Abstract
The implementation of beneficial microorganisms for plant protection has a long history. Many rhizobia bacteria are able to influence the immune system of host plants by inducing resistance towards pathogenic microorganisms. In this report, we present a translational approach in which we demonstrate the resistance-inducing effect of Ensifer meliloti (Sinorhizobium meliloti) on crop plants that have a significant impact on the worldwide economy and on human nutrition. Ensifer meliloti is usually associated with root nodulation in legumes and nitrogen fixation. Here, we suggest that the ability of S. meliloti to induce resistance depends on the production of the quorum-sensing molecule, oxo-C14-HSL. The capacity to enhanced resistance provides a possibility to the use these beneficial bacteria in agriculture. Using the Arabidopsis-Salmonella model, we also demonstrate that the application of N-acyl-homoserine lactones-producing bacteria could be a successful strategy to prevent plant-originated infections with human pathogens.
Introduction
The best understood mechanism of systemic resistance induced by beneficial microorganisms is the induced systemic resistance (ISR), where plants have a potentiated defensive capacity against future biotic challenges. Its mechanism requires the presence of an operable non-expressor of PR1 (NPR1) and components from ethylene (ET) and jasmonic acid (JA) signalling cascades. Together with systemic acquired resistance (SAR), which is usually associated with a previous pathogen attack, ISR and SAR are under intense study (Van Wees et al., 2008; Dempsey and Klessig, 2012; Fu and Dong, 2013; Shah and Zeier, 2013). Nevertheless, the molecular basis of ISR is not completely understood because, for example, the beneficial Pseudomonas fluorescens strain 89B61 induces resistance in a JA- and ET-independent manner (Ryu et al., 2003).
The exchange of signals between plants and nearby rhizobacteria contributes to the activation of ISR. Small signalling molecules, for example N-acyl-homoserine lactones (AHLs) from many Gram-negative bacteria, are used for their intra-population communication called quorum sensing (QS) (Kaplan and Greenberg, 1985; Fuqua and Winans, 1994). Remarkably, plants are able to detect and respond to bacterial QS molecules (Mathesius et al., 2003). The detection of AHLs and systemic response is an essential aspect of the establishment of mutualistic relationships (Bauer et al., 2005). Studies of plant responses to AHLs were first done in the model plant Medicago truncatula, where these molecules were found to affect extensive functions including cytoskeletal elements, transcriptional regulation and responses to defence, stress and hormones (Bauer et al., 2005). Another study on the interaction between Serratia liquefaciens and tomato (Solanum lycopersicum) provided also indications that QS molecules of rhizosphere bacteria influence plant defence responses (Schuhegger et al., 2006). In this study, authors used the S. liquefaciens strain MG1, which produces C4- and C6-homoserine lactones when colonizing the root surface (Gantner et al., 2006). Colonization of the roots with S. liquefaciens MG1 protected tomato plants against the leaf-pathogenic fungus Alternaria alternate, in contrast to the AHL-negative S. liquefaciens mutant MG44 that was not able to provide such protection (Schuhegger et al., 2006). Similarly, colonization with the AHL-producing Serratia plymuthica strain HRO-C48 protected cucumber plants (Cucumis sativus) from the damping-off disease caused by Pythium aphanidermatum, as well as tomatoes and beans (Phaseolus vulgaris) from the infection with the grey mould-causing fungus Botrytis cinerea (Pang et al., 2009). Comparable with the previous study, the AHL-negative splI- mutant of S. plymuthica could not confer protection against both pathogens. Moreover, the resistance induced by Ensifer meliloti (Sinorhizobium meliloti) against P. syringae in Arabidopsis plants was depended on AHL accumulation (Zarkani et al., 2013). These results provided indications that AHLs play a role in the modulation of the plant immune system. Opposite results were reported for Arabidopsis thaliana, where S. liquefaciens MG1 and its AHL-negative mutant MG44 induced similar resistance against the pathogenic bacterium Pseudomonas syringae, suggesting an AHL-independent effect (von Rad et al., 2008).
In addition, the application of commercial AHLs also had an impact on plant physiology. AHL application induced changes in gene expression, altered protein profiles, modified root development and enhanced resistance against bacterial and fungal pathogens. This effect leans on a stronger and prolonged activation of MPK6 (Mathesius et al., 2003; Ortiz-Castro et al., 2008; von Rad et al., 2008; Schikora et al., 2011a,b; Bai et al., 2012; Schenk et al., 2012). Furthermore, plant responses to different AHL molecules appear to be AHL specific. Proteome analysis revealed around 150 differentially accumulated proteins in response to the application of either the commercial oxo-C12-HSL or the oxo-C16:1-HSL isolated from a Sinorhizobium meliloti culture (Mathesius et al., 2003). Correspondingly, an application of three commercial AHLs (C6-HSL, oxo-C10-HSL, and oxo-C14-HSL) revealed specific transcriptional responses, depending on the length of the AHL molecule (Schenk et al., 2014). Interestingly, after exposure to the resistance-inducing oxo-C14-HSL and a further pathogen challenge, the plants expressed an increased accumulation of phenolic compounds, lignin and callose depositions in plant cell walls (Schikora et al., 2011a,b; Schenk et al., 2012; Zarkani et al., 2013). Additionally, accumulation of oxylipins in distal tissues promoted stomatal closure, thus enhancing plant resistance to bacterial infection (Schenk et al., 2014).
