Abstract
Objective
The first US Food and Drug Administration–approved clinical trial to treat amyotrophic lateral sclerosis (ALS) with neural stem cell–based therapy is in progress. The goal of the current study was to identify and assess the survival of human spinal cord–derived neural stem cells (HSSCs) transplanted into the spinal cord in patients with ALS.
Methods
Spinal cords transplanted with HSSCs were examined from six autopsy cases. Homogenized tissues were interrogated for the presence of donor versus recipient DNA using real-time PCR methods (qPCR). Fluorescence in situ hybridization (FISH) was performed using DNA probes for XY chromosomes to identify male donor HSSCs in one female case, and immunohistochemistry (IHC) was used to characterize the identified donor cells.
Results
Genomic DNA from donor HSSCs was identified in all cases, comprising 0.67–5.4% of total tissue DNA in patients surviving 196 to 921 days after transplantation. In the one female patient a “nest” of cells identified on H&E staining were XY-positive by FISH, confirming donor origin. A subset of XY-positive cells labeled for the neuronal marker NeuN and stem cell marker SOX2.
Interpretation
This is the first study to identify human neural stem cells transplanted into a human spinal cord. Transplanted HSSCs survived up to 2.5 years posttransplant. Some cells differentiated into neurons, while others maintained their stem cell phenotype. This work is a proof of concept of the survival and differentiation of human stems cell transplanted into the spinal cord of ALS patients.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rare, yet fatal neurodegenerative disease resulting from progressive degeneration of upper and lower motor neurons. ALS patients typically die within 3–5 years from diagnosis due to respiratory failure. Therapeutic options for ALS are limited to a single medication and supportive care, thus driving the search for innovative approaches to slow disease progression and improve survival.1–4 Our group is conducting the first US Food and Drug Administration–approved clinical trial to surgically transplant human spinal cord–derived stem cells (HSSCs) into the spinal cord of ALS patients. The details of the rationale, surgical methods, and phase I results of this trial have been published previously.5–8 Briefly, the injection of HSSCs into the spinal cord is safe, although the efficacy of this approach is not yet known.
Previous reports from therapeutic trials of intraspinal injection of stem cells were not able to demonstrate the presence or localization of cells in living patients due to the lack of intracellular markers that could be identified by imaging.9–12 Similarly, the stem cells used in our trial were not identifiable during life. This report focuses on postmortem identification of transplanted cells using qPCR and, in the one female patient autopsied to date, fluorescence in situ hybridization (FISH) using XY markers for the male donor cells.
Subjects and Methods
Subjects
The clinical trial design and initial results are published previously.5–7 This report focuses on six ALS patients who came to autopsy. Five males received injections of HSSCs into the lumbar spinal cord, and one female received injections into the cervical spinal cord.
Human neural stem cells and surgical injection into spinal cord
Details of the derivation, viability of the stem cells, and the clinical trial were described previously.5 Briefly, human neural stem cells (HSSCs) HSSC NSI-566RSC (Neuralstem, Inc., Rockville, MD) were derived from a single source 8-week gestation human fetal spinal cord, and serially expanded in culture. Of the six autopsy cases reported here, three patients received five unilateral injections and two patients received five bilateral injections (total 10) spaced 4 mm apart into the lumbar spinal cord at levels L2–L4. One patient received five unilateral injections into the cervical spinal cord at levels C3–C5. All injections contained a suspension of 100,000 cells in 10 μL volume.
Immunosuppression
All patients were placed on immunosuppressive therapy consisting of prednisone, basiliximab, mycophenolate mofetil, and tacrolimus.5 Tolerance of the immunosuppressive regimen was variable, and five of the six patients eventually stopped immunosuppressive medications. The period of time on immunosuppressive medications postoperatively and prior to death is presented in Table 1.
Table 1.
