Abstract
Fresh fruits and vegetables are increasingly recognized as important reservoirs of human pathogens, and therefore, significant attention has been directed recently to understanding mechanisms of the interactions between plants and enterics, like Salmonella. A screen of tomato cultivars for their susceptibility to Salmonella revealed significant differences in the ability of this human pathogen to multiply within fruits; expression of the Salmonella genes (cysB, agfB, fadH) involved in the interactions with tomatoes depended on the tomato genotype and maturity stage. Proliferation of Salmonella was strongly reduced in the tomato mutants with defects in ethylene synthesis, perception and signal transduction. While mutation in the ripening-related ethylene receptor Nr resulted only in a modest reduction in Salmonella numbers within tomatoes, strong inhibition of the Salmonella proliferation was observed in rin and nor tomato mutants. RIN and NOR are regulators of ethylene synthesis and ripening. A commercial tomato variety heterozygous for rin was less susceptible to Salmonella under the greenhouse conditions but not when tested in the field over three production seasons.
Introduction
From 1998 to 2007 fresh fruits, vegetables, spices and nuts were linked to more outbreaks of human gastroenteritis than either beef, or pork or poultry, with fresh produce sometimes ranked as the riskiest food (Batz et al., 2011). Non-typhoidal Salmonella has emerged as the most problematic human pathogen associated with fresh produce, nuts and complex foods containing them (deWaal et al., 2009; Mandrell, 2009; Batz et al., 2011). Despite the apparent importance of vegetables as a vehicle of human gastroenteritis, the approaches for reducing pathogen load in fresh produce could be further improved. At least in part, this lack of food safety solutions is due to the limited understanding of the mechanisms of interactions between enterics and plants.
The ability to colonize plants may be an effective survival strategy for Salmonella as it provides a direct route from its excretion in the environment back to its numerous herbivorous and omnivorous hosts (Brandl et al., 2013). Under laboratory conditions, multiple routes by which Salmonella enters plants' interior were characterized and include invasion of plant lesions, uptake by roots, ingress through hydathodes and stomata, and fruit colonization through the reproductive structures (Guo et al., 2001; Cooley et al., 2003; Brandl, 2008; Kroupitski et al., 2009; Lopez-Velasco et al., 2012; Gu et al., 2013). The outcomes of plant interactions with Salmonella to a significant extent depend on the host: colonization of plant tissues varied not only among plant species but also among genotypes of a given species (Jablasone et al., 2005; Klerks et al., 2007; Barak et al., 2011; Gu et al., 2013). Similarly, the plant genotype has an important role in controlling the proliferation of Escherichia coli O157:H7 in the lettuce phyllosphere (Quilliam et al., 2012). These observations suggest that the interactions between enterics and plants are determined, at least in part, by the host genotype and the associated difference in the biological, physiological and chemical properties of crops, as well as responses to pathogens and endophytes. A better understanding of the host genetic factors involved in restricting (or favouring) proliferation of enterics within plant tissues will be an important step towards devising innovative solutions for improving produce safety.
Even though Salmonella is not considered to be a plant pathogen (Barak and Schroeder, 2012; Brandl et al., 2013), plants are capable of recognizing it and the associated molecular patterns (Thilmony et al., 2006; Schikora et al., 2011; Meng et al., 2013). Exposure of Arabidopsis thaliana to Salmonella Typhimurium 14028 and E. coli elicited measurable and temporally distinct transcriptomic responses in the plant. One hundred sixty A. thaliana genes were commonly upregulated in response to Salmonella, E. coli K12 and a plant pathogen Pseudomonas syringae; however, the magnitude of responses to Salmonella or E. coli was significantly (50–100×) less than to P. syringae (Schikora et al., 2011). In another study, inoculation of A. thaliana with E. coli O157:H7 elicited responses that were distinct from those elicited by the plant pathogen P. syringae pv. tomato DC3000 but similar to those elicited by its attenuated mutants (Thilmony et al., 2006). The latter included genes belonging to hormone and stress response pathways (Thilmony et al., 2006). These observations suggest that plants recognize and respond to enteric pathogens. This conclusion was further supported by the recent characterization of the Salmonella Seflg22 as a microbe-associated molecular pattern specifically recognized by plants and leads to the activation of the pathogen-triggered immunity and callose deposition (Garcia et al., 2013; Hernandez-Reyes and Schikora, 2013; Meng et al., 2013).
There does not yet appear to be a unifying model of a molecular program with which plants respond to human pathogens; however, it is clear that some of the defence and hormone (auxin, ethylene, jasmonic acid) pathways are differentially regulated following interactions of plants with Salmonella or E. coli O157:H7 (Thilmony et al., 2006; Schikora et al., 2008; 2011). Salicylic acid-dependent and SA-independent responses have been reported to be involved in the outcome of the interactions of plants with human enteric pathogens [rev. (Brandl et al., 2013)]. For example, treatment of Medicago truncatula and wheat seedlings with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) strongly reduced endophytic populations of Salmonella (Iniguez et al., 2005). The effect of ACC on the endophytic populations of Salmonella depended on the presence of bacterial flagellar genes and Pathogenicity Island I genes encoding functions involved in effector translocation (Iniguez et al., 2005).
Further rationale for delineating the involvement of ethylene signalling in produce safety is provided by the fact that some of the commercial tomato varieties contain mutated alleles of ripening genes that are themselves a part of the ethylene signalling. Heterozygousity for rin (and, to a lesser extent, nor) is often used in tomato breeding programs (Giovannoni, 2007; Garg and Cheema, 2011; Klee and Giovannoni, 2011). NOR is a member of the NAC-domain transcription factor family, characterized by the N-terminal DNA-binding domain consisting of five subdomains and a transcriptional regulatory region at the C-terminal (Giovannoni, 2007). A functional LeMADS-RIN, a global regulator of ripening, is required to initiate ethylene biosynthesis in addition to ripening factors that cannot be complemented by exogenous ethylene (Vrebalov et al., 2002; Martel et al., 2011). While nor and rin appear to be in the same regulatory pathway (Rohrmann et al., 2011; Fujisawa et al., 2012), physiological and biochemical changes associated with ripening, accumulation of metabolites, and interactions with pathogens are distinct in the corresponding mutants (Cantu et al., 2009; Osorio et al., 2011; 2012). Therefore, with this study, we tested proliferation of Salmonella in at least a dozen tomato varieties and mutants, including those with defects in ethylene response and ripening processes. Furthermore, expression of the Salmonella genes, known to be involved in tomato colonization, was tested in tomato ethylene mutants to determine how and to what extent ethylene signalling affects expression of the known Salmonella genes involved in the interactions with tomatoes.