In this report, we present a translational approach in which the resistance-inducing effect of oxo-C14-HSL-producing S. meliloti strain expR+ on Arabidopsis was verified in crop plants. We show that this effect depends on the presence of AHL molecules, because the inoculation of plants with the AHL-negative S. meliloti strain attM, which expresses a lactonase that inhibits the accumulation of AHLs, had no consequences on the plant resistance towards the tested pathogens. We used three different crop plants, which have significant impact on the worldwide economy as well as on human health and nutrition. In addition to the plant protective action, using the Arabidopsis–Salmonella model, we demonstrate that the use of AHL-producing bacteria could be a successful method to prevent plant-originated infections with human pathogens.
Results
Barley and wheat can be primed by S. meliloti expR+ for enhanced resistance
Based on the observation that the inoculation of plants with AHL-producing bacteria induce resistance in plants towards diverse pathogens (Schuhegger et al., 2006; Pang et al., 2009; Zarkani et al., 2013), we tested the hypothesis that the induced resistance caused by S. meliloti in crop plants depends on AHL production in a similar way as in A. thaliana (Zarkani et al., 2013). For this purpose, we used two S. meliloti strains, the expR+ strain carrying the pWBexpR plasmid (M. McIntosh, pers. comm.), which allows the production of the long-chain oxo-C14-HSL, and the S. meliloti attM strain that is unable to accumulate AHLs due to the expression of the Agrobacterium tumefaciens lactonase gene attM from the pBBR2-attM plasmid, (Zarkani et al., 2013). Barley cultivar Golden Promise plants were grown on soil and inoculated with S. meliloti by watering three times during 2 weeks before the challenge with the powdery mildew fungus Blumeria graminis f. sp. hordei. The cultivar Golden Promise is susceptible to B. graminis, i.e. 50% of its epidermal cells allow fungal penetration causing the formation of elongated secondary hyphae (ESH) and subsequent disease symptoms (Fig. 1A and B). However, plants inoculated with the oxo-C14-HSL-producing S. meliloti strain expR+ (Fig. 1C) showed enhanced resistance as a result of the augmentation of hypersensitivity response (HR) reactions at the sites of fungal penetration, thus diminishing the number of developing pustules (Fig. 1B). Correspondingly, the lack of this enhanced HR response in plants inoculated with the attM strain (Fig. 1A) suggests that the increased resistance depends on the production of oxo-C14-HSL by S. meliloti.
Fig 1.
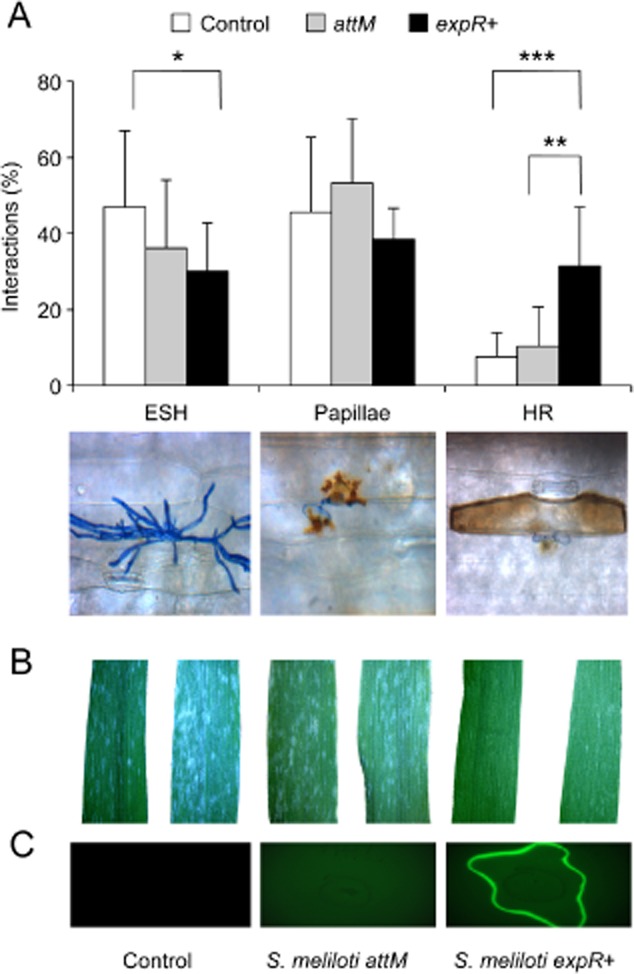
Oxo-C14-HSL produced by S. meliloti induced resistance against B. graminis in barley.A. Barley cv. Golden Promise plants were pretreated three times during 2 weeks with MgSO4 (control), the lactonase-expressing strain S. meliloti attM or the S. meliloti expR+ strain, which produces significant amount of oxo-C14-HSL, prior to inoculation with the powdery mildew causing fungus Blumeria graminis f. sp. hordei. The percentage of interaction sites resulting in elongated secondary hyphae (ESH) demonstrating susceptibility against the pathogen, papillae or hypersensitive response (HR), both indicating resistance, was assessed 2 days after inoculation. *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005 in Student's t-test. Experiment was repeated three times. Below the x-axis exemplary photographs are shown, presenting the possible results of interaction between barley leaf cells and B. graminis counted in A. From left: the formation of ESH, papillae and HR.B. Blumeria graminis mycelia developing on barley leaves 5 dai. Plants were treated like in A, representative photographs were taken using a standard binocular.C. Detection of oxo-C14-HSL produced by the S. meliloti strains used in A, using the biosensor bacterium Escherichia coli strain MT102.