Patient demographic data
| Patient number | Gender | HSSC injection and region of SC | Number of days on FK506 | Number of days on MMF | Number of days IM meds discontinued before death | Survival days | % Donor DNA |
|---|---|---|---|---|---|---|---|
| 1 | M | U/L | 177 | 165 | 216 | 394 | 0.06–5.40 |
| 2 | M | Bi/L | 107 | 503 | 67 | 572 | 0.18–0.93 |
| 3 | M | Bi/L | 259 | 259 | 0 | 259 | 0.03–2.39 |
| 4 | M | U/L | 189 | 192 | 133 | 325 | 0.07–4.20 |
| 5 | M | U/L | 94 | 283 | 638 | 921 | 0.14–0.67 |
| 6 | F | U/C | 139 | 134 | 57 | 196 | 0.06–0.96 |
HSSC, human neural stem cells; SC, spinal cord; FK506, tacrolimus; MMF, mycophenolate mofetil; IM, immunomodulatory; U, unilateral; Bi, bilateral; L, lumbar; C, cervical.
Spinal cord collection at autopsy
All patients were transported to Emory University Hospital for autopsy. The entire spinal cord was removed and the region of injection was identified by the location of the dural sutures overlying the transplantation field, as well as the matching of vascular anatomy to images taken at the time of surgery (see Fig. 2). The region of interest was cut in 0.5 cm sequential cross-section blocks (“bread loafed”) and alternate blocks were frozen on dry ice or fixed for 2–3 days in 4% paraformaldehyde. There were ~20 blocks for each spinal cord, 10 frozen and 10 fixed. The frozen blocks for each of the six patients were sampled for qPCR analysis by excising a core of anterior and lateral cord using the back end of a sterile micropipette tip, and depositing the tissue into a sterile 1.5 mL eppendorf tube. Two core samples were obtained from each block. The fixed blocks were embedded in paraffin and sectioned for routine histochemistry (hematoxylin and eosin stain, Luxol fast blue [LFB] stain), immunohistochemistry (IHC), and FISH.
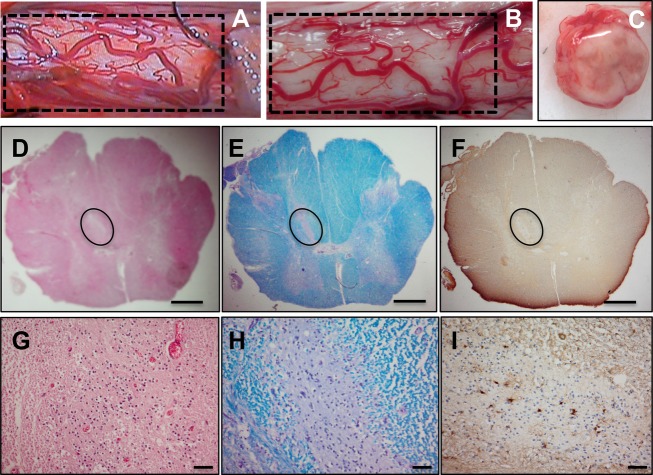
Figure 2.
Gross and histological analysis of male ALS spinal cord. Gross image of the spinal cord shows the cord surface at the site of HSSC transplant (A and B). The vascular anatomy between intraoperative videos (A) corresponds to the postmortem tissue (B). Cross section of the cord shows no visible tissue disruption (C). Histological staining with H&E (D and G), Luxol fast blue (E and H), and immunohistochemistry for GFAP (F and I) of 8-μm spinal cord sections from Patient 4 are shown. Nest of putative HSSCs are outlined in D–F. Scale bars: 1 mm (D–F); 50 μm (G–I). ALS, amyotrophic lateral sclerosis; HSSCs, human spinal cord–derived stem cells; GFAP, glial fibrillary acidic protein.
