Abstract
Allometry has been used to demonstrate a power–law scaling relationship in the brain of premature born infants. Forty-nine preterm infants underwent neonatal MRI scans and neurodevelopmental testing at age 2. Measures of cortical surface area and total cerebral volume demonstrated a power–law scaling relationship (α = 1.27). No associations were identified between these measures and investigated clinical variables. Term equivalent cortical surface area and total cerebral volume measures and scaling exponents were not related to outcome. These findings confirm a previously reported allometric scaling relationship in the preterm brain, and suggest that scaling is not a sensitive indicator of aberrant cortical maturation.
Introduction
Premature birth is associated with altered cerebral volumes, cortical surface area, and cortical folding at term equivalent postmenstrual age (PMA).1–4 One useful way to quantify these effects involves allometric analysis. In general, allometry assesses the degree to which the sizes and shapes of various body parts conform to isometric scaling relationships.5 By relating overall brain volume to surface area (and, indirectly, cortical volume), allometry has been used to investigate the evolutionary advantages of having a gyrencephalic (highly folded) cerebral cortex over a lissencephalic one.6 It can also provide a quantitative method for examining the changing geometry of the developing brain. Allometric relationships can be expressed mathematically as CSA = b(TCV)α where CSA is cortical surface area, TCV is total cerebral volume, b is a scaling factor and α is a scaling exponent related to the relative growth rates of CSA and TCV. An α with values less than 2/3 signifies a differential increase of TCV relative to CSA (negative allometry), and α greater than 2/3 signifies a differential increase of CSA to TCV (positive allometry). In a study of 113 premature infants, Kapellou and colleagues demonstrated that the expansion of CSA occurs at a much faster rate than the expansion of the tissue volume in infants, with a scaling exponent of α = 1.29,7,8 consistent with the rapid gyrification during this period. The measured scaling exponent was lower in males and infants who were more premature at birth, and was shown to have a positive relationship with neurodevelopmental outcome at 2 years of age. Here, we re-examined these issues in 49 preterm infants scanned at higher spatial resolution and analyzed using improved cortical surface-based methods.
Methods
Fifty-eight very preterm infants (born at <30 weeks gestation) were prospectively recruited within the first 24 h of life from the St. Louis Children’s Hospital Neonatal Intensive Care Unit between April 2007 and June 2010. Magnetic resonance imaging (MRI) was performed on each infant between 26 and 41 weeks PMA (range 1–4 scans, note that some infants missed time points if they were too clinically unstable to be scanned). All scans were performed using a 3-T TIM Trio system (Siemens, Erlangen, Germany) without sedating medications.9 T1- and T2-weighted images were acquired during each scanning session. T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) sequence parameters included TR 1500 msec and TE 3 msec with voxel size 1 × 0.7 × 1 mm. T2-weighted turbo-spin echo (TSE) sequence parameters included TR 8600 msec and TE 160 msec with voxel size 1 × 1 ×1 mm. Images were assessed for qualitative injury, and infants with grade III/IV intraventricular hemorrhage, moderate-severe cerebellar hemorrhage or periventricular leukomalacia were excluded from the analyses. Forty-nine infants were subsequently included in the final analysis. Developmental testing was undertaken at 2 years of age corrected using the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III).10 The Bayley-III provides composite scores of performance across cognitive, language, and motor domains. All aspects of the study were approved by the Human Research Protection Office of Washington University in St. Louis.
Volumetry was performed using the Advanced Normalization Tools (ANTs) software package (http://www.picsl.upenn.edu/ANTS) followed by manual correction using ITK-Snap software tools.11,12 TCV was calculated for each scan by summing the volume of white, gray and deep nuclear gray matter. Semi-automated cortical segmentation was based on the LIGASE method13 followed by manual editing. Surface-based morphometry was also performed (for full methodological account see Hill, et al.13). CSA was calculated using the mid-thickness surface for both the left and right hemispheres and summing the values for each hemisphere (Fig. 1A). For each scan, both CSA and TCV were plotted against PMA at time of scan. In addition, log-transformed values of CSA and TCV were entered into a linear regression model and TCV was plotted against CSA on a log-log scale to determine a power–law scaling relationship.
Figure 1.