Previously, we observed enhanced formation of papillae in barley plants pretreated with oxo-C14-HSL, which play a crucial role in resistance against fungal pathogens like B. graminis (Schikora et al., 2011a). Papillae are a complex structure between the plasma membrane and the plant cell wall, and depending of the plant species, the composition of these defence structures can consist of phenolics, reactive oxygen species (ROS) and cell wall proteins and polymers (Voigt, 2014). Hydrogen peroxide, one form of ROS that accumulates in forming papillae, is used by peroxidases to cause the cross-linking of proteins and phenolics for cell wall reinforcement. (Fig. 2A) (Hückelhoven, 2007; Deepak et al., 2005). In order to test whether oxo-C14-HSL influences this defence mechanism, we assessed the expression of one of the key enzymes in ROS production in barley, the peroxidase HvPRX7. To this end, barley plants were grown under sterile cultures for 10 days and the roots were pretreated with 6 μM oxo-C14-HSL or with the solvent control (acetone) for 3 days; subsequently, the first and second leaves were inoculated with B. graminis, and finally harvested for total RNA extraction after 24 and 48 h. Results from the quantitative reverse transcription polymerase chain reaction (RT-PCR) revealed that in contrast to control, plants pretreated with oxo-C14-HSL displayed a higher expression of HvPRX7 in response to B. graminis at 24 hai (Fig. 2B). Similarly, the expression of the Pathogenicity Related1 (HvPR1) gene was higher in oxo-C14-HSL pretreated plants, compared with the control (Fig. 2C). To substantiate our findings, we tested the impact of oxo-C14-HSL on ROS production by exploiting a different pathogen–host system. We used wheat plants cultivar Bobwhite grown and pretreated as described above before a challenge with the stem rust-causing fungus Puccinia graminis f. sp. tritici. Because P. graminis enters the interior of mesophyll tissues via stomata openings, the closure of stomatal pores is an effective protection mechanism against this fungus (Fig. 3A). In addition, guard cells of plants pretreated with oxo-C14-HSL presented an enhanced accumulation of H2O2, as indicated by the positive 3,3′-diaminobenzidine (DAB) staining (Fig. 3A and B). The disease symptoms observed on leaves at 11 days after inoculation were consistent with ROS accumulation (Fig. 3A, images on the right). These results suggested that oxo-C14-HSL primed barley and wheat plants for enhanced ROS production after a challenge with the pathogens. In the same manner, the oxo-C14-HSL-producing S. meliloti strain expR+ conferred protection against P. graminis in wheat plants (Fig. 3C). In comparison with the control and treatment with the S. meliloti attM strain wherein a high development of fungal pustules 5 dai was observed, S. meliloti expR+-treated plants presented lower number of developing pustules on leaves, suggesting that in analogy to barley (Fig. 1) and Arabidopsis (Zarkani et al., 2013), oxo-C14-HSL-producing bacteria protected wheat plants against P. graminis.
Fig 2.
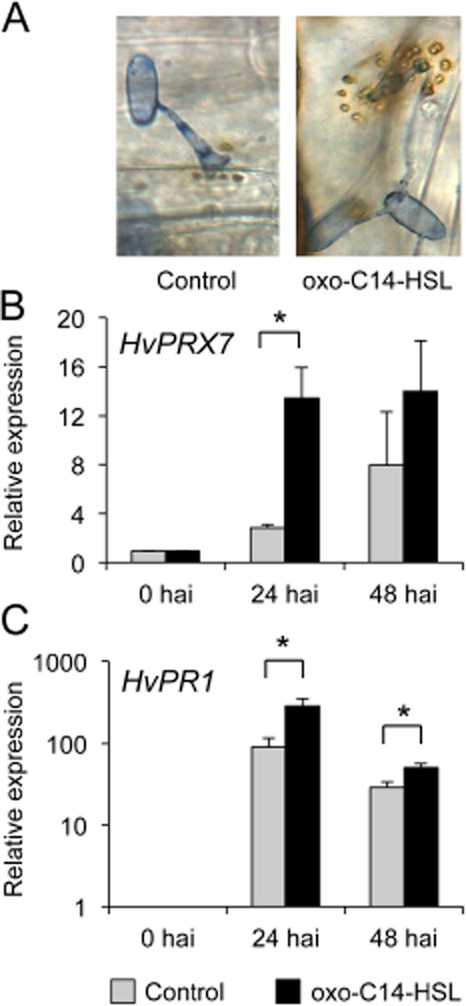
In oxo-C14-HSL-pretreated barley plants, papillae formation is associated with expression of Peroxidase 7 and Pathogenesis Related 1. Sterile-grown barley cv. Golden Promise plants were pretreated with oxo-C14-HSL for 3 days prior to inoculation with Blumeria graminis f. sp. hordei.A. Formation of papillae in oxo-C14-HSL-pretreated and control plants on sites of attempted penetration by B. graminis. Image was taken 48 h after inoculation (hai) with B. graminis.B–C. Relative expression of HvPRX7 (B) and HvPR1 (C) in control and oxo-C14-HSL-pretreated plants assessed in hai as indicated. Expression values were normalized to the expression of HvUBQ60 and 0 hai time point. *P ≤ 0.05 in Student's t-test. Experiment was repeated three times.
