Abstract
Objectives
Direct injury to the corticospinal tract (CST) is a major factor defining motor impairment after stroke. Diffusion tensor imaging (DTI) tractography allows definition of the CST. We sought to determine whether DTI-based assessment of the degree of CST damage correlates with motor impairment at each phase of ischemic stroke.
Methods
We evaluated patients at the acute (3–7 days), subacute (30 days), and chronic (90 days) phases of ischemic stroke with DTI and clinical motor scores (upper extremity Fugl-Myer test [UE-FM], motor items of the National Institutes of Health Stroke Scale [mNIHSS]). The CST was identified and virtual fiber numbers (FN) were calculated for the affected and contralateral CST. We used Spearman correlation to study the relationship of FN ratio (FNr) (affected/unaffected CST) with motor scores at each time point, and the regression model to study the association of the acute parameters with chronic motor scores.
Results
We studied 23 patients. Mean age was 66.7 (±12) years. FNr correlated with UE-FM score in the acute (r = 0.50, P = 0.032), subacute (r = 0.57, P = 0.007), and chronic (r = 0.67, P = 0.0008) phase, and with mNIHSS in the acute (r = −0.48, P = 0.043), subacute (r = −0.58, P = 0.006), and chronic (r = −0.75, P = 0.0001) phase. The combination of acute NIHSS and FNr significantly predicted chronic UE-FM score (r = 0.74, P = 0.0001).
Interpretation
DTI-defined degree of CST injury correlates with motor impairment at each phase of ischemic stroke. The combination of baseline FNr and NIHSS predicts motor outcome. DTI-derived CST assessment could become a surrogate marker of motor impairment in the design of neurorestorative clinical trials.
Introduction
Motor impairment is the main element of poststroke disability, present in 50% of survivors older than 65 years at 6 months after stroke onset.1 The severity of disability at the outcome phase of stroke has been associated with several factors, including baseline stroke severity,2 volume and location of ischemic lesion,3 and treatment with thrombolysis in the acute phase.4,5 Despite major efforts in improving and expanding acute-phase therapies, the treatment options for ischemic stroke remain limited. A new treatment paradigm, perhaps focusing on brain repair and reorganization after the acute phase of stroke, is needed. Developing reproducible and valid biomarkers of the underlying motor impairment as well as the brain processes contributing to motor recovery from stroke will be an important step toward organizing rational restorative treatment paradigms.
Magnetic resonance imaging (MRI) has the unparalleled ability to provide both structural and physiological information on the brain.6 Diffusion tensor imaging (DTI) is based on the movement of water and permits quantification of the diffusion characteristics of water molecules in vivo and thereby delineation of the anatomic connectivity of white matter pathways,7 providing thus a means for detecting pathological tract disruption. The diffusion measurements can be used to calculate the fractional anisotropy (FA),8 a metric that reflects the degree of directional preference in water diffusion. FA is directly correlated with histological markers of myelination9; high FA values signify white matter tract integrity; loss of anisotropy is typically seen in the setting of acute ischemic stroke.10
Three-dimensional images of white matter fiber tracts can be reconstructed based on DTI data, a process named diffusion tensor tractography (DTT). This process is based on connecting the fiber orientation information pixel-by-pixel. In this way, the proximity of a tract to the ischemic lesion can be visualized. The corticospinal tract (CST) is the most studied tract in the context of motor function after stroke. Previous investigations on CST have primarily focused on patients in the chronic phase of stroke. Unilateral reductions of CST integrity gave rise to interhemispheric asymmetries in FA values11 due to either local tissue damage or Wallerian degeneration. Previous studies examined small homogeneous samples of subcortical stroke patients and found that larger asymmetries in FA were associated with poorer motor recovery.12,13 In a larger study, also addressing patients with chronic ischemic stroke, structural integrity of the CST motor fibers on DTI was found to correlate with motor impairment.14
It remains uncertain whether DTI-derived measures of CST injury in the early stages of ischemia correlate with the early motor impairment and also whether they correlate with the final motor outcome after ischemic stroke. The presence of edema within the ischemic lesion has been offered as a possible explanation for poor correlations.15 In addition, it remains uncertain how the CST changes evolve as the ischemic lesion evolves from the acute to subacute and chronic stages.