Quantitative real-time PCR
Using core samples from the frozen blocks of spinal cord (each block separated by 1 cm) from six patients, the presence of the genomic DNA sequence unique to the donor HSSCs NSI-566RSC was determined by qPCR on the 7500 SDS System (Applied Biosystem, Foster City, CA). DNA from frozen tissue was extracted using the QIAamp DNA Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). During the screening test, a 96-well screening plate (#5002645; Celera, Alameda, CA) containing two sets of 34 chimerism assays (CA001 to CA034 qPCR primers and fluorescence probes) plus an additional assay (CA999) were used for negative and positive controls. One set was used for the recipient DNA and one set for the donor DNA. Each assay well contained a final 25 μL reaction volume of 5 ng of DNA in a 5 μL PCR Master Mix (#5002681; Celera) containing buffer and DNA polymerase. Recipient and donor-specific informative markers were identified by the AlleleSEQR Screening Software, Celera, Alameda, CA following the PCR. One or more recipient-specific informative markers (CA001 to CA034, #5002646 to #5002679; Celera) were then used for quantitation. CA999 (#5002680; Celera), the primer/probe set for RNase P gene, was included as the reference assay. Each DNA sample was tested at 50–250 ng per reaction. A pretransplant recipient sample served as a 100% reference. A total of four PCR reactions were performed: presample/informative marker, postsample/informative marker, presample/CA999, and postsample/CA999. Presample is the recipient’s genomic DNA prior to transplant, postsample is the genomic DNA extracted from the spinal cord tissue. Each reaction was run in triplicate and the mean threshold cycle (CT) was applied in the formula for accurate quantitation. CT is the point on the qPCR curve where the amplification becomes exponential and is a relative measure of the target concentration in the PCR reaction. The calculations were performed by the AlleleSEQR Quantitation Software based on the relative quantitation method (2-ΔΔCT). The sensitivity for detecting DNA chimerism is 0.01–0.1%.
Fluorescence in situ hybridization
One of the six patients was female, while the neural stem cells were derived from a male donor. Cervical spinal cord from this patient containing the HSSCs injection region and noninjection lumbar regions were embedded in paraffin, sectioned at 4 μm, and mounted on Superfrost plus slides. Sections were used for dual-color FISH targeting X and Y chromosomes and processed according to the manufacturer’s protocol (Abbott Molecular, Inc., Des Plaines, IL) at Emory University Hospital Oncology Cytogenetics Laboratory. CEP X Spectrum orange (X-chromosome) and Y Spectrum green (Y-chromosome) direct-labeled fluorescent DNA probe kit was used (Abbott Molecular). Briefly, slides were preheated on a hot plate at 56°C overnight, deparaffinized three times 5 min each in Americlear, twice in 100% EtOH 1 min each, 0.2 N HCl for 20 min, and rinsed in dH2O for 3 min. Slides were pretreated using Pretreatment Reagent (Abbott Molecular) for 30 min at 80°C, rinsed in dH2O for 3 min, and digested in Protease I (Abbott Molecular) for 40 min at 37°C. Sections were rinsed in dH2O for 3 min, fixed in 10% buffered formalin for 10 min, rinsed in dH2O for 3 min, dehydrated in a series of graded ethanol (70%, 85%, 100%) 1 min each, and air dried. XY probes were added onto the sections, covered by coverslip, sealed with rubber cement, codenatured, and incubated at 37°C for 14–16 h. Rubber cement and coverslips were removed from the slides and posthybridization washes were conducted as follows: preheated 2X SSC/0.3% NP-40 at 72 ± 1°C for 2 min, air dried, DAPI II (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) (Abbott Molecular, Abbott Park, Illinois) was added, and sections were re-covered with coverslips. Slides were stored in the dark at −20°C overnight before imaging. Fluorescently labeled sections were analyzed using an Olympus microscope with the appropriate filters (Olympus, Melville, NY). Images were captured using CytoVision® (Leica Biosystems, Buffalo Grove, IL). For quantitative analysis, hematoxylin and eosin (H&E)-stained sections containing nests of putative stem cells were identified and outlined. Corresponding regions were marked on FISH sections and 100 cells each within these regions were counted by two independent readers. The percentage of XY- and XX-positive cells was computed from the total 200 cells counted.
Immunohistochemistry
Formalin-fixed, paraffin-embedded spinal cord sections (4 μm) were deparaffinized and IHC was performed on a DAKO Autostainer using antibodies for NeuN (mouse monoclonal, 1:800; Millipore, Billerica, MA), SOX2 (goat polyclonal, 1:50; R&D Systems, Minneapolis, MN), glial fibrillary acidic protein (GFAP) (mouse monoclonal, 1:100; Dako, Carpinteria, CA), OLIG2 (rabbit polyclonal, 1:100; Lifespan Biosciences, Seattle, WA), and LCA (CD45; monoclonal, 1:640; Dako, Carpinteria, CA) and costained with hematoxylin. Avidin–biotin–peroxidase complex was used to detect the antibodies using 3, 3′-diaminobenzidine (DAB) as the chromogen. Standard positive controls and normal sera without primary antibodies as negative controls were used.