Results and discussion
Screen of the existing tomato varieties and mutants for susceptibility to Salmonella
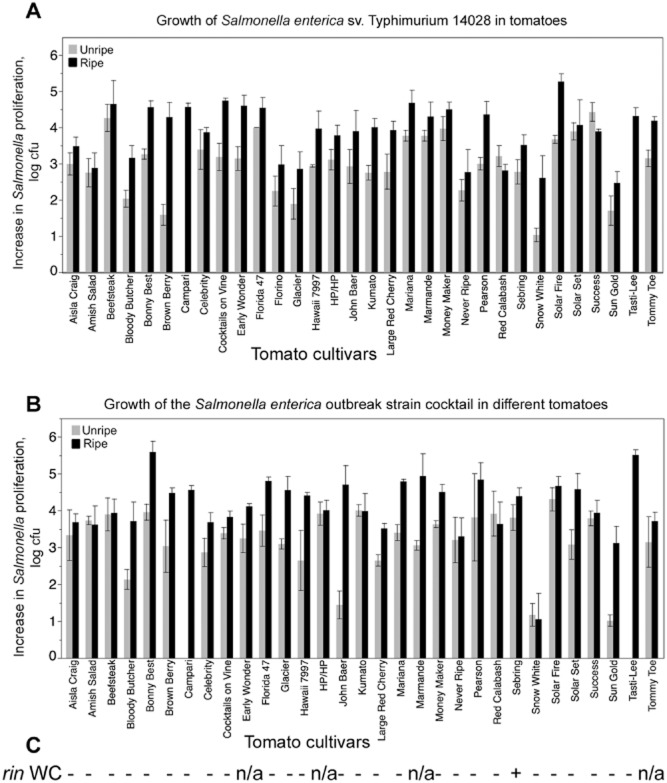
Our screen of 31 tomato varieties was not comprehensive; however, we aimed to include heirloom and commercial varieties with a number of characteristics that could conceivably affect how conducive tomatoes are to Salmonella. We have included tomato varieties with known resistances to plant pathogens, including a universally susceptible variety Bonny Best. Because at least one outbreak of salmonellosis was linked to roma-type tomatoes, sampled varieties included beefsteaks, standard-size tomatoes, and roma and cherry types (Fig. 1). Interestingly, cherry tomatoes were generally less conducive to proliferation of Salmonella (Supporting Information Fig. S1); however, this observation was not pursued further. In general, it is clear that none of the tested tomato varieties is completely ‘resistant’ to Salmonella; however, 10- to 100-fold differences in the populations reached by Salmonella within fruits of different cultivars were readily observed (Fig. 1, Supporting Information Table S1).
Fig 1.
Proliferation of Salmonella in ripe and unripe tomatoes of various genotypes. Tomatoes were grown either in the greenhouse or under the field conditions under standard production practices. Red ripe tomatoes of cvs. Campari and Tasti-Lee were purchased in local supermarkets. Tomatoes were inoculated with 100–1000 cells of either S. enterica sv. Typhimurium 14028 (A) or a cocktail of the Salmonella strains recovered from human outbreaks of illness (B). An increase in proliferation is a log-transformed ratio of the recovered cfu versus inoculum dose. For each variety, at least three technical and three biological tests were done in at least two production seasons. Errors bars are standard errors. (C) A test for heterozygosity revealed that only cv. Sebring is heterozygous for rin.
There were differences in the proliferation of the type strain Salmonella Typhimurium 14028 and the cocktail of the outbreak strains (S. enterica svs. Javiana ATCC BAA-1593, Montevideo LJH519, Newport C6.3, Braenderup 04E01347, 04E00783, 04E01556) in tomatoes of different varieties, but the differences in the proliferation of the type strain and the Salmonella cocktail appear cultivar-specific. This observation is consistent with the reports that under some conditions, strong Salmonella serovar-dependent differences in the proliferation within tomatoes, however, these differences were not always reproducible when tomatoes of different varieties were tested (Shi et al., 2007; Noel et al., 2010a; Marvasi et al., 2013a,b).
Strong differences in the Salmonella ability to colonize tomatoes at different maturity stages have been reported (Shi et al., 2007; Marvasi et al., 2013a,b) and are consistent with the observation that ripe fruits are generally more susceptible to opportunistic pathogens (Prusky, 1996). Differences in proliferation of Salmonella in mature and immature tomatoes were also observed in this study (Fig. 1). To follow-up on this observation and to attempt to determine the basis underlying this phenomenon, we tested the ability of Salmonella to multiply in fruits of the tomatoes with known mutations in ripening-related functions, such as differences in pigmentation, or in ethylene production or perception. For example, the brown colour of fruits of cv. Kumato is due to a green-flesh mutation (reduced chlorophyll degradation in ripening fruits (Hu et al., 2011). As shown in Fig. 1, populations of Salmonella in ripe tomatoes of this variety increased by 104. Proliferation of Salmonella Typhimurium ATCC14028 in smaller fruited Brown Berry was reduced in immature tomatoes, but not in mature tomatoes. Even though the nature of the mutation leading to the brown pigmentation in Brown Berry is not defined, these observations collectively suggest that the chlorophyll remaining in the mature fruit tissues is not what is responsible for the reduced proliferation of the pathogen in immature tomatoes. The final cell numbers reached by Salmonella in cv. Snow White and Sun Gold (both cherries lacking red pigment when mature) were generally lower than in most tomato varieties and even in some of the red-fruited cherries (e.g. Tommy Toe and Cocktails on the Vine). The deep red colour of mature fruit of cv. Tasti Lee is due to hyperpigmentation (determined by the HP mutation) (Wang et al., 2008). Even though the cocktail of the outbreak strains was able to increase 5.5 logs in ripe fruit, proliferation of the Salmonella Typhimurium 14028 in the ripe fruit of Tasti Lee was an order of magnitude lower. However, when growth of salmonellae in the HP/HP mutant and the isogenic wild-type Aisla Craig were compared directly, there were no statistical differences in the proliferation of the pathogen in response to the increased red pigmentation (Fig. 1, Supporting Information Table S1).
