presentation, there were night vision deficits, retinitis pigmentosa, peripheral neuropathy and a history of falls thought to be related to deranged proprioception.
Prior to conception, she was restarted on supplementation with vitamins A 750 mg and D 200 IU daily, and vitamin E 1600 mg and vitamin K 5 mg twice weekly.
The patient was always poorly compliant with supplementation, and it is noteworthy that she entered labour with a preterm premature rupture of membranes, delivering a 1722 g baby boy with significant bilateral colobomata of the irides and choroid.
During her second pregnancy, she was highly compliant with supplementation, apart from vitamin E, and she delivered a healthy baby boy, even though there was again preterm premature rupture of membranes.
In the first instance, hypovitaminosis A can be a likely link to the manifested fetal ophthalmic defects. Vitamin A is thought to be necessary for growth and cell differentiation in the developing fetus. Deficiency has been shown to be teratogenic in lower animals, causing skeletal anomalies.8 Furthermore, there is good evidence that in humans, maternal hypovitaminosis A is linked to congenital reduction in visual acuity and to the emergence of colobomata.9
In both pregnancies, there was premature rupture of membranes, with no recognised predisposing factors. Research has been showing that vitamin E might be necessary to protect placental integrity and that hypovitaminosis E and C might predispose to premature rupture of membranes.10
Finally, a 46-year-old woman of Northern European descent was diagnosed with abetalipoproteinemia at age 11, when she presented with right ptosis.11 She was started on high oral doses of fat-soluble vitamins approximately 9 years after her diagnosis. As an adult, she had developed neurological symptoms such as decreased visual acuity on the right side, a wide-based gait, decreased vibration sense in upper and lower limbs, decreased position sense in the lower limbs and absent knee reflexes.
At age 34, she had given birth to a full-term healthy baby boy. During her pregnancy she was advised to stop all intake of vitamin A to avoid the potential risk of vitamin A teratogenicity, despite the fact that her serum β-carotene concentration was below the lower limit of the normal range. Postpartum, she developed a right corneal ulcer that required corneal transplantation after a year. She was restarted on vitamin A postpartum. Her corneal transplant failed and, of note, she was not able to achieve a second pregnancy despite multiple attempts.
Considering the above discussed cases, it would be easy to be enthusiastic about maximising the supplementation therapy protocols in abetalipoproteinemia. It is, nevertheless, prudent to remember that liposoluble vitamin replacement is a double-edged sword, and hypervitaminosis A and D can have serious consequences both in the mother and the fetus. We include some possible clinical consequences of hypovitaminosis and hypervitaminosis A, D, E and K, in mother and child (table 5).
Table 5.
Maternal and fetal consequences of hypovitaminosis and hypervitaminosis A, D, E and K (adapted from Gaudet et al7)
| Fat-soluble vitamin | Maternal risks of deficiency | Maternal risks of excess | Fetal risks of deficiency | Fetal risks of excess |
|---|---|---|---|---|
| A | Follicular hyperkeratosis Impaired resistance to infection Night blindness Xerophthalmia Preeclampsia |
Hyperostosis Hepatotoxicity Alopecia Increased cerebrospinal fluid pressure Hypercalcaemia |
Ophthalmic defects | Neural tube defects Craniofacial, central nervous system, cardiac and thymic malformations |
| D | Osteomalacia | Hypercalcaemia | Hypocalcaemia Rickets |
Aortic stenosis Calcium deposition in brain and other organs |
| E | Ophthalmoplegia Peripheral neuropathy Hyporeflexia Decreased proprioception Ataxia |
Bleeding Impaired leucocyte function |
Low birth weight | None documented |
| K | Bleeding disorders Possible effects on bone density |
None documented | Neonatal bleeding Increased risk of spontaneous abortion |
None documented |
Taking this discussion into account, it is reasonable to assume that early diagnosis and institution of vitamin replacement followed by strict compliance with therapy optimises adult patient outcomes, including in the obstetric setting.
Ultimately, how well an adult abetalipoproteinemia patient will do in terms of neurological sequelae depends on the extent of nerve tissue damage and degeneration, which is only halted by adequate vitamin E supplementation. Since vitamin E appears to be safe in large doses apart from patients with coagulopathy and those taking specific anticoagulant medication, it is wise to follow the current guidelines for vitamin E (approximately 100 mg/kg or 5–10 g/day)12 and to continue the treatment throughout pregnancy. On the other hand, there appears to be no advantage in heterozygous familial hypobetalipoproteinemia patients receiving vitamin E supplementation.13
When our patient started attempts at conception, vitamin E supplementation was not only continued but increased from 3000 mg daily to 4000 mg, with the goal of preventing placental complications.