Results
Subject demographics
Demographic data for the six cases, five males and one female, are presented in Table 1. Five patients received tacrolimus for 94–259 days and mycophenolate mofetil for 134–503 days posttransplant. One patient was on both immunosuppressive drugs until the time of death. Survival posttransplant surgery ranged from 196 to 921 days. Patients 1, 4, and 5 received unilateral lumbar injections, Patients 2 and 3 bilateral lumbar, and Patient 6 unilateral cervical injections.
Identification of donor DNA
To assess HSSCs graft survival, the presence of donor DNA within the recipient spinal cord was measured by qPCR. Sixteen core samples, eight from each side of the spinal cord, were analyzed from each case. In all cases, donor NSI-566RSC DNA was identified in several of the samples, with maximum percentage of donor DNA in each case ranging from 0.67% to 5.4% of total DNA (Fig. 1).
Figure 1.
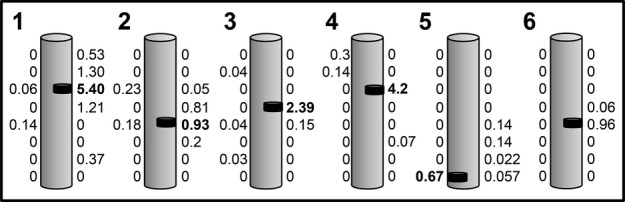
Identification of donor DNA in ALS spinal cord. Schematic diagram showing the presence of donor genomic DNA from spinal cord autopsy samples in six patients (1–6). Donor genomic DNA was extracted from alternating frozen blocks therefore the distance between each value is 1 cm. The numbers adjacent to each schematic cord represent the percentage of donor DNA in that tissue homogenate. HSSCs were unilaterally injected in the lumbar spinal cord in Patients 1, 4, and 5, bilateral lumbar in Patients 2 and 3, and unilateral cervical in Patient 6. The black bar identifies the region containing the highest percentage of donor DNA, which ranged from 0.67% to 5.4%. ALS, amyotrophic lateral sclerosis; HSSCs, human spinal cord–derived stem cells.
Neuropathology and localization of transplanted cells
At autopsy, localization of the site of transplant was accomplished by the presence of dural sutures and matching of the vascular anatomy between intraoperative videos and postmortem tissue. Gross inspection of the cord surface (Fig. 2A and B) and of the cross sections (Fig. 2C) did not reveal any tissue disruption, discoloration, or cavitation. Indeed, the sites of injection could not be grossly identified. Each paraffin-embedded block was sectioned through its entirety and stained with H&E. In three of the six cases we could identify one or more needle tracks corresponding to injection sites (Fig. S1). There was otherwise no tissue disruption, discoloration, or cavitation of sectioned tissue. In four cases (three male, one female), we identified “nests” of round cells with little cytoplasm that did not correspond to normal microanatomy; these cells did not stain with the GFAP or with the neuronal precursor protein doublecortin. Representative images from male Patient 4 spinal cord show histological staining with H&E (Fig. 2D and G) and LFB (Fig. 2E and H). IHC shows a lack of labeling with GFAP (Fig. 2F and I). Based on location and staining properties, these cells were interpreted to be transplanted HSSCs.
A “nest” of putative HSSCs was identified in one female patient as previously reported7 and shown in Figure 3A and B. This region was devoid of GFAP staining (Fig. 3C). Thus, taking advantage of the gender differences in male donor HSSCs transplanted in one female ALS patient, we targeted XY chromosomes for FISH analysis. Vysis Spectrum orange X probe and Spectrum green Y probe and counterstained with DAPI were used for FISH on two regions of the spinal cord – one region from the HSSCs injection site and one from a noninjected site that served as a negative control (Fig. S2).
Figure 3.