Because statistically significant differences in the proliferation of Salmonella in tomatoes of different varieties grown under greenhouse conditions were observed (Fig. 1, Supporting Information Fig. S2 and Table S1), field tests were carried out with tomatoes of four cultivars that represented varying levels of ‘resistance’ to Salmonella under greenhouse condition (Bonny Best, Florida 47, Sebring and Solar Fire). While tomatoes of the cv. Sebring grown in the greenhouse were less conducive to proliferation of Salmonella, a similar trend was not observed in tomatoes of this variety harvested in the field (Supporting Information Fig. S2). Under the field conditions, ripe tomatoes of cv. Florida 47, Bonny Best and Solar Fire were less conducive to Salmonella proliferation compared with Sebring (Supporting Information Fig. S2). The mechanism responsible for these observed differences is not yet clear; differences in crop production practices, the diversity of the associated phytomicrobiota are all known to affect the outcomes of interactions between enterics and crops (Gutierrez-Rodriguez et al., 2012; Lopez-Velasco et al., 2012; Marvasi et al., 2013a; Poza-Carrion et al., 2013; Williams et al., 2013).
In vivo Expression of the Salmonella tomato-specific genes
A suite of the Salmonella genes differentially regulated in tomatoes has been partially characterized, and their expression was shown to be dependent on the genotype of the plant or fruit's maturity state (Noel et al., 2010a; Marvasi et al., 2013b). Therefore, with this study, we tested the expression of the Salmonella tomato-specific genes within tomatoes of varieties with different levels of ‘susceptibility’ to Salmonella, identified in Fig. 1. These reporters included those in cysB (a regulator of cysteine biosynthesis and swarming), agfB (curli nucleator) and fadH (a 2,4-dienoyl-CoA reductase, an iron-sulfur flavoenzyme required for the metabolism of unsaturated fatty acids with double bonds at even carbon positions).
Consistent with previous reports, activity of the cysB Recombinase in vivo Expression Technology (RIVET) reporter was not strongly affected by the maturity of the fruit; however, there were cultivar-level differences in the activity of the reporter (Fig. 2). Because it was previously observed that the expression of cysB was highest in the tomato variety with a known resistance to a plant pathogen (Noel et al., 2010a) and because CysB regulon is known to be involved in antibiotic resistance (Turnbull and Surette, 2008; 2010), it was hypothesized that the regulation of this gene may correlate with the ability of the tomato variety to sustain proliferation of the pathogen. Consistent with this hypothesis, regression analyses indicate that the expression of the cysB reporter in red tomatoes of the tested varieties correlated (R2 = 0.14038) with the levels reached by the pathogen in red tomatoes; however, no similar trend was observed for green tomatoes (Supporting Information Fig. S3). The highest resolution of the reporter was observed, however, inside red tomatoes of the varieties that tended to sustain higher populations of Salmonella. Activity of the agfB reporter was generally higher in green tomatoes, compared with red, with the exception of the cultivar Sebring (rin heterozygote), in which expression of the agfB reporter was higher in red tomatoes (Fig. 2).
Fig 2.

Expression of the Salmonella tomato-specific genes in tomatoes of different genotypes. Activity of the Recombinase in vivo Expression Technology (RIVET) reporters in the Salmonella genes (cysB, fadH, agfB) previously shown to be differentially regulated in tomatoes. Reporters were inoculated into ripe or unripe fruits of the tomatoes of 11 varieties and recovered after a week-long incubation at 24°C. ‘Resolved’ constructs were scored by patching onto tetracycline-containing medium.
Generally consistent with the previous report that the expression of the fadH gene was highest in green tomatoes, likely in response to the accumulation of linoleic acid (Noel et al., 2010a), resolution of the fadH reporter was highest in green tomatoes, with few exceptions. In tomatoes of the cv. Kumato (in which chlorophyll is retained while ripening of the fruit due to a green-flesh mutation), mean resolution of the fadH reporter was higher in red tomatoes (Fig. 2). In red tomatoes of cvs. Sebring and Solar Fire, resolution of the reporter was higher than in other tomatoes and statistically indistinguishable from the resolution of the reporter in green tomatoes of the same varieties.
Proliferation of Salmonella in tomato ethylene mutants
Because the differences in the pigmentation per se do not appear to account for the increased proliferation of the pathogen in red ripe tomatoes compared with green tomatoes and because expression of the Salmonella tomato-specific genes in fruit of the variety (Sebring) heterozygous for rin was consistently distinct from those with the wild-type ethylene production and detection pathways (Fig. 2), our follow-up experiments focused on determining the contribution of the plant ethylene signalling to the interactions with Salmonella.
Under the greenhouse conditions, proliferation of Salmonella Typhimurium 14028 and of the cocktail of the outbreak strains in green and red tomatoes of cv. Sebring was generally lower than in fruits of other varieties but also statistically indistinguishable from some of the varieties, which are known to have both wild-type alleles of Rin (Fig. 1). Salmonella Typhimurium ATCC 14028 and the cocktail of the outbreak strains grew to significantly lower numbers in the mature tomatoes of the Never Ripe mutant compared with the mature fruits of the isogenic parent, cv. Pearson (Fig. 1). The effects of the mutations in the ethylene production and perception are known to depend on the genetic background of tomato (Garg and Cheema, 2011). Therefore, to further standardize experimental conditions, rin, nor and the Nr mutants in the Ailsa Craig background were used for all follow-up experiments.
When inoculated into developmentally synchronized rin and nor fruits harvested at 46 or 59 DPA, populations of Salmonella increased only 50- to 100-fold. The numbers of the pathogen increased to a similar extent in green (34 dpa) tomatoes of the wild-type Ailsa Craig; however, an increase in the red Ailsa Craig tomatoes (46 or 59 dpa) was 105- to 106-fold. The phenotype of the nor tomato mutants was the most severe, and the levels of Salmonella within 46 or 59 DPA fruit were similar to those reached by the pathogen in green (34 DPA) fruits of the isogenic parent, Ailsa Craig (Fig. 3). The phenotype of the rin mutant was partially relieved at later maturity stages (46 and 59 DPA). In fruits of the Nr tomato mutant, which lacks one of the ethylene receptors involved in fruit ripening, Salmonella populations increased by approximately 1000-fold, which was higher than in nor or rin mutants, but significantly less than in the wild type (Fig. 3). Generally, similar trends were observed for the cocktail of the outbreak strains (Supporting Information Fig. S4).