Fig 3.
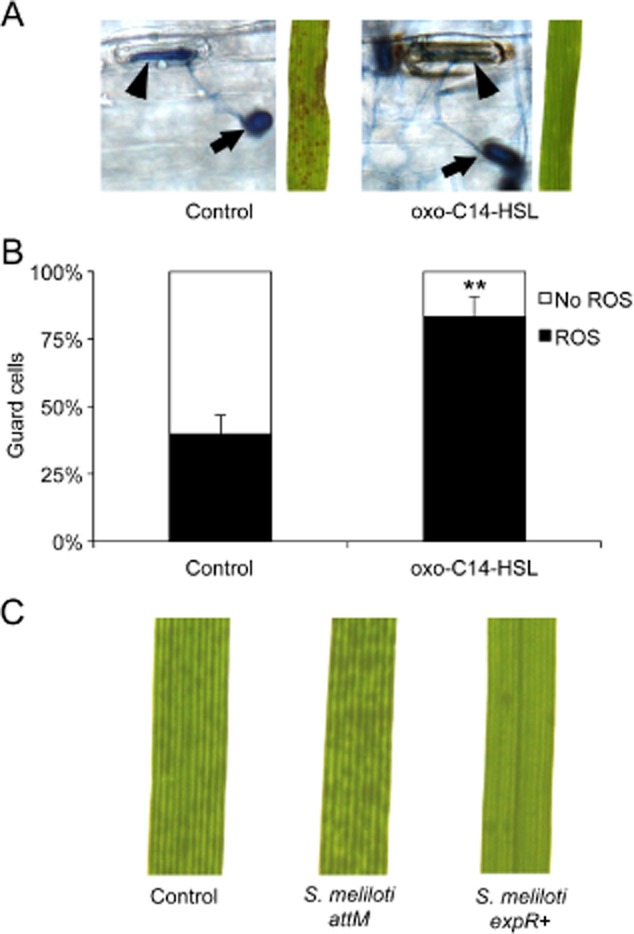
Enhanced accumulation of hydrogen peroxide in wheat guard cells after pretreatment with oxo-C14-HSL.A. Control leaves showing urediniospore (arrow) germination with directional growth of the hyphae towards stomatal opening (arrowhead) (control). Pathogen interaction with guard cells of oxo-C14-HSL pretreated leaves results in a significant accumulation of H2O2 (oxo-C14-HSL). Sterile-grown wheat cv. Bobwhite plants were pretreated with AHL solvent (control) or oxo-C14-HSL. Subsequently, leaves were inoculated with Puccinia graminis f. sp. tritici. DAB staining was performed 2 days after inoculation. On the right: exemplary leaves at 11 dai showing the differences in pustules development between control and oxo-C14-HSL-treated plants.B. The percentage of guard cells with higher accumulation of H2O2 as shown in A, 2 dai with P. graminis. **P ≤ 0.005 in Student's t-test. Experiment was repeated four times.C. Pustules development on wheat plants grown on soil for 10 days and inoculated three times with MgSO4 (control), S. meliloti attM or S. meliloti expR+. Representative images were taken 5 dai with P. graminis using a standard binocular.
Inoculation with oxo-C14-HSL-producing S. meliloti protects tomato from late blight disease
We extend our analysis from monocots to a dicot crop plant with high agronomic interest. We tested the induced resistance in tomato against Phytophthora infestans, which is nowadays one of the principal pathogens causing the late blight disease and a worldwide damage of 6 billion US dollars each year (Nowicki et al., 2011). Similar to the experiments with barley, soil-grown tomato plants cultivar Moneymaker were inoculated four times with S. melioti during 4 weeks prior to the challenge with P. infestans; control plants were pretreated with MgSO4. Disease symptoms were assessed 4 and 7 days after the challenge with P. infestans and the efficacy of the pretreatment was calculated using Abbott's formula (Abbott, 1925). In accordance to previous results, inoculation with S. meliloti strain expR+ induced resistance against P. infestans in tomato plants (Fig. 4A and B). Moreover, we observed differences between the inoculation with the oxo-C14-HSL-producing expR+ strain and the AHL-negative attM strain (Fig. 4C), implying that as in the case of fungal pathogens, resistance towards this Oomycete depends on the production of AHL.
Fig 4.