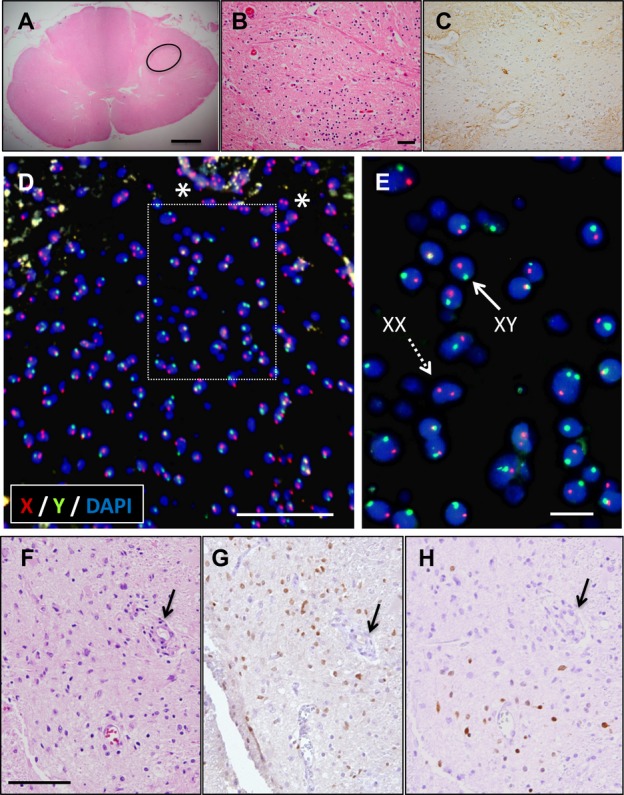
Donor HSSC localization and characterization using XY chromosome FISH and IHC, respectively, in a female ALS patient. H&E staining shows nests of cells in the female spinal cord (A) (circle). High-power image corresponding to the nest of cells outlined in (A) is shown in (B). Proximal sections stained with GFAP show lack of labeling of nest of cells (C). FISH labeling shows numerous X (red) Y (green)–positive cells counterstained with DAPI (blue) (D). Asterisks shows XX–positive recipient cells in the surrounding regions. Inset image from (D) is shown in (E). Donor HSSCs are positive for XY (solid arrow). H&E labeling of HSSCs graft (arrow) (F) label with SOX2 and (G) and NeuN (H). Scale bars: 1 mm (A), 50 μm (B–D), 10 μm (E), 100 μm (F–H). HSSC, human spinal cord–derived stem cell; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; ALS, amyotrophic lateral sclerosis; GFAP, glial fibrillary acidic protein.
Spinal cord sections from the injection site showed many XY-chromosome–positive cells within the region containing the putative HSSCs (Fig. 3D and E). XX-chromosome–positive cells were also noted in close proximity to XY-chromosome–positive cells (asterisks). Visual assessment of the DAPI labeling of XY-chromosome–positive cells showed that the nuclear morphology was intact and appeared normal with no evidence of condensation or fragmentation. Control sections from the lumbar spinal cord, distant from the injection site, showed exclusively XX-chromosome–positive cells (Fig. S2). Quantification of randomly selected areas within the injection site showed 36% XX- and 64% XY-chromosome–positive cells, while noninjection site regions had 100% XX-chromosome–positive cells. The female patient survived 196 days postsurgery demonstrating that many transplanted HSSCs survived long term. Taken together, these data demonstrate the identification and survival of intraspinal transplanted HSSCs into the spinal cord of ALS patients.
Characterization of transplanted HSSCs
Tissue sections proximate to those demonstrating the presence of donor cells by FISH were interrogated with antibodies for various cell fate markers and standard H&E staining (Fig 3F). Sections were stained for the transcription factor SOX2, a marker of multipotent stem cells in embryos and in adults, NeuN (differentiating neurons), OLIG2 (developing and differentiated oligodendrocytes), and GFAP (astrocytes). There were many SOX2-positive cells in regions of the spinal cord corresponding to the locations of XY-positive donor cells (Fig. 3G). Visual analysis of the sections showed many more XY-chromosome–positive cells compared to SOX2-positive cells. There were also NeuN-positive cells located within regions containing XY-positive donor cells (Fig. 3H). There was no labeling of OLIG2 suggesting that the HSSCs did not take on oligodendrocyte fate (data not shown). There was also no labeling of HSSCs with LCA (Fig. S4) suggesting the absence of leukocyte infiltration to the graft region. GFAP labeling was observed throughout the spinal cord sections, but did not appear to colocalize with the XY-positive cells. Thus, there were many more XY-positive cells than NeuN-positive and SOX2-positive cells combined, suggesting that transplanted HSSCs had differentiated beyond stem cell pluripotency but not to a specific neuronal or glial population.