We performed the present prospective observational study in order to evaluate whether DTI-based quantitative assessment of the degree of ischemic injury to the CST correlates with the motor impairment clinical scores at each phase of ischemic stroke in patients with various locations of their ischemic lesions.
Methods
This was a prospective observational study conducted in a tertiary care academic hospital. The study was approved by the Institutional Review Board of the Henry Ford Health System. Written informed consent was obtained from all participating patients (or their next of kin, when the patient was unable to provide informed consent).
Patients
We prospectively enrolled patients fulfilling the following criteria: (1) diagnosis of ischemic stroke, (2) National Institutes of Health Stroke Scale (NIHSS) score ≥4, (3) age ≥18 years, (4) informed consent was obtained.
Major exclusion criteria were: (1) prior history of stroke or other neurological disorder that would hamper accurate clinical assessment of poststroke recovery, (2) deemed unlikely to present for follow-up by the investigator, (3) life expectancy less than 90 days, (4) prestroke modified Rankin score was >2 (due to any non-neurological disorder), (5) any underlying serious illness that could make the clinical assessments unreliable, and (6) unable to undergo MRI studies for any reason.
Clinical assessments
Each patient was evaluated with DTI and clinical scores at three time points: (1) acute: 3–7 days from stroke onset, (2) subacute: 4 weeks (±7 days) from stroke onset, and (3) chronic: 3 months (±1 week) from stroke onset.
The total clinical neurological deficit was graded using the NIHSS score.16 The degree of motor impairment of the affected arm was graded using the upper extremity Fugl-Myer test (UE-FM),17 and the score for the affected arm and leg motor strength of the NIHSS (items 5 and 6) was used as a measure of the severity of hemiparesis (mNIHSS). For the latter, we did not include the scores for facial palsy and dysarthria, because these deficits result from damage to the corticobulbar tract and not CST tract that was reconstructed in the present study. The UE-FM is a 30-item clinical scale representing conventional testing of muscle strength used in the evaluation of motor function of stroke patients. Its range of score is 0–66, with higher scores indicating better motor function of the arm.
Magnetic resonance imaging
MRI scans were obtained at each one of the three time points that the patient underwent the clinical assessments.
Image acquisition
All MRI studies were acquired in 3T GE Signa MRI scanner on the same day (±24 h) as the clinical assessments.
The image acquisition protocol included: (1) DTI using dual echo-planar imaging (EPI) sequence with TR = 8000 msec, TE = 86.5 msec, FOV = 24 cm, imaging matrix 96 × 96, slice thickness 4 mm (2.5 × 2.5 × 4 mm3 voxel size), 31 slices, b-value of 1500 sec/mm2, 55 diffusion attenuate directions, 1 average; (2) T2-weighted imaging (T2WI) using fast spin-echo sequence with effective TE of 30 and 117 msec, TR of 2500 msec, imaging matrix 320 × 224, 4 mm thick; (3) T1-weighted imaging (T1WI) using gradient echo sequence with TE of 14 msec, TR of 500 msec, imaging matrix 320 × 224, 4 mm thick.
Image analysis
The MRI data were transferred to a computer workstation with Microsoft Windows operating system and were processed using 3D Slicer (version 3) open source image analysis software platform (www.slicer.com). The first step was to convert the diffusion weighted (DWI) DiCom images to nearly raw raster data (NRRD) format. Then, the DWI image was filtered in the mean squared error sense using a Rician noise model for noise reduction.18 Diffusion tensors were calculated for all voxels within the brain with the least squares method. The diffusion tensor D in the voxel can be visualized as an ellipsoid, with the eigenvectors indicating the directions of the principal axes, and the square root of the eigenvalues defining the ellipsoidal radii.19 FA was calculated for each tensor. FA is useful to characterize the shape of the diffusion ellipsoid.20
DTT was performed to reconstruct the CST based on DTI data and using a single tensor module.21 Fiber tracts were traced using the streamline algorithm, which connects neighboring voxels by propagating the ends of fiber tracts from user-defined seed voxels. The orientation of the fibers is collinear with the direction of the principal eigenvector, which is the eigenvector with the largest eigenvalue. The threshold for terminating the tracking process was: FA < 0.1 and cosine of the turning angle >0.8. The fibers represent the streamline composing a tract.