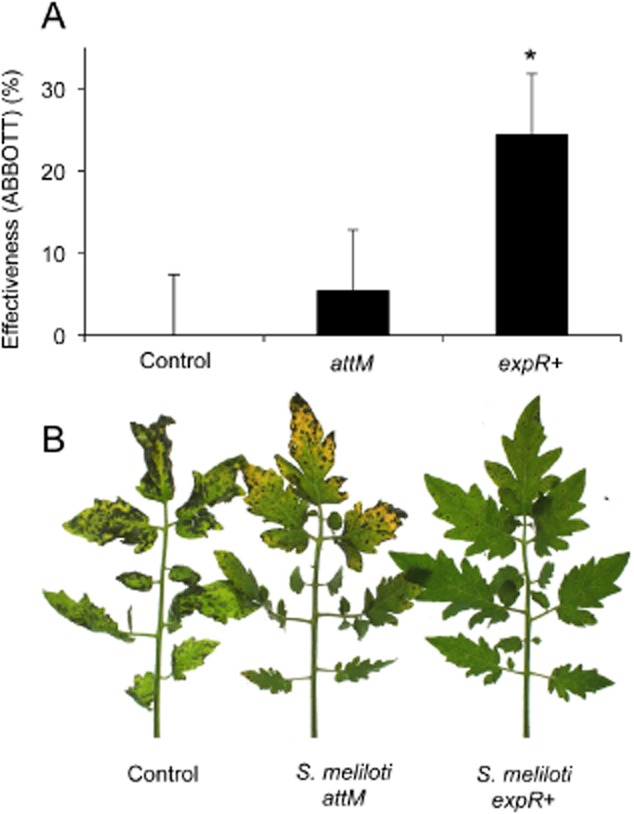
Treatment with S. meliloti strain producing oxo-C14-HSL increases resistance against late blight in tomato. Roots of tomato cv. Moneymaker plants were inoculated via watering with the oxo-C14-HSL-producing S. meliloti strain expR+, the attM lactonase-expressing S. meliloti attM strain or MgSO4 (control) for 4 weeks prior to inoculation with the oomycete Phytophthora infestans. Disease symptoms were assessed 1 week after inoculation. Data represent mean from three independent repetitions.A. Efficiency of treatment assessed using the Abbott formula. *P ≤ 0.05 in ANOVA.B. Macroscopic symptoms caused by the late blight agent P. infestans on tomato plants.
AHL-producing bacteria can promote resistance towards human pathogens
Salmonella are Gram-negative bacteria that are able to colonize humans and plants. These bacteria are the causal agents of gastroenteritis and typhoid fever in humans due to the ingestion of contaminated food or water (Pang et al., 1995). Additionally, in recent years the proportion of raw-food related outbreaks in the USA reached 25% (Rangel et al., 2005). The increasing number of infections related to the consumption of fresh fruits and vegetables contaminated with these bacteria is very alarming and suggests that plants may be a substantial reservoir for Salmonella. Many reports proposed a complex interaction between Salmonella and the host plant because the plant immune system seems to play a key role in the outcome of the colonization (Schikora et al., 2011b; 2012; Shirron and Yaron, 2011). For this reason, the induction of defence mechanisms by oxo-C14-HSL-producing S. meliloti was tested as a potential measure to reduce the risk of plant-related infections. According to the experiments above, soil-grown A. thaliana Col-0 plants were watered four times with S. meliloti or with the respective controls before defying the plants with Salmonella enterica serovar Typhimurium strain 14028s. The proliferation of S. Typhimurium was assessed during 6 days after syringe infiltration. Interestingly, Arabidopsis plants pretreated with the S. meliloti expR+ caused a lower Salmonella proliferation than plants pretreated with S. meliloti attM or MgCl2 (Fig. 5A), which corresponds to the diminished disease symptoms in leaves from S. meliloti expR+ pretreated plants (Fig. 5B and C). This suggest that in line with the effects seen in barley and tomato, the production of AHLs allowed S. meliloti to prime Arabidopsis plants for an enhanced defence against Salmonella.
Fig 5.