Fig 3.
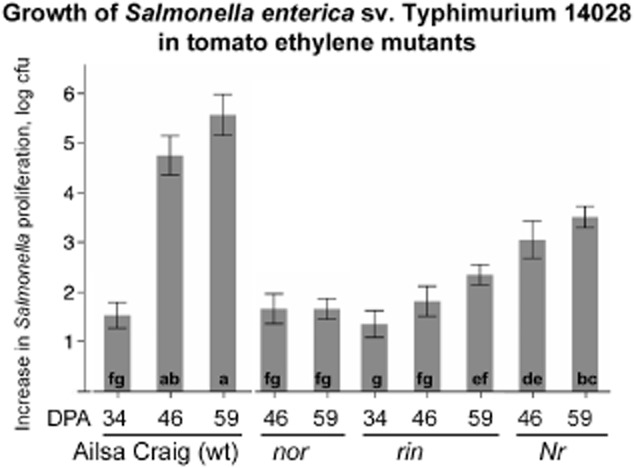
Proliferation of Salmonella Typhimurium 14028 in tomato ethylene mutants. Tomato ethylene mutants defective in ethylene perception (Nr) or ethylene synthesis and signal transduction (rin, nor) along with the isogenic parent Ailsa Craig were tested for their ability to support growth of the pathogen. Tomatoes were harvested at 34, 46 or 59 days post-anthesis and 100–1000 cells of Salmonella were inoculated into tomatoes and then recovered after a week-long incubation at 24°C. An increase in proliferation is expressed as a log-transformed ratio of the recovered cfu versus the inoculum. Each experiment included at least three technical and three biological replicas; error bars are standard errors. Letters at the bottom of each bar graph represent the Tukey-means separation. Different letters correspond to significantly different means (P < 0.05).
Effect of ethylene on proliferation of Salmonella in tomato mutants
At 34 dpa, Salmonella grew the least in rin tomatoes and reached approximately the same final numbers in nor, Nr and wild-type tomatoes (Fig. 4). Treatment with ethylene resulted in the development of the red colouration of the 34 dpa wild-type tomatoes, and a slight orange colour was apparent in the Nr fruits, while rin and nor mutants remained green. Exposure to ethylene at 34 dpa promoted proliferation of Salmonella in the wild type, rin and Nr mutants but not in nor (Fig. 4). It is important to note, however, that the treatment of the wild type, while resulting in the development of the red colour, did not lead to the increase in the Salmonella proliferation to the levels observed in red ripe tomatoes.
Fig 4.
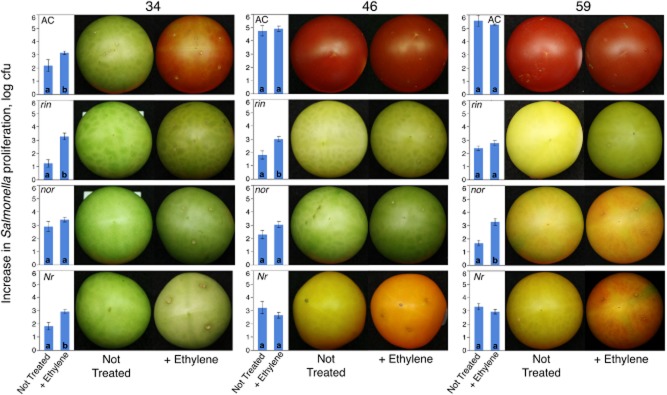
The effect of exogenous ethylene on Salmonella proliferation in tomato ethylene mutants. Fruit of the ethylene mutants (Nr, rin, nor) and isogenic parent Ailsa Craig (AC) were harvested at 34, 46 or 59 days post-anthesis, inoculated with Salmonella and incubated in a chamber where ethylene was applied to reach 12 ppm every 48 h following a brief venting. As a control, tomatoes were similarly incubated in a chamber-only without supplementation with exogenous ethylene. Tomatoes were sampled after a week-long incubation at 24°C. Blue bars indicate an increase in Salmonella Typhimurium 14028 numbers with or without ethylene. Photographs of tomatoes before and after the treatment are also included. Letters within the bars represent results of the pairwise comparisons (P < 0.05). Different letters indicate significantly different means.
At 46 dpa, fruits of the wild-type Ailsa Craig were fully red, and Salmonella numbers increased by 104–105, which is at least 100-fold higher than in green fruit (34 dpa) and approximately 10-fold higher than in green tomatoes treated with ethylene. Treatment of 46 dpa Ailsa Craig tomatoes with ethylene did not further promote the development of the red colour, nor did it significantly increase proliferation of Salmonella (Fig. 4). Treatment of nor or rin tomatoes did not lead to an increase in the red colour but increased proliferation of the Salmonella in the rin mutant. Exposure of the 46 dpa Nr tomatoes to ethylene increased pigmentation of the fruit but did not lead to an increased proliferation of Salmonella.
At 59 dpa, in fruits of the wild-type Ailsa Craig, Salmonella further increased by ∼10-fold (compared to 46 dpa); however, treatment with ethylene did not have an impact on further promotion of proliferation of the pathogen (Fig. 4). In rin tomatoes, Salmonella cell numbers similarly increased by ∼10-fold (compared with 46 dpa), and the treatment with ethylene had only modest effect on the proliferation of the pathogen. In nor tomatoes, Salmonella populations did not increase compared with 46 dpa, and the treatment with ethylene strongly promoted proliferation of the pathogen in treated fruit. Compared with 46 dpa, there was no further increase in Salmonella cell numbers in Nr tomatoes at 59 dpa, regardless of the exposure to ethylene (Fig. 4).
These observations suggest that the ability of Salmonella to persist in tomatoes depends on the maturity of the fruit and, to some extent, on functionality of the ethylene signalling pathways. The use of the tomato mutants with specific defects in the ethylene synthesis and perception suggests that the ethylene signalling pathways mediated by RIN and NOR (MADS box and SPBP transcriptional factors) are more consequential that those that rely on the ethylene response sensor-like ethylene receptor Nr. In tomatoes, in addition to controlling a common set of transcripts of metabolites, these divergent, but partially overlapping ethylene signalling pathways also control distinct changes in secondary product synthesis, hormone and polyamine metabolism as well as protein turnover (Osorio et al., 2011). It is not yet known which of the compounds differentially accumulated in response to ethylene affect proliferation of Salmonella in tomatoes. Even though rin and nor are within the same regulatory pathway (Klee and Giovannoni, 2011; Martel et al., 2011; Fujisawa et al., 2012; 2013), the corresponding mutations do not have the same effect on the outcomes of tomato interactions with pathogens. For example, NOR, but not RIN, was required for the control of the susceptibility of tomatoes to Botrytis cinerea (Cantu et al., 2009).