A multiple region of interest (ROI) approach was used to reconstruct the CST, which requires knowledge of the anatomical brain landmarks that the CST passes through (corona radiata, posterior limb of internal capsule [PLIC], and cerebral peduncle). For identification of the anatomical landmarks, DTI color-coded maps were used. In the DTI color maps green, blue, and red colors were assigned to anterior–posterior, superior–inferior, and left-to-right orientations, respectively. The areas of corona radiata, posterior limp of internal capsule, and cerebral peduncle are blue in color since the fibers that are passing through are having superior–inferior direction. The blue color of DTI color maps in combination with knowledge of neuroanatomy guided us to standardize the placement of ROIs. To standardize the size of the ROIs we calculated the number of voxels that each of them covered. In some cases the ischemic lesion was disrupting a large segment of the anatomical landmarks. In these cases, the disrupted segment was appearing as a black, rather than blue, area on the DTI color maps. This factor in combination with guidance from the unaffected contralateral area allowed us to standardize the placement of the ROI in those cases as well.
The first ROI was drawn symmetrically in the bilateral corona radiata; this ROI was used as a seed point to generate the fibers of the CST (Fig. 1). The second ROI was drawn symmetrically in the posterior limb of bilateral internal capsules (Fig. 1). A logical AND function was used to generate a tract with fibers that pass only through both of the corona radiata and PLIC.22 A third ROI was drawn symmetrically in the bilateral cerebral peduncle (Fig. 1). A logical AND function was also used to reconstruct a tract with fibers that pass through all three of the corona radiata, PLIC, and cerebral peduncle (Fig. 2). These three ROIs correspond to the anatomical landmarks along the course of the CST. We excluded fibers that coursed in and out of the cerebellum by drawing ROIs in the middle cerebellar peduncle and superior cerebellar peduncle and applying a logical NOT function in conjunction with the other ROIs. Additionally, we excluded fibers that crossed to the contralateral side at the level of the pons by drawing an ROI and applying a logical NOT function.
Figure 1.
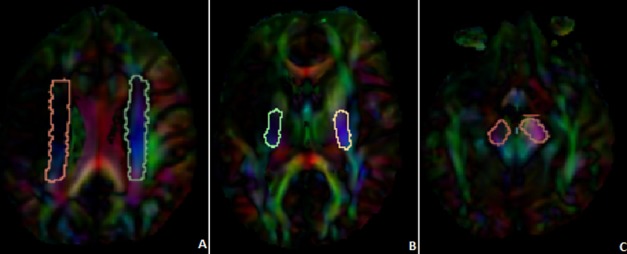
Demonstration of the ROIs used for CST analysis. The first ROI was drawn in the corona radiata (A), the second ROI in the PLIC (B), and the third ROI in the cerebral peduncle (C). ROIs, regions of interest; CST, corticospinal tract; PLIC, posterior limb of internal capsule.
Figure 2.
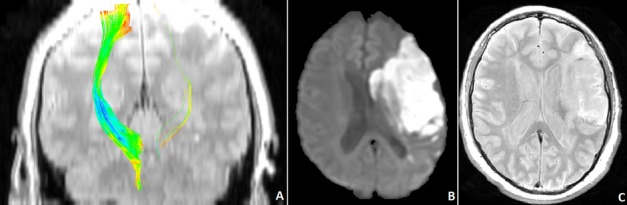
Demonstration of the affected and unaffected corticospinal tracts from a 67-year-old man with a left hemispheric infarct in the distribution of the middle cerebral artery, at the subacute phase (30 days after onset) (A). The infarct location and size are shown in the acute (diffusion weighted [DWI] image) (B) and subacute (proton density image) (C) phase of stroke.
The number of virtual fibers of the ipsilesional (FNi) and contralesional (FNc) CST as well as the fiber number ratio (FNr) were calculated, the latter by dividing the FNi by FNc (FNi/FNc).