Discussion
There are three major findings from this autopsy series of ALS patients undergoing spinal cord transplantation with HSSCs. First, DNA analysis focused on the regions of transplant identified DNA from donor HSSCs in all patients up to almost 3 years following surgery. Only one of these patients tolerated full immunosuppression until the time of death, suggesting that continuous immunosuppression is not necessary for continued survival of transplanted cells, though partial rejection cannot be excluded. Second, FISH analysis using Y-chromosome probes was able to identify and localize HSSCs in one female patient. Third, immunohistochemical labeling of the HSSCs showed evidence of neuronal differentiation with the expression of NeuN by some of the XY-positive cells. Other cells continued to express the stem cell marker SOX2, which is a prominent marker of these HSSCs prior to transplantation. We did not see labeling with oligodendrocyte marker OLIG2, and the astrocyte marker GFAP was difficult to interpret due to the diffuse expression throughout the spinal cord. However, there was a focal reduction in GFAP staining identified in areas of deposition of the transplanted cells (Figs. 2, 3). Taken together, this is the first study showing HSSCs graft survival and differentiation following transplantation into human spinal cord.
A critical component to the success of this clinical trial is the survival of transplanted HSSCs in the spinal cord. Our data show that donor HSSC DNA was present 196–921 days posttransplant survival. Because this is a first-in-human trial, it is not clear whether immunosuppressive therapy is necessary for long-term survival of the transplanted cells. Interestingly, donor DNA was detected by qPCR 57–638 days posttransplant after the discontinuation of immunosuppression therapy, and FISH analysis identified donor cells in situ at 196 days posttransplant. In this instance, the subject had not been on immunosuppressive drugs for 57 days prior to death. We found no correlation of DNA content to survival period after discontinuation of immunosuppressant medications. These data demonstrate that transplanted HSSCs can survive for a prolonged period, even in the absence of immunosuppression, and raise the interesting question of how long, if at all, a subject requires immunosuppressive medication following HSSC transplantation.
The presence of donor DNA using qPCR methods complimented the histological assessments of HSSC survival after transplantation. Our H&E staining identified non-native nests of cells near the injection site in the spinal cord of three males and one female, which we suspected to be of donor origin.7 In the female patient, XY-chromosome–positive labeling with FISH confirmed that the non-native nests of cells were the transplanted HSSCs. Due to the fact that the H&E labeling identified similar nests of cells in the male patients, we suspect that all transplanted ALS patients had successful HSSCs graft survival. Five of six patients were males, thus limiting our ability to use FISH analysis to distinguish HSSCs based on gender. Taken together, this is the first therapeutic trial localizing HSSCs following intraspinal injection. This finding is important as it demonstrates that human spinal cord provides a permissive microenvironment for allogenic fetal-derived transplant and the feasibility of FISH analysis for future clinical trials.
While the current approach demonstrated the survival of graft cells, it is difficult to rigorously quantify the percentage of grafted cells surviving. Such a measure is relevant to the question of how much immunosuppression to use and for how long. It is also relevant to assessing the effectiveness of the graft. That is, one would expect that the number of surviving cells would directly impact the therapeutic potential of the treatment. We have currently begun using iron oxide nanoparticle loading of donor cells prior to transplantation into large animals. This approach appears not to perturb cell health or differentiation in vitro. It also allows for visualization of the grafts to assess surgical accuracy in the immediate postoperative period. Finally, postmortem iron staining allows for quantification of graft survival, distribution, and accuracy (N. N. Boulis, pers. comm. 2014).