Experimental procedures
Bacterial strains and culture conditions
The following wild-type strains were used in this study: S. enterica sv Typhimurium ATCC14028, Salmonella Javiana ATCC BAA-1593, Salmonella Montevideo LJH519, Salmonella Newport C6.3, Salmonella Braenderup 04E01347, 04E00783, 04E01556 (the latter six strains were linked to the human outbreaks of salmonellosis resulting from consumption of tomatoes). All strains were maintained as frozen glycerol stocks.
For the tomato infections, bacteria were individually grown overnight at 37°C in Luria Bertani (LB) (Fisher Scientific) broth with shaking at 200 r.p.m. They were then washed twice in PBS (pH 7.0), and the strains from the outbreaks were combined into a six-strain ‘cocktail’ as suggested by the Framework for Evaluation of Microbial Hazards (Harris et al., 2012; 2013). These inocula were further diluted in sterile water and 3 μl of the suspension [containing between 102 and 103 colony-forming units (cfu)] were spotted onto three shallow (∼1 mm) wounds in tomato epidermis. Infected tomatoes were incubated at room temperature for a week. Upon completion of the incubation, tomatoes were macerated in an equal volume of 9.8 g l−1 of PBS (Fisher Scientific) using a stomacher (Sevard) (200 r.p.m. for 1 min), and the suspensions were plated onto a Xylose Lysine Deoxylate (XLD) agar (Beckton, Dickinson and Company) and incubated at 37–42°C overnight. Proliferation was calculated by dividing the total cfu recovered from each tomato by the total cfu inoculated into each fruit. This provided an accounting for differences in tomato sizes and for the fact that the colonization of a tomato fruit by Salmonella is not uniform. The ratios were further subjected to the log10 transformation. XLD plates on which there were no Salmonella colonies upon completion of the incubation were treated based on the rules of Most Probable Number analysis.
Reporter assays
RIVET reporters were used for the quantification of Salmonella gene expression in tomatoes. Activation of a promoter of interest cloned upstream of the promoterless tnpR recombinase gene was determined by scoring the frequency with which TnpR excised an antibiotic resistance cassette cloned in between the ‘res’ sites that are recognized and acted upon by TnpR (Angelichio and Camilli, 2002). For the RIVET assays in tomatoes, Salmonella cultures were grown at 37°C overnight in LB supplemented with the appropriate antibiotic(s) (Noel et al., 2010a). Bacterial cultures were then pelleted, washed three times in an equal volume of sterile PBS. Approximately 102 cfu (in 3 μl of water) were inoculated onto superficial 1 mm deep wounds on surfaces of unwaxed fruits. At least two technical (individual infections) and three biological (tomatoes from different plants) replications were carried out for each experiment. Unless otherwise stated, infected tomatoes were incubated at 22°C in vented chambers. All RIVET assays were carried out for a week. To harvest samples, 15 × 0.5 mm cores were removed from fruits, homogenized in PBS, and aliquots were then plated onto XLD agar (Oxoid) with appropriate antibiotics. Individual colonies were then patched on LB agar with tetracycline (10 μl ml−1) to detect constructs in which TnpR recombinase was active.
Plant material
For the screen of tomato varieties, tomatoes were grown in the field (two locations/seasons: Citra, FL in Fall 2010 (conventional) and Archer, FL in Spring 2012 (transitional organic) or in the roof-top greenhouse (during the breaks between production seasons). For each variety, field and greenhouse-grown tomatoes were sampled, and the combined data are presented. Seeds were purchased from commercial suppliers. Tomato maturity at harvest was assessed visually. Note that at maturity (corresponding to the USDA chart stages 5 and 6), fruits of Amish Salad, Bonny Best, Celebrity, Red Calabash, Sebring, Solar Fire, Mariana and Bloody Butcher turn red, Brown Berry and Kumato are brown, and Snow White are ivory, while Sun Gold are yellow.
Cultivar Ailsa Craig and lines nearly isogenic for the rin, nor and Nr mutations (Yen et al., 1995; Vrebalov et al., 2002) were grown in the roof-top greenhouse. To track developmental stages of the fruits, each developing fruit was tagged when it first reached exactly 1 cm in diameter, equal to 7 days post-anthesis (d.p.a.) (Alba et al., 2005). In the greenhouse, plants were grown from seed in Miracle-Gro Potting Soil and fertilized biweekly with Miracle-Gro Tomato Plant Food (18-21-21) (Marysville, OH).
Rin genotyping
For genotyping experiments, plants were grown in the greenhouse from seed to approximately four to six true leaf stage. DNA was extracted with a PowerPlant DNA isolation kit (MoBio) according to the manufacturer's instructions. Genotyping for Rin/rin alleles was conducted by polymerase chain reaction using primers ATACGATAATGTACAACCCGAAAATG and TCAACTTGAACACACATAAAAAGGAA yielding a 330 bp fragment diagnostic of the wild-type Rin allele and primers CTTTCAAACATCATGGCATTGTGGTG and ATATCATTGGCGGAACTTGACGTGAG yielding a 765 bp fragment diagnostic for the mutant rin allele.
Field tomato production
For some experiments, tomatoes (cvs. Bonny Best, Florida 47, Sebring and Solar Fire) were grown in the field over three production seasons in two locations in Florida. Generally, recommended practices for Florida tomato production were used for this research (Olson et al., 2012). A cover crop (15 cm tall) of rye (Secale cereale L.) was rototilled in preparation for tomato production. Pre-plant fertilizer (13N-2P-10K) was applied at 840 kg ha−1 to the bed area and rototilled into the soil prior to bedding and fumigating. The soil at each site was formed into raised beds and fumigated with a mixture of 50% methyl bromide: 50% chloropicrin to control soil-borne pests and weeds. Pre-emergence herbicides were applied carefully to the soil surface in the alleys between beds to control weeds. Black polyethylene mulch was applied to the beds for the spring crops and silver-on-black for the fall. Drip irrigation was applied under the mulch to maintain volumetric water content in the sandy soil (measured by time domain reflectometry) at 8–10% (Munoz-Carpena, 2012). Soluble fertilizer solution (ammonium nitrate and potassium chloride) was injected in 6 biweekly amounts to supplement the pre-plant fertilizers. Total-season N application was 224 kg ha−1 N and total-season K application was 210 kg ha−1 as K. During the season, fungicides, bactericides and insecticides were applied for pest control as recommended by field scouting and consistent with commercial tomato production practices. For experiments with Salmonella, tomatoes were harvested and sorted following normal commercial harvesting practices and brought to the lab for infections with Salmonella within 2–24 h of the field harvest.