Statistical analysis
To study the relationship of the degree of damage to CST (FNi, FNc and FNr) with the UE-FM score and the mNIHSS at each time point, Spearman correlation was used, since both clinical assessments, UE-FM and mNIHSS, were not normally distributed. The LASSO (Least Absolute Shrinkage and Selection Operator) approach, a shrinkage and selection method for linear regression, was used to identify a combination of acute and subacute variables that is associated with chronic UE-FM score. The NIHSS score and the UE-FM score were included in the regression analysis.
Results
We enrolled 23 patients with ischemic stroke, 14 (61%) were women. Mean age was 66.7 (±12) years. The data of these patients are summarized in Table 1. Four patients underwent the acute-phase study in a 1.5T scanner according to the institutional clinical protocol and not in the 3T scanner according to the study protocol and therefore no CST results could be produced. Two patients did not return for the subacute and chronic phase studies and therefore no clinical or CST data could be produced.
Table 1.
Demographics and stroke location of the entire study population
| Variable | Response | (N = 23) |
|---|---|---|
| Age | N | 23 |
| Mean (±SD) | 66.7 (±12) years | |
| Gender | F | 14 (61%) |
| M | 9 (39%) | |
| Affected hand | L | 10 (44%) |
| R | 12 (52%) | |
| None | 1 (4%) | |
| Handedness | L | 1 (4%) |
| R | 22 (96%) | |
| Hemisphere | L | 12 (52%) |
| R | 11 (48%) | |
| Stroke location | Left subcortical | 2 (9%) |
| Left hemispheric, cortically based | 10 (43%) | |
| Right subcortical | 3 (13%) | |
| Right hemispheric, cortically based | 7 (30%) | |
| cerebellar/brainstem | 1 (4%) |
The mean NIHSS score was: 10.7 (±5.5) in the acute phase, 6.1 (±5.7) in the subacute phase, and 4.8 (±4.6) in the chronic phase. The mean UE-FM score was: 25 (±22) in the acute phase, 39 (±24) in the subacute phase, and 45 (±23) in the chronic phase (Fig. 3B). The mean mNIHSS was: 4.2 (±2.9) in the acute phase, 2.1 (±2.6) in the subacute phase, and 1.8 (±2.4) in the chronic phase of ischemic stroke.
Figure 3.
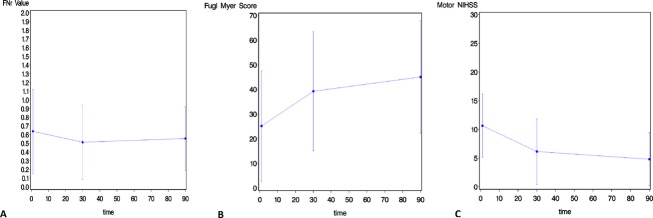
Graphs demonstrating the change in FNr value (A), upper extremity Fugl-Myer score (B), and motor NIHSS score (C) from the acute to subacute and chronic phase of ischemic stroke. FNr, fiber number ratio; NIHSS, National Institutes of Health Stroke Scale.
The evolution of the FNr, UE-FM, and mNIHSS scores from the acute to subacute to chronic stages of stroke is depicted in Figure 3A–C, respectively.
The location of the ischemic lesion was as follows: cortically based left hemispheric (n = 10, 43%), left subcortical (n = 2, 9%), cortically based right hemispheric (n = 7, 30%), right subcortical (n = 3, 13%), and cerebellar/brainstem (n = 1, 4%) (Table 1).
The FNr was significantly correlated with the UE-FM score at each phase of ischemic stroke: acute (r = 0.50, P = 0.032, n = 18), subacute (r = 0.57, P = 0.007, n = 21), and chronic (r = 0.67, P = 0.0008, n = 21). Interestingly, these correlations became stronger when moving from the acute to subacute and chronic phases of stroke (Table 2).
Table 2.
