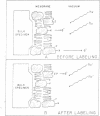
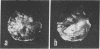
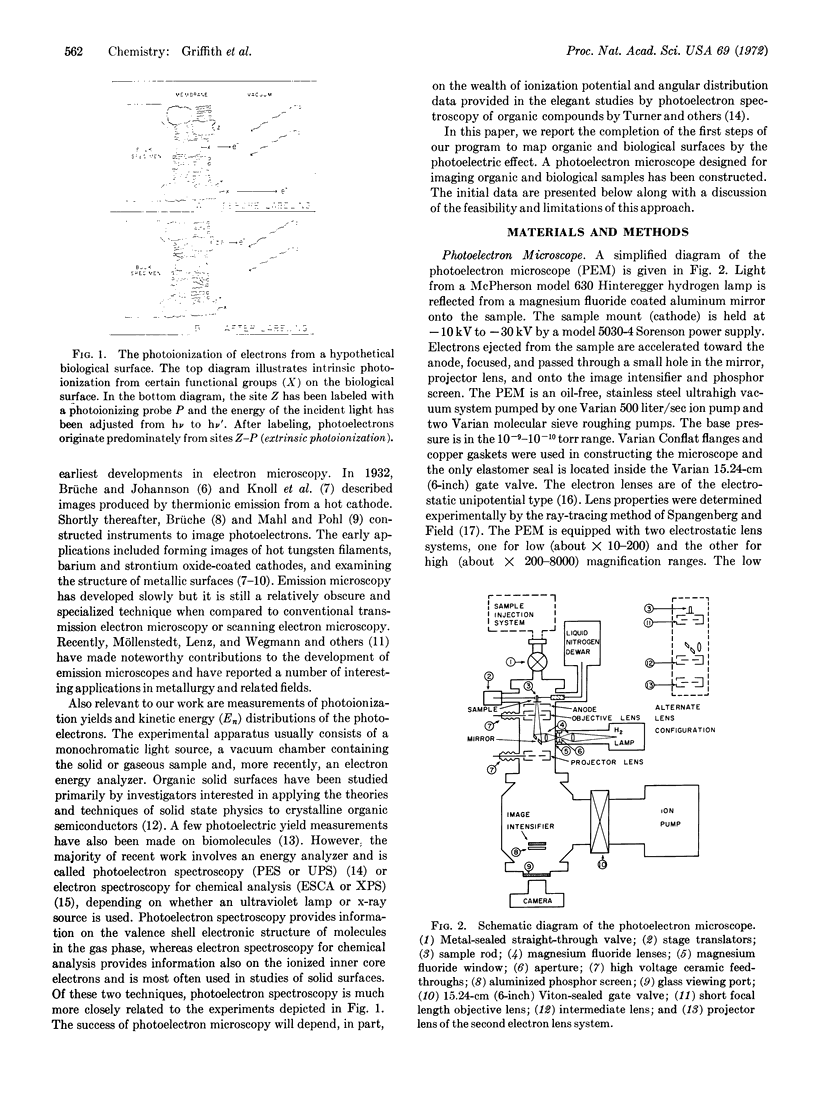
Abstract
A general method of imaging organic and biological surfaces based on the photoelectric effect is reported. For the experiments, a photoelectron emission microscope was constructed. It is an ultrahigh vacuum instrument using electrostatic electron lenses, microchannel plate image intensifier, cold stage, hydrogen excitation source, and magnesium fluoride optics. The organic surfaces examined were grid patterns of acridine orange, fluorescein, and benzo(a)pyrene on a Butvar surface. A biological sample, sectioned rat epididymis, was also imaged by the new photoelectron microscope. Good contrast was obtained in these initial low magnification experiments. These data demonstrate the feasibility of mapping biological surfaces according to differences in ionization potentials of exposed molecules. A number of technical difficulties, such as the intensity of the excitation source, must be solved before high resolution experiments are practical. However, it is probable that this approach can be useful, even at low magnifications, in determination of the properties of organic and biological surfaces.
Keywords: photoionization, photoemission, fluorescence microscopy, membranes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalieri E., Calvin M. Molecular characteristics of some carcinogenic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1251–1253. doi: 10.1073/pnas.68.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Reiner L. ON THE CHEMICAL ALTERATION OF PURIFIED ANTIBODY-PROTEINS. Science. 1930 Nov 7;72(1871):483–484. doi: 10.1126/science.72.1871.483. [DOI] [PubMed] [Google Scholar]
- Roberts G. C., Jardetzky O. Nuclear magnetic resonance spectroscopy of amino acids, peptides, and proteins. Adv Protein Chem. 1970;24:447–545. doi: 10.1016/s0065-3233(08)60246-6. [DOI] [PubMed] [Google Scholar]
- SETLOW R. Ultraviolet wave-length-dependent effects on proteins and nucleic acids. Radiat Res. 1960;Suppl 2:276–289. [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]