The therapeutic concept of cell transplantation into the spinal cord is based on the idea that these cells may survive, possibly differentiate, and provide trophic support, acting as “nurse cells” for endogenous motor neurons. This concept is supported by preclinical studies in animals showing that these HSSCs survive, differentiate, and integrate into the recipient spinal cord environment. In the SOD1 rat model of ALS these stem cells differentiated into glial cells and interneurons that functionally integrated into preexisting neural circuitry.13 In these studies, double labeling with IHC showed 70.4% of the human nuclear protein (HNu) colabeled with class III β-tubulin (TUJ1), 19.2% with stem cell marker Nestin, and 1.3% with GFAP suggesting extensive neuronal differentiation. In addition, these HSSCs produced glial-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factors (BDNF), which may also provide local trophic support for motor neurons.13–15 In each of these experimental paradigms there was a positive effect of spinal cord stem cell transplantation on animal survival.
In this first-in-human study, characterization of HSSCs transplanted in human ALS spinal cord showed evidence of neuronal differentiation and maintenance of stem cell markers. Pretransplant examination of cytospin prepared donor cells and stained with ICC (immunocytochemistry) revealed all were positive for SOX2, very few were positive for OLIG2, and no cells labeled with NeuN (Fig. S3). This finding is similar to previous work characterizing this HSSC cell line as 100% SOX1, 93.8% Nestin, 8% βIII-tubulin, and 0.75% OLIG2-positive cells.16 The one female patient where donor cells could be identified by FISH showed populations of transplanted cells that were labeled with SOX2, suggesting maintenance of the stem cell properties, and NeuN demonstrating differentiation into neuronal lineage after transplantation, which is consistent with the data from animal models. Future studies in human tissue will address the integration of donor cells into the spinal cord, and their effects on the environment of endogenous neurons.
In conclusion, this demonstration of survival and differentiation of transplanted HSSCs in ALS patients is an essential positive step to test the potential for therapeutic efficacy of using HSSCs as neuroprotective and/or neurorestorative treatment for ALS and possibly other neurodegenerative disorders.
Acknowledgments
This study was funded by Neuralstem, Inc. and by a grants from the National Institute of Neurological Disorders and Stroke (R01 NS077982), the National Institute of Aging (P50 AG025688), and the National Institute of Environmental Health Sciences (T32 ES12870). We are grateful to the patients and their families for participating in this trial for the development of ALS.
Author Contributions
T. T., J. D. G.: experimental procedures, data analysis, and original draft of manuscript. M. G., D. S., D. S., D. B., R. B., H. G., C. H., E. F.: experimental procedures, data analysis, and final editing of manuscript. N. B., J. R., T. F.: surgical procedures and final editing of manuscript. K. J., T. H.: cell production and characterization and final editing of manuscript. M. P., J. B.: patient care and data collection and final editing of manuscript.
Conflict of Interest
N. M. B.: personal fees, NeuralStem Inc., outside the current study; patents, licensed to NeuralStem Inc. T. H.: reports works for Neuralstem Inc. patents issued NeuralStem Inc. C. H.: personal fees from Roche Molecular Systems, Inc., unrelated to the current study. K. J.: personal fees from Neuralstem, Inc., during the study; personal fees and other from Neuralstem, Inc., outside the current study, patent issued Neuralstem, Inc. J. D. G.: grants from Neuralstem Inc.
Dr. Boulis reports personal fess from NeuralStem Inc, Outside the submitted work; in addition, Dr. Boulis has a patent floating cannula licensed to NeuralStem Inc, and a patent Spinal Platform licensed to NeuralStem Inc. Dr. Feldman reports grants from ALS Association, grants from NINDS. Dr. Glass reports grants from Neuralstem Inc, grants from NINDS. Dr. Hazel has a patent Transplantation of human neural cells for treatment of neurodegenerative conditions issued, and a patent Methods of treating amyotrophic lateral sclerosis (ALS) issued. Dr. Hill reports personal fees from Roche Molecular Systems.
Dr. Johe reports personal fees from Neuralstem, Inc., during the conduct of the study; personal fees and other from Neuralstem, Inc., outside the submitted work; In addition, Dr. Johe has a patent Neuralstem, Inc. issued.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
H&E staining of a spinal cord section from Patient 6. Representative images show low and high power of needle tracks (arrows) corresponding to the injection site (A and B).