Correlation analysis (correlation and P-values) between imaging parameters and motor function scores at the acute, subacute, and chronic phases of ischemic stroke
| Motor score | Phase | FNr |
|---|---|---|
| UE-FM | Acute | 0.50 |
| 0.032 | ||
| Subacute | 0.57 | |
| 0.007 | ||
| Chronic | 0.67 | |
| 0.0008 | ||
| mNIHSS | Acute | −0.48 |
| 0.043 | ||
| Subacute | −0.58 | |
| 0.006 | ||
| Chronic | −0.75 | |
| 0.0001 |
FNr, fiber number ratio; UE-FM, upper extremity Fugl-Myer test; mNIHSS, motor items of the National Institutes of Health Stroke Scale.
Similarly, there were significant negative correlations between FNr and mNIHSS at each phase of ischemic stroke: acute (r = −0.48, P = 0.043, n = 18), subacute (r = −0.58, P = 0.006, n = 21), and chronic (r = −0.75, P = 0.0001, n = 21). As seen in the correlations between FNr and UE-FM scores, these correlations became stronger when moving from the acute to subacute to chronic phase of stroke (Table 2).
In addition, we found that the FNr at both the acute and subacute phases, the acute mNIHSS, the acute NIHSS, and the acute UE-FM score were significantly associated with the UE-FM score at the chronic phase (Univariate analysis P < 0.05). Of them, acute mNIHSS, acute NIHSS, and acute UE-FM score were highly correlated (r in the range of 0.76-0.93) and they were included in the initial multivariable LASSO regression model separately along with FNr at both the acute and subacute phases. After the model selection, two variables (NIHSS and FNr at acute phase) were retained in the model. The combination of NIHSS and FNr regression, 61.93-2.4 × NIHSS + 14.28 × FNr (at acute phase) resulted in R2 = 0.5426 (r = 0.74) based on observed data and 0.4276 (r = 0.64) based on cross-validation data (see Fig. 4).
Figure 4.
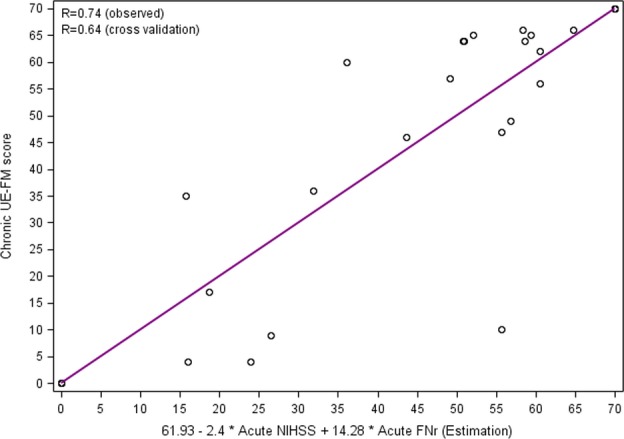
Scatterplot displaying the results of regression analysis for prediction of chronic UE-FM score on the basis of acute-phase data. The y-axis represents the UE-FM at 90 days, and the x-axis represents the estimated point based on regression 61.92867 - 2.4077 × NIHSS at acute phase + 14.2827 × FNr at acute phase. UE-FM, upper extremity Fugl-Myer test; NIHSS, National Institutes of Health Stroke Scale; FNr, fiber number ratio.
Discussion
We performed quantitative assessment of the CST in patients with ischemic stroke and found significant correlations between the degree of DTT-defined CST damage and the severity of motor impairment in every phase (acute, subacute, and chronic) of ischemic stroke. We also found a significant correlation between the degree of CST damage at baseline and motor scores at the outcome phase of stroke.
Our findings indicate that we can reliably define ischemia-induced CST damage and associated motor impairment in stroke patients by measuring virtual fibers of CST. Our study complements previous studies that investigated the correlation between various parameters, such as spatial relationship of CST lesion,23 regional FA and diffusivities in CST-relevant ROIs,14,24 and the degree of motor impairment in patients with chronic stroke, and others that reported on the dynamic evolution of CST changes from baseline to 3 months poststroke25 and the Wallerian degeneration of the CST from baseline to 1 year poststroke26 and the correlations with standardized motor or neurological scores. Our study benefits from providing these correlations at three time points in a larger sample size and addressing all three phases of ischemic stroke and therefore expands previously presented knowledge.