All other liposoluble vitamins should also ideally be replaced from childhood and should not be discontinued during pregnancy, since fetal malformations, eclampsia and maternal bleeding, ophthalmic and musculoskeletal complications can occur.
Essential fatty acids (EFA), especially n−3 EFA, are pivotal for pregnancy and neurological development of the fetus. Our patient did not receive oral supplementation of EFA, nor were maternal or newborn plasmatic levels monitored. This might be an appropriate routine strategy to manage this type of patient, with the topic being worthy of further studies.
Patient's perspective.
I think other patients with my condition might be at risk of complications, since I believe that doctors might not explain properly to patients how important it is to take the vitamins every day. Ordinary people usually think vitamin supplementation is, as the name implies, something that is optional and that they can do without.
I would say doctors should reinforce that this is an exceptional situation. I was lucky enough to be treated by good clinicians who always stressed how important it is to be compliant with treatment, and this is one of the reasons why I am free of complications and my child is a healthy boy.
Learning points.
- Consider the diagnosis of abetalipoproteinemia in failure to thrive
- Early vitamin replacement and dietary modifications improve clinical outcome, lessening the extent of permanent irreversible neurological sequelae, which are highly debilitating.
- Check concordance with treatment
- As very relevantly mentioned by our patient, most patients might be misled to believe that omitting simple vitamins is of no relevance, since they might underestimate their therapeutic power. Liquid formulations might ensure better compliance as opposed to many different capsules.
- Ensure therapeutic ranges of vitamins A, D, K and E
- Vitamin supplementation must not be discontinued during pregnancy.
- Preeclampsia, premature rupture of membranes and fetal malformations are all possible complications. Bear in mind the opposite end of the scope, where hypervitaminosis is teratogenic. An appropriate strategy to avoid hypervitaminosis is the frequent monitoring of plasmatic levels and dosing in accordance.
- It is likely that increasing the usual vitamin E dose supplementation during pregnancy is adequate and safe to prevent placental complications in most cases, but further studies are needed.
- Infertility investigation
- Abetalipoproteinaemia patients might benefit from progesterone supplementation, and further studies on this and hormonal profiles would benefit this pool of patients.
- Optimising the nutritional profile and body mass index of female patients will undoubtedly contribute to better chances at conception.
Acknowledgments
The authors wish to thank our patient for her generous contribution; Mr Christopher Davey, Pharmacist; Mr Stephen Ayre, Library Services Manager; and Mr Martin Hewitt, Medical Photographer.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Burnett JR, Bell DA, Hooper AJ et al. Clinical utility gene card for: abetalipoproteinaemia. Eur J Hum Genet 2012;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welty FK. Hypobetalipoproteinemia and abetalipoproteinemia. Curr Opin Lipidol 2014;25:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper AJ, Burnett JR. Recent developments in the genetics of LDL deficiency. Curr Opin Lipidol 2013;24:111–15. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mahdili HA, Hooper AJ, Sullivan DR et al. A mild case of abetalipoproteinaemia in association with subclinical hypothyroidism. Ann Clin Biochem 2006;43: 516–19. [DOI] [PubMed] [Google Scholar]
- 5.Illingworth DR, Corbin DK, Kemp ED et al. Hormone changes during the menstrual cycle in abetalipoproteinemia: reduced luteal phase progesterone in a patient with homozygous hypobetalipoproteinemia. Proc Natl Acad Sci USA 1982;79:6685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triantafillidis JK, Kotaras G, Peros G et al. Endocrine function in abetalipoproteinemia, a study of a female patient of Greek origin. Ann Ital Chir 2004;75:683–90. [PubMed] [Google Scholar]
- 7.Gaudet LM, MacKenzie J, Smith GN. Fat-soluble vitamin deficiency in pregnancy: a case report and review of abetalipoproteinemia. J Obstet Gynaecol Can 2006;28:716–19. [DOI] [PubMed] [Google Scholar]
- 8.Rader DJ, Brewer HB Jr. Abetalipoproteinemia: new insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease. JAMA 1993;270:865–9. [DOI] [PubMed] [Google Scholar]
- 9.Seeliger MW, Biesalski HK, Wissinger B et al. Phenotype in retinal deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci 1999;40:3–11. [PubMed] [Google Scholar]
- 10.Woods JR, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:5–10. [DOI] [PubMed] [Google Scholar]
- 11.Zamel R, Khan R, Pollex RL et al. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis 2008;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traber MG, Shils ME, Shike M et al. Modern nutrition in health and disease. 10th edn Baltimore, MD: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 13.Clarke MA, Hooper AJ, Headlam HA et al. Assessment of tocopherol metabolism and oxidative stress in familial hypobetalipoproteinemia. Clin Chem 2006;52:1339–45. [DOI] [PubMed] [Google Scholar]