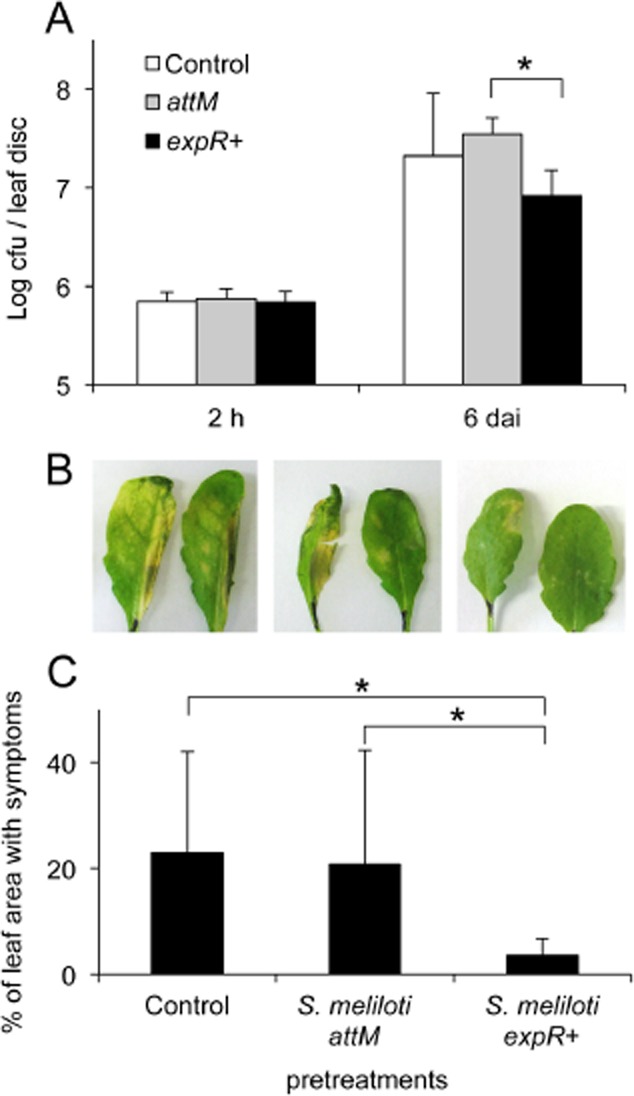
Treatment with oxo-C14-HSL-producing S. meliloti strain enhances resistance against the human pathogen Salmonella enterica serovar Typhimurium in Arabidopsis. Roots of Arabidopsis Col-0 plants were inoculated via watering with the oxo-C14-HSL-producing S. meliloti strain expR+, the attM lactonase-expressing S. meliloti attM strain or MgCl2 (control) four times during 4 weeks prior to syringe infiltration of leaves with Salmonella Typhimurium bacteria.A. Proliferation of Salmonella Typhimurium in Arabidopsis leaves assessed at 2 h and 6 days after inoculation (hai and dai respectively). *P ≤ 0.05 in Student's t-test. Data present a mean from three biological repetitions.B. Macroscopic symptoms caused by Salmonella Typhiumurium on Arabidopsis leaves.C. Quantification of symptoms caused by S. Typhimurium on Arabidopsis laves was performed using the algorithm described in (Schikora et al., 2012). *P ≤ 0.05 in Student's t-test.
Discussion
In this report, we present the impact of oxo-C14-HSL-producing bacteria on the plant immune system. We demonstrated that the previously described AHL-priming (Schenk et al., 2014) and the effect of oxo-C14-HSL-producing bacteria is not restricted to the commercial molecule, nor to the model plant A. thaliana (Zarkani et al., 2013). In a translational approach, we showed that the use of AHL-producing bacteria could be a potential method to improve plant resistance and to decrease the yield loss caused by many pathogens. Moreover, AHL-induced resistance may reduce the risk of plant-originated outbreaks of salmonellosis in addition to other possible related diseases.
AHLs used by Gram-negative bacteria may vary in the length of the lipid side chain and in the substitution of the C3-atom (O- or OH- group). The length of the lipid side chain is essential for the effect on plants; for example, C4-HSL, C6-HSL, oxo-C6-HSL and oxo-C8-HSL promoted growth in Arabidopsis (von Rad et al., 2008; Liu et al., 2012; Schenk et al., 2012), whereas oxo-C10-HSL induced the formation of adventitious roots in mung beans (Bai et al., 2012). On the other hand, only some AHL molecules were reported to have resistance-inducing attributes. A comparison of plant responses with different AHLs at the transcriptome and proteome levels revealed that just long-chain AHLs could induce resistance-related changes at the transcriptome and proteome levels (Mathesius et al., 2003; Miao et al., 2012; Schenk et al., 2014). The molecule oxo-C14-HSL and to a lesser extend OH-C14-HSL induced resistance in Arabidopsis and barley plants towards biotrophic and hemibiotrophic pathogens (Schikora et al., 2011a,b). Sinorhizobium meliloti produces different long-chain AHLs, like oxo-C14-HSL (Teplitski et al., 2003; Zarkani et al., 2013), and therefore we decided to use this bacterium in this work to study the interaction between crop plants and AHL-producing rhizobacteria. Besides the acknowledged benefit that the interaction between S. meliloti and its native host M. truncatula results in nodulation and N2-fixation, the oxo-C14-HSL-producing S. meliloti strain induced resistance in the non-host plant Arabidopsis against Pseudomonas syringae pathovar tomato (Zarkani et al., 2013). For this reason, to ascertain our translational approach, we used economically important non-host crop plants of S. meliloti to study the impact of AHLs. The resistance induced by beneficial bacteria is referred as ISR, and it has been exhaustively studied employing P. fluorescens and Bacillus spp. bacteria; for review, see Pieterse and colleagues (2014). Today, the mechanism of ISR is relatively well understood; it requires NPR1 and components of the JA- and ET-signalling pathways. The transcription factor MYB72 was postulated to play a key role in ISR and link JA- and ET-signaling pathways (Van der Ent et al., 2008). However, the AHL-induced resistance, termed AHL priming, seems to depend on other mechanism. Resent findings indicated that instead of MYB72 and JA/ET pathway(s), the salicylic acid/oxylipin pathway influenced the AHL priming (Schenk et al., 2014). Moreover, the resistance-inducing effect of the long-chain AHLs in Arabidopsis was reflected in the reinforcement of the cell wall through the accumulation of callose, phenolic compounds and lignins, as well as to an intensified stomatal closure in response to bacterial attack. Likewise, we observed that the inoculation with oxo-C14-HSL-producing S. meliloti strain, as well as pretreatment with the pure oxo-C14-HSL molecule, primed barley and wheat plants for enhanced ROS production. Membrane-bound NADPH oxidase and apoplastic peroxidase proteins usually contribute to this transiently increased production of toxic ROS known as oxidative burst. In addition, ROS act as secondary messengers, which allocates them a central role in plant defence mechanisms (Marino et al., 2012).