In the acute phase of stroke, the FNr was moderately correlated with the UE-FM score (r = 0.50, P = 0.032) and the mNIHSS (r = −0.48, P = 0.043). Our results complement those of Nelles et al., who demonstrated significant correlation between fiber disruption on spatial CST analysis and motor scores in patients with acute anterior choroidal artery infarcts.27 In contrast, Liu et al. reported that FA ratio and ipsilateral FA values at 2 weeks after stroke correlated with motor outcome, while there was no correlation in the first 2 weeks28; interestingly, they found elevated FA values in the first hours after stroke, possibly secondary to myelin fiber swelling, followed by a phase of significant FA reduction possibly due to loss of structural integrity of fiber tracts.15 The evolving major changes of brain architecture during the first days after stroke likely result in FA and FN values which may not be a true reflection of the structural integrity of the CST. This FA change likely results from change in the surrounding brain architecture rather than the architecture of the CST itself. In our study, we attempted to eliminate these factors by acquiring the baseline (acute phase) study at 3-7 days after symptom onset.
We also found significant correlation between FNr and clinical motor scores in the late subacute phase (UE-FM [r = −0.57, P = 0.007], mNIHSS [r = −0.58, P = 0.006]). Similar observations were made by Puig et al. who reported a significant correlation between FA ratio and motor deficit at 30 days after stroke.29 The decreased CST FNr in the late subacute phase of stroke likely indicates decreased FA values of the ischemic white matter.
In the chronic phase of stroke, the correlation between FNr and clinical motor scores became stronger than those in the other two phases (UE-FM [r = 0.67, P = 0.0008], mNIHSS [r = −0.75, P = 0.0001]). Our results are in accordance with those of Thomalla et al., who reported a significant correlation between anisotropy decrease and motor deficit at the 90 days after stroke.13 In contrast, Schaechter et al. reported only modest, nonsignificant correlations between affected hand motor function and tractography-based CST integrity in chronic stroke along with reduced fiber numbers of the reconstructed CST.30 Reduced CST FNr after stroke can be related to low FA values in the regions of the tract, possibly due to direct injury by the infarct leading to axonal degeneration and gliosis which affect white matter sites close but also remote to the infarct.13 The strong correlation between FNr and clinical scores at the outcome phase indicates that the FNr reflects the motor skills of stroke patients in this phase.
Our study has a significant advantage over other studies because it provides longitudinal data from three different phases of stroke in a rather large sample size. It is worth noting that the correlations between FNr and motor scores remain significant, and they even become stronger, in the transition from acute to subacute and outcome phases of stroke. Therefore, this DTT scalar can define stroke-related motor impairment independent of time and could become a useful method for monitoring the progress of motor recovery after stroke and perhaps a surrogate marker in stroke recovery clinical trials.
Work in experimental stroke models provides evidence of axonal remodeling after stroke and its association with functional recovery. Compensatory sprouting of axons arising from nondamaged fibers can be found.31 Liu et al. showed significant recovery in CST density in the denervated side of mice spinal cord at 32 days after middle cerebral artery occlusion and significant correlation between axonal density and functional outcome,32 further supporting the notion that DTT of the CST could become a reliable marker of progression of axonal remodeling and perhaps a marker of white matter response to neurorestorative treatments after stroke.
An additional finding of the present study was the significant association of the baseline combination of FNr and NIHSS with the UE-FM score at the chronic phase, based on multivariate LASSO regression approach. The correlation between the acute stroke status and motor recovery at chronic phase was improved from 0.5 for FNr and −0.48 for mNIHSS, respectively, to 0.74 through the FNr and NIHSS combination (Fig. 4) based on the observed data and 0.64 based on the cross-validation results. This confirms findings of others who demonstrated that motor outcome is strongly dependent on lesion location within the CST and involvement of regions such as the PLIC.33 Most experimental interventions in ischemic stroke are more effective when applied in the acute phase, when the potential for positive effect is the greatest. In the future, we could possibly define an FNr and NIHSS threshold that would indicate a good outcome and could be used as an inclusion or exclusion criterion for clinical stroke therapeutic trials. However, given the rather small sample size in our study, we cannot confirm a predictive relationship between baseline FNr and motor outcome after stroke.