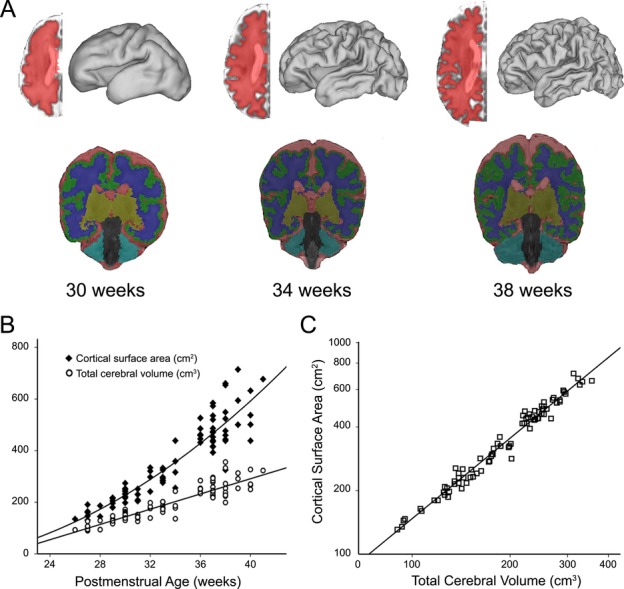
(A) Midthickness cortical segmentations and volumetric segmentations for an individual infant born at 26 weeks gestation scanned at (from left to right) 30, 34 and 38 weeks postmenstrual age. Each cortical segmentation was used to generate a three-dimensional fiducial surface. Volumetric segmentations were used to identify total volumes of cerebrospinal fluid (red), grey matter (green), white matter (blue), deep nuclear grey matter (yellow) and cerebellum (teal). Total cerebral volume was determined as the summation of grey, white and deep nuclear grey matter measures. (B) Plot demonstrating expansion of cortical surface area and total cerebral volume as a function of postmenstrual age at time of scan. Note the quadratic relationship for measures of cortical surface area and the linear relationship for measures of total cerebral volume. (C) Total cerebral volume plotted against cortical surface area on a log-log scale demonstrating a significant power–law scaling relationship with α = 1.27 (r = 0.98, P < 0.001).
Linear mixed models were used to determine if several clinical factors were independently related to either CSA or TCV. Investigated variables included gestational age, sex, birthweight, and postnatal growth. Each model accounted for repeated scans within subjects and PMA at scan. A set of variables modeling the interaction between log-transformed TCV values and each clinical factor was created. All clinical factors and the set of interactions with log-transformed TCV were incorporated into a final linear mixed model with log-transformed CSA as the dependent variable to examine the interaction of clinical variables with the power–law scaling relationship. Linear regressions were used to determine if CSA or TCV calculated from the term equivalent scan (PMA at scan ≥36 weeks; n = 27) were related to Bayley-III composite scores, controlling for PMA at scan. For the 13 infants with at least two scans, including a term equivalent scan and available outcome data, an individual scaling exponent was generated. Partial correlations and linear regressions controlling for PMA at term equivalent scan were used to explore relationships between the individual scaling exponents and composite scores of the Bayley-III.
Results
Forty-nine very preterm infants underwent a total of 80 MRI scans for which cortical surface reconstruction and volumetric segmentation were completed. This included 31 infants with one scan, nine infants with two scans, five infants with three scans and four infants with four scans. Demographic information for the cohort is included in Table 1.
Table 1.
Demographic information for the studied cohort (n = 49)
| Mean ± SD, n (%) median (interquartile range) | |
|---|---|
| Gestational age (weeks) | 27 ± 1.6 |
| Male | 21 (43) |
| Caucasian | 28 (57) |
| Birthweight (g) | 908 ± 239 |
| CRIB Score | 4 ± 3 |
| Vaginal delivery | 13 (27) |
| Hours on ventilator | 48 (24–240) |
| Received postnatal steroids | 15 (31) |
| PDA surgical LIGATION | 7 (14) |
| Necrotizing enterocolitis | 3 (6) |
| Length of stay (days) | 90 ± 30 |
| Cognitive composite | 87 ± 9 |
| Language composite | 90 ± 11 |
| Motor composite | 85 ± 10 |
As a function of PMA, CSA expansion conformed to a quadratic relationship, whereas TCV followed a linear expansion (Fig. 1B). When CSA was plotted against TCV on a log-log scale, a power–law relationship was found between CSA and TCV with a scaling exponent (α) of 1.27, F(1,78) = 3084.65, P < 0.001 (r = 0.98) (95% CI 1.22–1.31) (Fig. 1C).
No clinical variables were found to have a significant relationship with CSA, TCV or a significant influence on the power–law scaling relationship (Table 2). Two post hoc linear mixed-models (sufficiently powered at 0.85) were used to examine the relationship between sex and power-law scaling and GA and power-law scaling, and no significant relationship was found in our cohort. No relationship was found between Bayley-III developmental domains and term equivalent measures of CSA (cognitive, β = −0.02, P = 0.94; language, β = 0.15, P = 0.52; motor, β = 0.19, P = 0.39), or TCV (cognitive, β = 0.11, P = 0.63; language, β = 0.03, P = 0.89; motor, β = −0.20, P = 0.38). Scaling exponents also showed no relationship with Bayley-III scores across developmental domains (cognitive, β = 0.19, P = 0.57; language, β = −0.14, P = 0.68; motor, β = −0.23, P = 0.44).