Ethylene add-back experiments
Tagged, developmentally synchronized tomatoes (Ailsa Craig wild-type and isogenic rin/rin, nor/nor, Nr/Nr mutants) were harvested at 34, 46 and 59 d.p.a. Tomatoes were inoculated with Salmonella Typhimurium 14028 exactly as previously mentioned and then placed inside a 40 × 40 × 20 cm lidded air-tight aquarium, into which 0.39 ml of 100% ethylene were injected with a syringe for a treatment concentration of 12 ppm. Tomatoes were incubated for 1 week, and ethylene injections were repeated every 48 h, following a brief (∼10 min) venting to reduce accumulation of CO2. Tomatoes were harvested, and the total proliferation was calculated as described earlier.
Conflict of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Proliferation of Salmonella in cherry-type tomatoes. Proliferation of the type strain of the sv. Typhimurium 14028 and a cocktail of the outbreak strains in cherry-type tomatoes was compared with other varieties (e.g. roma, beefsteaks, etc.). In box plots, rectangles include the lower and upper quartiles, thick lines within the box are medians, and whiskers indicate the degree of dispersion of the data. Outlier data are shown as dots. Letters above box plots represent results of the pairwise comparisons (P < 0.05). Different letters correspond to significantly different means.
Fig. S2. Susceptibility of the tomatoes to Salmonella as affected by cultivar and method of production (greenhouse versus field). Tomatoes Bonny Best, Florida 47, Sebring and Solar Fire were grown in the greenhouse over at least two production seasons and in the field, as indicated in Experimental procedures. In the field, tomatoes were produced in three production seasons in two geographical locations in Florida. Harvested tomatoes were brought into the lab and inoculated either with Salmonella Typhimurium 14028 or with the outbreak strain cocktail. An increase in the Salmonella numbers was measured after a week. Only green (immature) and red (mature) tomatoes are included in the assessment. Tomatoes that ripened during the experiment were excluded from the comparison. In box plots, rectangles include the lower and upper quartiles, thick lines within the box are medians, and whiskers indicate the degree of dispersion of the data. Outliers are shown as dots. Letters above box plots represent Tukey means separation. Different letters correspond to significantly different means (P < 0.05).
Fig. S3. Correlation between expression of cysB, fadH, agfB and the overall proliferation of Salmonella in tomatoes. Resolution of the RIVET reporters in cysB, fadH and agfB (see Fig. 2) was correlated with the overall phenotype (Fig. 1). Red squares are mature (red) tomatoes, and green circles are immature (green) tomatoes. Continuous and dotted lines represent the linear regression for mature and immature tomatoes respectively. The coefficients of determination (R2) for the linear regressions were cysB 0.14038, 0.01494; fadH 0.13372, 0.03229; and agfB 0.00512, 0.00261 for mature and immature tomatoes respectively.
Fig. S4. Proliferation of the cocktail of Salmonella outbreak strains in tomato ethylene mutants. Tomato ethylene mutants defective in ethylene perception (Nr) or ethylene synthesis and signal transduction (rin, nor) along with the isogenic parent Ailsa Craig were tested for their ability to support growth of the pathogen. Tomatoes were harvested at 34, 46 or 59 days post-anthesis and 100–1000 cells of Salmonella were inoculated into tomatoes and then recovered after a week-long incubation. An increase in proliferation is expressed as a log-transformed ratio of the recovered cfu versus the inoculum. Each experiment included at least three technical and three biological replicas; error bars are standard errors. Letters at the bottom of each bar graph represent the Tukey-means separation. Different letters correspond to significantly different means (P < 0.05).
Table S1. Proliferaton of Salmonella enterica in tomatoes of different varieties: Tukey–Kramer means separation.
References
- Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, et al. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell. 2005;17:2954–2965. doi: 10.1105/tpc.105.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelichio MJ. Camilli A. In vivo expression technology. Infect Immun. 2002;70:6518–6523. doi: 10.1128/IAI.70.12.6518-6523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak JD. Schroeder BK. Interrelationships of food safety and plant pathology: the life cycle of human pathogens on plants. Annu Rev Phytopathol. 2012;50:241–266. doi: 10.1146/annurev-phyto-081211-172936. [DOI] [PubMed] [Google Scholar]
- Barak JD, Kramer LC. Hao LY. Colonization of tomato plants by Salmonella enterica is cultivar dependent, and type 1 trichomes are preferred colonization sites. Appl Environ Microbiol. 2011;77:498–504. doi: 10.1128/AEM.01661-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz MB, Hoffman S. Morris JG. Ranking the Risks: The 10 Pathogen-Food Combinations with the Greatest Burden on Public Health. Gainesville, FL, USA: University of Florida, Emerging Pathogens Institute; 2011. [Google Scholar]
- Brandl MT. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl Environ Microbiol. 2008;74:5285–5289. doi: 10.1128/AEM.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl MT, Cox CE. Teplitski M. Salmonella interactions with plants and their associated microbiota. Phytopathology. 2013;103:316–325. doi: 10.1094/PHYTO-11-12-0295-RVW. [DOI] [PubMed] [Google Scholar]
- Cantu D, Blanco-Ulate B, Yang L, Labavitch JM, Bennett AB. Powell AL. Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiol. 2009;150:1434–1449. doi: 10.1104/pp.109.138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MB, Miller WG. Mandrell RE. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl Environ Microbiol. 2003;69:4915–4926. doi: 10.1128/AEM.69.8.4915-4926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deWaal CS, Tian XA. Plunkett D. 11th edition. 2009. pp. 1–24. Outbreak Alert!: Center for Science in Public Interest, December 2009. [WWW document]. URL http://cspinet.org/new/pdf/outbreakalertreport09.pdf.