The main limitation of our study relates to the question whether the actual number of CST axons can be determined on the basis of DTI data, and whether the number of reconstructed streamlines is a true measurement of the number of actual fibers, as they would be shown on histology. In diffusion MRI, a signal change can be measured when a motion-sensitizing gradient is applied along a given axis. Myelination, axonal density, axonal diameter, membrane permeability, as well as the architectural layout of axons within the voxel are factors that modulate this signal.34 Even if all factors are kept normal, a difference in the intravoxel architectural paradigm will result in difference in anisotropy. Simple models for diffusion, such as that used in the present work, may not be capable of mapping the layout of axons within a voxel, because they only use information from outside the voxel.34–36
The success of reconstructing a continuous path through the DTI data field can also be influenced by additional factors, including errors related to physiological noise and deterministic errors.34,35 During reconstruction of a tract, a “quantitative” score can be calculated and found to correlate with the performance on a task in a group of individuals, as was the case here between CST FNr and UE-FM or mNIHSS scores. We cannot be absolutely certain that the change in those scores is being driven by a specific biological process, but we can indicate that there is a difference in our ability to form a continuous path through the data field, as the result of a series of measurements of signal loss.34 For reconstructing the local fiber orientations with reasonable accuracy, it is important not only to measure signal attenuation along a sufficiently large number of unique orientations but also to model the data in a way that preserves orientation-dependent information. When there are more than one fiber populations in the voxel, the diffusion tensor model is inadequate for this purpose, unless all other features of the pathway and experimental conditions are kept the same so that the only thing that differs is the number of axonal projections.34 It may be more appropriate if we use the term “reconstructed streamlines count” rather than “fiber number,” but, for reasons of simplicity and uniformity with recent publications, we elected to use the term “fiber number,” which is more familiar to the clinician than any of the other terms. Despite these limitations, DTI is extremely sensitive to microstructural changes of brain tissue and could provide a useful first assessment in investigations of white matter disease and repair. A DTI setup of the type used in the current study could also provide q-space DTI information which could allow for additional investigation of the effects of crossing axonal bundles with minimum acquisition time and superior quality of DTI maps than the routine clinical DTI.
We considered including a control group of age-matched, healthy controls, instead of using the contralateral unaffected side of the brain, in order to calculate the FNr, primarily because of the possibility of confounding effect of the white matter hyperintensities (WMH) on the investigated relationship. However, we decided against this because: (1) age-matched, nonstroke controls may also have a significant degree of underlying white matter disease, which would not have made the comparisons more reliable, and (2) real-time results would be needed for these results to be of use in the future design of restorative trials. Using the unaffected CST measurements as control can accomplish the latter purpose.
In conclusion, our study demonstrates that the degree of CST damage by ischemic stroke on DTT correlates with the severity of motor impairment at each phase of ischemic stroke (acute, subacute, and chronic), and that the baseline FNr correlates with the motor outcome. DTT could possibly become an in vivo marker of the severity of stroke-related motor impairment and the response to possible neurorestorative treatments.
Acknowledgments
This work was supported by National Institutes of Health-NINDS Grant R01 NS070922 to w Dr. Mitsias and the Harris Stroke Fund to the Henry Ford Neurosciences Institute. The authors wish to express their gratitude to Joyce Jones, RN, and Theresa Holmes for the coordinating the study and to the Residents of the Department of Neurology, Henry Ford Hospital, for their assistance in recruiting patients for this study.
Authors’ Contributions
Dr. Maraka: acquisition of data, analysis of imaging data, writing major portions of manuscript, and critical revision of the manuscript for important intellectual content. Dr. Jiang: acquisition of imaging data, analysis of imaging data, and study concept and design. Dr. Jafari-Khouzani and Dr. Li: acquisition of imaging data and analysis and interpretation. Dr. Malik: acquisition of data and critical revision of the manuscript for important intellectual content. Hajar Hamidian: acquisition and analysis of imaging data. Talan Zhang: analysis and interpretation. Dr. Lu and Dr. Soltanian-Zadeh: analysis of imaging data and critical revision of the manuscript for important intellectual content. Dr. Chopp: critical revision of the manuscript for important intellectual content. Dr. Mitsias: study concept and design, study supervision, acquisition of data, data analysis and interpretation, writing majority of manuscript, and critical revision of the manuscript for important intellectual content.