Table 2.
Results from final linear mixed model examining the influence of clinical factors on the power–law scaling relationship between log-transformed TCV and CSA, using CSA as the dependent variable
| Variable | F | P-value | 95% CI | |
|---|---|---|---|---|
| Independent | TCV | 38.917 | <0.001 | 1.048 to 2.035 |
| Gestational age (weeks) | 0.247 | 0.62 | −0.030 to 0.049 | |
| Sex | 0.173 | 0.68 | −0.157 to 0.240 | |
| Birthweight SDS | 0.797 | 0.38 | −0.182 to 0.069 | |
| Postnatal growth | 2.609 | 0.11 | −0.324 to 0.034 | |
| Interactions | TCV × gestational age (weeks) | 0.895 | 0.35 | −0.026 to 0.009 |
| TCV × sex | 0.413 | 0.52 | −0.059 to 0.116 | |
| TCV × birthweight SDS | 0.807 | 0.37 | −0.030 to 0.079 | |
| TCV × postnatal growth | 3.164 | 0.08 | −0.008 to 0.144 |
The model accounts for repeated measures and PMA at scan. TCV, total cerebral volume; CSA, cortical surface area; PMA, postmenstrual age.
Discussion
The allometric relationship between CSA and TCV helps illustrate the geometry of cortical development in very preterm infants. We found a power-law scaling exponent that was virtually identical to that described by Kapellou and colleagues,8 despite using a considerably higher spatial resolution in our study (1-mm vs. 4-mm slice thickness), which allows improved fidelity for following the curving cortical surface and calculating its surface area. However, unlike the report by Kapellou and colleagues, gestational age and sex were not related to measures of cortical expansion and folding in our study. While our study was not sufficiently powered to determine that there was no influence of clinical factors in our final power-law scaling model, we can conclude that the addition of these clinical variables did not affect the significance of the primary allometric scaling relationship. Sex and GA had no influence on the power-law scaling relationship in separate mixed-models either. In addition, measures of surface area and volume at term equivalent were not related to neurodevelopmental outcomes at age 2 years across developmental domains, nor were the individual scaling exponents for infants with serial scans. While the median gestational age at birth for our cohort was similar to that in Kapellou, our study included fewer scans, employed differing measures of neurodevelopmental performance, and based upon prior published reports,14 our population exhibited lower performance rates at age 2 years. These factors might account for why our findings did not replicate the evidence for effects of gestational age and sex. While this investigation confirms a strong allometric scaling relationship in the preterm brain, it suggests that this relationship is not a sensitive indicator of atypical cortical development in preterm infants or affected by clinical factors. In addition, on a single-patient basis, it is unlikely to provide additional information regarding prognosis.
Acknowledgments
This study was supported by the National Institutes of Health (grant numbers R01 HD057098, UL1 TR000448, KL2 TR000250, R01MH60974, and K12 NS001690), the McDonnell Center for Systems Neuroscience, the Intellectual and Developmental Disabilities Research Center at Washington University (National Institutes of Health/NICHD P30 HD062171) and the Doris Duke Foundation. We also thank Tara Smyser for assistance with manuscript editing and Brian Avants for his guidance in surface-based analysis and the many infants and families for their generous assistance and dedication.
Conflict of Interest
Dr. Alexopoulos, Dr. English, Dr. Inder, Dr. Meyer, Dr. Neil, Dr. Paul, Dr. Rogers, Dr. Smyser, Dr. VanEssen, Dr. Wallendorf reports grants from National Institutes of Health.
References
- Ajayi-Obe M, Saeed N, Cowan FM, et al. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356:1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18:1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Scaling: why is animal size so important? Cambridge: Cambridge University Press; 1984. [Google Scholar]
- Prothero JW, Sundsten JW. Folding of the cerebral cortex in mammals. Brain Behav Evol. 1984;24:152–167. doi: 10.1159/000121313. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Geometric similarity in allometric growth: a contribution to the problem of scaling in the evolution of size. Am Nat. 1971;105:113–136. [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:1382–1390. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Neil JJ, McKinstry RC, et al. Transport, monitoring and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development. 3. San Antonio: Harcourt Assessment Inc; 2006. [Google Scholar]
- Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]