Intriguingly, S. meliloti expR+ induced plant resistance towards Salmonella, which is generally considered an animal or human pathogens. Until now, the infection mechanism(s) used by Salmonella to successfully and simultaneously colonize diverse hosts like animals and plants are poorly understood. Stomata openings were identified as possible entry points of bacteria into the inner layers of the mesophyll (Kroupitski et al., 2009). Remarkably, although some plant species (e.g. arugula) allowed Salmonella to internalize, others (e.g. parsley) seemed to prevent internalization (Golberg et al., 2011). The plant immune system appears to play a central role during colonization of Salmonella as indicated by the induction of defence mechanisms after inoculation with these bacteria (Schikora et al., 2008; Meng et al., 2013; Garcia et al., 2014) and by the fact that Salmonella can actively suppress those mechanisms in tobacco (Nicotiana tabacum) and Arabidopsis plants (Schikora et al., 2011a,b; Shirron and Yaron, 2011). Accordingly, the use of beneficial bacteria with the ability to enhance defence mechanisms in crop plants that are susceptible to infection with human pathogens could be an alternative to lower the risk of disease outbreaks associated with contaminated fruits or vegetables.
Experimental procedures
Plant growth
Barley (Hordeum vulgare) cultivar Golden Promise, wheat (Triticum aestivum) cultivar Bobwhite and tomato (S. lycopersicum) cultivar Moneymaker were grown on soil (for pathogenesis assays) or under sterile conditions (for transcriptional analyses and oxo-C14-HSL treatments) in a long day photoperiod at 19°C (barley and wheat plants) or at 25°C, 80% humidity (tomato plants). For the sterile system, 1 l of jars were used and plants grew on partially solidified 1/10 strength plant nutrient medium (PNM) (0.5 mM KNO3, 2 mM MgSO4, 0.2 mM Ca(NO3)2, 0.43 mM NaCl, 0.14 mM K2HPO4, 2 ml l−1 Fe-EDTA [20 mM FeSO4, 20 mM Na2EDTA]). Arabidopsis thaliana Colombia-0 plants were grown on soil at 22°C with 150 μmol m−2 s−1 light in 8/16 h day/night photoperiod.
Seed disinfection
For sterile growth, barley (H. vulgare) cv. Golden Promise and wheat (T. aestivum) cv. Bobwhite seeds were soaked shortly in sterile water and then in 70% ethanol. Subsequently, the seeds were immersed for 90 min in 6% sodium hypochlorite with continuous stirring. Seeds were then rinsed two times with sterile water at pH 3.0 and several times with sterile water at pH 7.0 until no trace of sodium hypochlorite was detected. For germination, the seeds were placed on wet sterile filter paper for 3 days. Arabidopsis thaliana Col-0 seeds were surface-disinfected with 50% ethanol/0.5% Triton X-100 for 30 min and briefly rinsed with 95% ethanol. For germination, seed were placed for 10 days on sterile half-strength MS medium supplied with 0.4% gelrite and 1% sucrose.
Oxo-C14-HSL treatment
Sixty millimolar stock solution of oxo-C14-HSL (Sigma-Aldrich) was prepared by dissolving the molecule in acetone. Ten-day-old barley or wheat plants cultivated on 1/10 PNM medium under sterile conditions were treated with oxo-C14-HSL at final concentration of 6 μM. All experiments were performed with the solvent control acetone.
Sinorhizobium meliloti inoculation
Sinorhizobium meliloti (Ensifer meliloti) Rm2011 expR+ containing the pWBexpR plasmid (M. McIntosh, pers. comm.) and S. meliloti (pBBR2-attM) carrying the lactonase gene attM from Agrobacterium tumefaciens were used. The rhizosphere was inoculated with S. meliloti expR+, S. meliloti attM (both OD600 = 0.2) or watered with 10 mM MgSO4 as control. Extraction of AHLs originated from S. meliloti liquid cultures was performed by vortexing with CHCl3 and discarding the aqueous phase after centrifugation. The CHCl3 phase was then evaporated using an ultra-speed vacuum centrifuge. The remaining residue was dissolved in acetone. Detection of oxo-C14-HSL was accomplished by dropping 10 μl of the extracted AHLs onto reporter bacteria: Escherichia coli strain MT102 carrying the pJBA89 plasmid [Apr; pUC18Not-luxR-PluxI-RBSII-gfp(ASV)-T0-T1] (Andersen et al., 2001). After 2 h, the fluorescence was observed using an ex: 480/40 nm and em: 510-nm filters.