Conflict of Interest
Dr. Maraka, Dr. Li, Dr. Malik, Talan Zhang, and Dr. Lu report no disclosures. Dr. Jiang is funded by National Institutes of Health Grant R01 064134. Dr. Jafari-Khouzani, Hajar Hamidian, and Dr. Soltanian-Zadeh are funded by National Institutes of Health Grant R01-EB013227. Dr. Chopp is funded by National Institutes of Health Grant R01 AG 037506. Dr. Mitsias is funded by National Institutes of Health Grant R01 NS070922, the Harris Stroke Fund to Henry Ford Neurosciences Institute, and Michigan Department of Community Health – Michigan Stroke Registry and Quality Improvement Plan.
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel MR, Morgenstern LB, Kwiatkowski T, et al. Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology. 2000;55:952–959. doi: 10.1212/wnl.55.7.952. [DOI] [PubMed] [Google Scholar]
- Phan TG, Demchuk A, Srikanth V, et al. Proof of concept study: relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebrovasc Dis. 2013;35:560–565. doi: 10.1159/000351147. [DOI] [PubMed] [Google Scholar]
- Group NIoNDaSr-PSS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1588. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Moseley M, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14:330–346. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Berman JI, Chung SW, et al. Diffusion tensor MR imaging and Fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008;29:632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, et al. Independent component analysis applied to diffusion tensor MRI. Magn Reson Med. 2002;47:354–363. doi: 10.1002/mrm.10046. [DOI] [PubMed] [Google Scholar]
- Bockhorst KH, Narayana PA, Liu R, et al. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J Neurosci Res. 2008;86:1520–1528. doi: 10.1002/jnr.21607. [DOI] [PubMed] [Google Scholar]
- Ozsunar Y, Grant PE, Huisman TA, et al. Evolution of water diffusion and anisotropy in hyperacute stroke: significant correlation between fractional anisotropy and T2. AJNR Am J Neuroradiol. 2004;25:699–705. [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Honda Y, Fujii Y, et al. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg. 2001;94:955–960. doi: 10.3171/jns.2001.94.6.0955. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury – a review. NMR Biomed. 2002;15:561–569. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- Lyden P, Raman R, Liu L, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke. 2005;36:2446–2449. doi: 10.1161/01.STR.0000185725.42768.92. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Aja-Fernandez S, Niethammer M, Kubicki M, et al. Restoration of DWI data using a Rician LMMSE estimator. IEEE Trans Med Imaging. 2008;27:1389–1403. doi: 10.1109/TMI.2008.920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kabasawa H, Masutani Y, Abe O, et al. Quantitative diffusion tensor analysis using multiple tensor ellipsoids model and tensor field interpolation at fiber crossing. Acad Radiol. 2008;15:84–92. doi: 10.1016/j.acra.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bammer R, Auer M, Keeling SL, et al. Diffusion tensor imaging using single-shot SENSE-EPI. Magn Reson Med. 2002;48:128–136. doi: 10.1002/mrm.10184. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlinska B, Ghinani S, Leppert IR, et al. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75:1048–1054. doi: 10.1212/WNL.0b013e3181f39aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller M, Frandsen J, Andersen G, et al. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol Neurosurg Psychiatry. 2007;78:587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, et al. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47:451–458. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Nelles M, Gieseke J, Flacke S, et al. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol. 2008;29:488–493. doi: 10.3174/ajnr.A0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tian W, Qiu X, et al. Correlation analysis of quantitative diffusion parameters in ipsilateral cerebral peduncle during Wallerian degeneration with motor function outcome after cerebral ischemic stroke. J Neuroimaging. 2012;22:255–260. doi: 10.1111/j.1552-6569.2011.00617.x. [DOI] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31:1324–1330. doi: 10.3174/ajnr.A2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang RL, Li Y, et al. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32:857–863. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, King MD, et al. Limitations and requirements of diffusion tensor fiber tracking: an assessment using simulations. Magn Reson Med. 2002;47:701–708. doi: 10.1002/mrm.10116. [DOI] [PubMed] [Google Scholar]