- Fujisawa M, Shima Y, Higuchi N, Nakano T, Koyama Y, Kasumi T. Ito Y. Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses. Planta. 2012;235:1107–1122. doi: 10.1007/s00425-011-1561-2. [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y. Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell. 2013;25:371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AV, Charrier A, Schikora A, Bigeard J, Pateyron S, de Tauzia-Moreau ML, et al. Salmonella enterica flagellin is recognized via FLS2 and activates PAMP-triggered immunity in Arabidopsis thaliana. Mol Plant. 2013;7:657–674. doi: 10.1093/mp/sst145. [DOI] [PubMed] [Google Scholar]
- Garg N. Cheema DS. Assessment of fruit quality attributes of tomato hybrids involving ripening mutants under high temperature conditions. Sci Hortic (Amsterdam) 2011;131:29–38. [Google Scholar]
- Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol. 2007;10:283–289. doi: 10.1016/j.pbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gu G, Cevallos-Cevallos JM. van Bruggen AH. Ingress of Salmonella enterica Typhimurium into tomato leaves through hydathodes. PLoS ONE. 2013;8:e53470. doi: 10.1371/journal.pone.0053470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chen J, Brackett RE. Beuchat LR. Survival of salmonellae on and in tomato plants from the time of inoculation at flowering and early stages of fruit development through fruit ripening. Appl Environ Microbiol. 2001;67:4760–4764. doi: 10.1128/AEM.67.10.4760-4764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Rodriguez E, Gundersen A, Sbodio AO. Suslow TV. Variable agronomic practices, cultivar, strain source and initial contamination dose differentially affect survival of Escherichia coli on spinach. J Appl Microbiol. 2012;112:109–118. doi: 10.1111/j.1365-2672.2011.05184.x. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Bender J, Bihn EA, Blessington T, Danyluk MD, Delaquis P, et al. A framework for developing research protocols for evaluation of microbial hazards and controls during production that pertain to the quality of agricultural water contacting fresh produce that may be consumed raw. J Food Prot. 2012;75:2251–2273. doi: 10.4315/0362-028X.JFP-12-252. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Berry ED, Blessington T, Erickson M, Jay-Russell M, Jiang X, et al. A framework for developing research protocols for evaluation of microbial hazards and controls during production that pertain to the application of untreated soil amendments of animal origin on land used to grow produce that may be consumed raw. J Food Prot. 2013;76:1062–1084. doi: 10.4315/0362-028X.JFP-13-007. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reyes C. Schikora A. Salmonella, a cross-kingdom pathogen infecting humans and plants. FEMS Microbiol Lett. 2013;343:1–7. doi: 10.1111/1574-6968.12127. [DOI] [PubMed] [Google Scholar]
- Hu ZL, Deng L, Yan B, Pan Y, Luo M, Chen XQ, et al. Silencing of the LeSGR1 gene in tomato inhibits chlorophyll degradation and exhibits a stay-green phenotype. Biol Plant. 2011;55:27–34. [Google Scholar]
- Iniguez AL, Dong YM, Carter HD, Ahmer BMM, Stone JM. Triplett EW. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant Microbe Interact. 2005;18:169–178. doi: 10.1094/MPMI-18-0169. [DOI] [PubMed] [Google Scholar]
- Jablasone J, Warriner K. Griffiths M. Interactions of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system. Int J Food Microbiol. 2005;99:7–18. doi: 10.1016/j.ijfoodmicro.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- Klerks MM, Franz E, van Gent-Pelzer M, Zijlstra C. van Bruggen AH. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J. 2007;1:620–631. doi: 10.1038/ismej.2007.82. [DOI] [PubMed] [Google Scholar]
- Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D. Sela S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol. 2009;75:6076–6086. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Velasco G, Sbodio A, Tomas-Callejas A, Wei P, Tan KH. Suslow TV. Assessment of root uptake and systemic vine-transport of Salmonella enterica sv. Typhimurium by melon (Cucumis melo) during field production. Int J Food Microbiol. 2012;158:65–72. doi: 10.1016/j.ijfoodmicro.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mandrell R. Enteric human pathogens associated with fresh produce: sources, transport, and ecology. In: Fan X, Niemira BA, Doona CJ, Feeherry FE, Gravani RB, editors. Microbial Safety of Fresh Produce. Ames, Iowa: Blackwell Publishing and the Institute of Food Technologies; 2009. pp. 5–41. [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P. Giovannoni JJ. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol. 2011;157:1568–1579. doi: 10.1104/pp.111.181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvasi M, Cox CE, Xu Y, Noel JT, Giovannoni JJ. Teplitski M. Differential regulation of Salmonella Typhimurium genes involved in O-antigen capsule production and their role in persistence within tomato fruit. Mol Plant Microbe Interact. 2013a;26:793–800. doi: 10.1094/MPMI-09-12-0208-R. [DOI] [PubMed] [Google Scholar]
- Marvasi M, Hochmuth GJ, Giurcanu MC, George AS, Noel JT, Bartz J. Teplitski M. Factors that affect proliferation of Salmonella in tomatoes post-harvest: the roles of seasonal effects, irrigation regime, crop and pathogen genotype. PLoS ONE. 2013b;8:e80871. doi: 10.1371/journal.pone.0080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Altier C. Martin GB. Salmonella colonization activates the plant immune system and benefits from association with plant pathogenic bacteria. Environ Microbiol. 2013;15:2418–2430. doi: 10.1111/1462-2920.12113. [DOI] [PubMed] [Google Scholar]
- Munoz-Carpena R. 2012. Field devices for monitoring soil moisture content. University of Florida Cooperative Extension Service. Bull 343. Univ of Florida/IFAS.
- Noel JT, Arrach N, Alagely A, McClelland M. Teplitski M. Specific responses of Salmonella enterica to tomato varieties and fruit ripeness identified by in vivo expression technology. PLoS ONE. 2010a;5:e12406. doi: 10.1371/journal.pone.0012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SM, Dittmar PJ, Vallad GE, Webb SE, Smith SA, McAvoy EJ, et al. 2012. Tomato production in Florida. In: EDIS. UF/IFAS. University of Florida Extension Circ HS739: University of Florida/IFAS.