Fluorescence in situ hybridization (FISH) staining using DNA probes CEP X Spectrum orange (X chromosome) and Y Spectrum green (Y chromosome) in a spinal cord section of a female patient. A “noninjected” lumbar spinal cord section of the same female patient that received cervical injections shown in Figure 3 served as a negative control. H&E staining of the lumbar cord shows an absence of “nest” of cells (A and B). FISH staining shows exclusively red XX chromosome labeling counterstained with DAPI in all the cells (C, inset i & ii). Cervical spinal cord section from the “injected” region shows cells from the central canal with H&E (D), FISH (E), and a blood vessel (F) with XX chromosome labeling. These images show the specificity of the XY probe labeling shown in Figure 3. Scale bars: 1 mm (A), 50 µm (B–F), 10 µm (i, ii).
Immunocytochemistry staining of cytospin prepared HSSC. All the HSSCs show positive staining for SOX2 (A). There are very few cells (circles) that have positive staining for OLIG2 (B). There are no NeuN-positive cells (C).
IHC staining of a cervical spinal cord section from a female patient. There is no LCA staining in the “nest” of putative HSSCs (A and B). In the same section, a blood vessel shows numerous LCA-positive labeling demonstrating the specificity of the antibody (C).
References
- Faravelli I, Riboldi G, Nizzardo M, et al. Stem cell transplantation for amyotrophic lateral sclerosis: therapeutic potential and perspectives on clinical translation. Cell Mol Life Sci. 2014;71:3257–3268. doi: 10.1007/s00018-014-1613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen GM, Gowing G, Svendsen S, Svendsen CN. The past, present and future of stem cell clinical trials for ALS. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.02.021. In Press, Corrected Proof, Available online 6 March 2014. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales PL, Revilla A, Ocana I, et al. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev. 2013;9:685–699. doi: 10.1007/s12015-013-9443-6. [DOI] [PubMed] [Google Scholar]
- Appel SH, Engelhardt JI, Henkel JS, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71:1326–1334. doi: 10.1212/01.wnl.0000327668.43541.22. [DOI] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Riley J, Federici T, Polak M, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71:405–416. doi: 10.1227/NEU.0b013e31825ca05f. discussion 16. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Boulis NM, Hur J, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75:363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulis NM, Federici T, Glass JD, et al. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8:172–176. doi: 10.1038/nrneurol.2011.191. [DOI] [PubMed] [Google Scholar]
- Blanquer M, Moraleda JM, Iniesta F, et al. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. 2012;30:1277–1285. doi: 10.1002/stem.1080. [DOI] [PubMed] [Google Scholar]
- Deda H, Inci MC, Kurekci AE, et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11:18–25. doi: 10.1080/14653240802549470. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Feron F, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(Pt 9):2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- Xu L, Yan J, Chen D, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ryugo DK, Pongstaporn T, et al. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Johe K, Molnar P, et al. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med. 2010;4:181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H&E staining of a spinal cord section from Patient 6. Representative images show low and high power of needle tracks (arrows) corresponding to the injection site (A and B).
Fluorescence in situ hybridization (FISH) staining using DNA probes CEP X Spectrum orange (X chromosome) and Y Spectrum green (Y chromosome) in a spinal cord section of a female patient. A “noninjected” lumbar spinal cord section of the same female patient that received cervical injections shown in Figure 3 served as a negative control. H&E staining of the lumbar cord shows an absence of “nest” of cells (A and B). FISH staining shows exclusively red XX chromosome labeling counterstained with DAPI in all the cells (C, inset i & ii). Cervical spinal cord section from the “injected” region shows cells from the central canal with H&E (D), FISH (E), and a blood vessel (F) with XX chromosome labeling. These images show the specificity of the XY probe labeling shown in Figure 3. Scale bars: 1 mm (A), 50 µm (B–F), 10 µm (i, ii).
Immunocytochemistry staining of cytospin prepared HSSC. All the HSSCs show positive staining for SOX2 (A). There are very few cells (circles) that have positive staining for OLIG2 (B). There are no NeuN-positive cells (C).
IHC staining of a cervical spinal cord section from a female patient. There is no LCA staining in the “nest” of putative HSSCs (A and B). In the same section, a blood vessel shows numerous LCA-positive labeling demonstrating the specificity of the antibody (C).