- Osorio S, Alba R, Damasceno CM, Lopez-Casado G, Lohse M, Zanor MI, et al. Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 2011;157:405–425. doi: 10.1104/pp.111.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Alba R, Nikoloski Z, Kochevenko A, Fernie AR. Giovannoni JJ. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 2012;159:1713–1729. doi: 10.1104/pp.112.199711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poza-Carrion C, Suslow TV. Lindow SE. Resident bacteria on leaves enhance survival of immigrant cells of Salmonella enterica. Phytopathology. 2013;103:341–351. doi: 10.1094/PHYTO-09-12-0221-FI. [DOI] [PubMed] [Google Scholar]
- Prusky D. Pathogen quiescence in postharvest diseases. Annu Rev Phytopathol. 1996;34:413–434. doi: 10.1146/annurev.phyto.34.1.413. [DOI] [PubMed] [Google Scholar]
- Quilliam RS, Williams AP. Jones DL. Lettuce cultivar mediates both phyllosphere and rhizosphere activity of Escherichia coli O157:H7. PLoS ONE. 2012;7:e33842. doi: 10.1371/journal.pone.0033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann J, Tohge T, Alba R, Osorio S, Caldana C, McQuinn R, et al. Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J. 2011;68:999–1013. doi: 10.1111/j.1365-313X.2011.04750.x. [DOI] [PubMed] [Google Scholar]
- Schikora A, Carreri A, Charpentier E. Hirt H. The dark side of the salad: Salmonella Ryphimurium overcomes the innate immune response of Arabidopsis thaliana and shows an endopathogenic lifestyle. PLoS ONE. 2008;3:e2279. doi: 10.1371/journal.pone.0002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Virlogeux-Payant I, Bueso E, Garcia AV, Nilau T, Charrier A, et al. Conservation of Salmonella infection mechanisms in plants and animals. PLoS ONE. 2011;6:e24112. doi: 10.1371/journal.pone.0024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Namvar A, Kostrzynska M, Hora R. Warriner K. Persistence and growth of different Salmonella serovars on pre- and postharvest tomatoes. J Food Prot. 2007;70:2725–2731. doi: 10.4315/0362-028x-70.12.2725. [DOI] [PubMed] [Google Scholar]
- Thilmony R, Underwood W. He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;46:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AL. Surette MG. L-Cysteine is required for induced antibiotic resistance in actively swarming Salmonella enterica serovar Typhimurium. Microbiology. 2008;154:3410–3419. doi: 10.1099/mic.0.2008/020347-0. [DOI] [PubMed] [Google Scholar]
- Turnbull AL. Surette MG. Cysteine biosynthesis, oxidative stress and antibiotic resistance in Salmonella Typhimurium. Res Microbiol. 2010;161:643–650. doi: 10.1016/j.resmic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu J, Feng Y, Niu X, Giovannoni J. Liu Y. Altered plastid levels and potential for improved fruit nutrient content by downregulation of the tomato DDB1-interacting protein CUL4. Plant J. 2008;55:89–103. doi: 10.1111/j.1365-313X.2008.03489.x. [DOI] [PubMed] [Google Scholar]
- Williams TR, Moyne AL, Harris LJ. Marco ML. Season, irrigation, leaf age, and Escherichia coli inoculation influence the bacterial diversity in the lettuce phyllosphere. PLoS ONE. 2013;8:e68642. doi: 10.1371/journal.pone.0068642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HC, Lee SY, Tanksley SD, Lanahan MB, Klee HJ. Giovannoni JJ. The tomato Never-Ripe locus regulates Ethylene-inducible gene expression and is linked to a homolog of the Arabidopsis Etr1 gene. Plant Physiol. 1995;107:1343–1353. doi: 10.1104/pp.107.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Proliferation of Salmonella in cherry-type tomatoes. Proliferation of the type strain of the sv. Typhimurium 14028 and a cocktail of the outbreak strains in cherry-type tomatoes was compared with other varieties (e.g. roma, beefsteaks, etc.). In box plots, rectangles include the lower and upper quartiles, thick lines within the box are medians, and whiskers indicate the degree of dispersion of the data. Outlier data are shown as dots. Letters above box plots represent results of the pairwise comparisons (P < 0.05). Different letters correspond to significantly different means.
Fig. S2. Susceptibility of the tomatoes to Salmonella as affected by cultivar and method of production (greenhouse versus field). Tomatoes Bonny Best, Florida 47, Sebring and Solar Fire were grown in the greenhouse over at least two production seasons and in the field, as indicated in Experimental procedures. In the field, tomatoes were produced in three production seasons in two geographical locations in Florida. Harvested tomatoes were brought into the lab and inoculated either with Salmonella Typhimurium 14028 or with the outbreak strain cocktail. An increase in the Salmonella numbers was measured after a week. Only green (immature) and red (mature) tomatoes are included in the assessment. Tomatoes that ripened during the experiment were excluded from the comparison. In box plots, rectangles include the lower and upper quartiles, thick lines within the box are medians, and whiskers indicate the degree of dispersion of the data. Outliers are shown as dots. Letters above box plots represent Tukey means separation. Different letters correspond to significantly different means (P < 0.05).
Fig. S3. Correlation between expression of cysB, fadH, agfB and the overall proliferation of Salmonella in tomatoes. Resolution of the RIVET reporters in cysB, fadH and agfB (see Fig. 2) was correlated with the overall phenotype (Fig. 1). Red squares are mature (red) tomatoes, and green circles are immature (green) tomatoes. Continuous and dotted lines represent the linear regression for mature and immature tomatoes respectively. The coefficients of determination (R2) for the linear regressions were cysB 0.14038, 0.01494; fadH 0.13372, 0.03229; and agfB 0.00512, 0.00261 for mature and immature tomatoes respectively.
Fig. S4. Proliferation of the cocktail of Salmonella outbreak strains in tomato ethylene mutants. Tomato ethylene mutants defective in ethylene perception (Nr) or ethylene synthesis and signal transduction (rin, nor) along with the isogenic parent Ailsa Craig were tested for their ability to support growth of the pathogen. Tomatoes were harvested at 34, 46 or 59 days post-anthesis and 100–1000 cells of Salmonella were inoculated into tomatoes and then recovered after a week-long incubation. An increase in proliferation is expressed as a log-transformed ratio of the recovered cfu versus the inoculum. Each experiment included at least three technical and three biological replicas; error bars are standard errors. Letters at the bottom of each bar graph represent the Tukey-means separation. Different letters correspond to significantly different means (P < 0.05).
Table S1. Proliferaton of Salmonella enterica in tomatoes of different varieties: Tukey–Kramer means separation.