Blumeria graminis treatment
Three days after the last treatment with S. meliloti strains or MgSO4, barley leaves (cv. Golden Promise) were inoculated with Blumeria graminis f. sp. hordei by blowing fresh spores originated from infected barley leaves (∼ 100 conidia/cm2). The inoculated leaves were kept on 1% water-agar plates at room temperature under low-light conditions for 2 days.
Puccinia graminis treatment
Urediniospores of Puccinia graminis f. sp. tritici were collected from infected plants (density of ∼ 106 spores ml−1) and sprayed on 10-day-old wheat plants (cv. Bobwhite) that were previously pretreated with oxo-C14-HSL or control (acetone) for 3 days. The inoculated plants were placed for 12 h in the dark. Subsequently, inoculated plants were exposed to normal light condition and kept for 11 days in a growth chamber with an average of 19°C and 90% relative air humidity.
Phytophthora infestans treatment
The P. infestans isolate was originally obtained from infected potato foliage. To maintain its virulence, it was invigorated by monthly passage through potato tubers and the P. infestans cultures (16–22 days old) were maintained on solid V8 juice agar in the dark at 15°C. In order to obtain P. infestans spore solution, the culture was flooded with sterile, distilled water. The spore density was counted using a Fuchs–Rosenthal counting chamber. To improve the zoospore release, the sporangial suspension was placed at 5°C for 3 h and the final solution was adjusted to a density of about 80,000 spores ml−1. For the treatment, plants were drenched with the inoculation solution using a pneumatic spray gun and kept at 16°C in the dark with 100% relative air humidity. After 48 h, plants were exposed to a dark/light regime of 16/8 h and 65% relative air humidity. The disease severity was assessed by visual estimation of the infested leaf area and documented with digital pictures. The scale for rating was 1, 5, 10, 20, 30, 50, 60, 70, 80, 90 and 100%. The rating was done 4 and 7 days after inoculation. Each plant was rated separately and means were calculated of five replications per treatment. The formula adapted from Abbott (1925): EF = (Mtr – Mte)/(100 – Mte), in which EF is the percentage of treatment efficiency, Mtr is the percentage of treatment severity and Mte is the percentage of control severity, was used to calculate the efficiency of the treatment.
Salmonella Typhimurium treatment
In order to assess the Salmonella proliferation rate in plants, soil-grown 4-week-old A. thaliana Col-0 plants were pretreated as indicated before, and thereafter infiltrated using syringe infiltration with wild-type S. enterica serovar Typhimurium strain 14028s carrying the pEC75 plasmid conferring resistance to ampicillin. Bacteria were grown until the early log phase in LB medium, washed and resuspended in 10 mM MgCl2. Infiltration solution was adjusted to OD600 = 0.1, (1.7 × 108 bacteria ml−1). Bacterial population was monitored during 6 days post infiltration using selective LB medium containing ampicillin, as described in (Schikora et al., 2008).
DAB staining
Leaves were partially submerged in DAB-staining solution (pH 3.8) at a concentration of 1 mg ml−1 for 6 h. Thereafter, leaves were distained with ethanol : chloroform : trichloroacetic acid (4:1:0.15%) solution for 2 days and transferred to 50% glycerol until cytological observations. Development of ESH, formation of papillae or production of ROS were evaluated using an Axioplan 2 (Zeiss, Germany) microscope.
Transcriptional analyses
Barley cv. Golden Promise leaves pretreated with oxo-C14-HSL or acetone, and subsequently inoculated with Blumeria graminis f. sp. hordei were harvested at 0, 24 and 48 h after inoculation (hai). Plant material was homogenized and the total RNA was extracted using the Trizol system. cDNA synthesis was perform using 2 μg of total RNA according to qScript cDNA Synthesis Kit (Quanta BioScience Inc.), quantitative RT-PCR was performed using primers listed in Table S1. All expression values were normalized to expression of HvUBQ60 (Genbank: M60175.1).
Conclusions
We showed that the resistance-inducing effect of S. meliloti in crop plants depends on the production of oxo-C14-HSL. In three different crop plants of worldwide economic importance and relevant for the food chain, oxo-C14-HSL-producing bacteria enhanced their resistance against specific pathogens. In addition, using the Arabidopsis–Salmonella model, we demonstrate that the same strategy could be a successful method to prevent outbreaks of food-borne diseases originated from plants.
Acknowledgments
We are grateful to M. McIntosh and A. Becker (Centre of Synthetic Microbiology, Marburg) for proving the plasmid pWBexpR and the strain S. meliloti Rm 2011. We would like to thank M. Schikora for his help in the quantification of symptoms. The E. coli reporter strain used in this study was a kind gift from A. Hartmann.
Conflict of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1. List of primers used in quantitative RT-PCR. Annealing temperature for all primers was set at 60°C.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [Google Scholar]
- Andersen JB, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen BB, et al. gfp-Based N-Acyl Homoserine-lactone sensor systems for detection of bacterial communication. Appl Environ Microbiol. 2001;67:575–585. doi: 10.1128/AEM.67.2.575-585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Todd CD, Desikan R, Yang Y. Hu X. N-3-Oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol. 2012;158:725–736. doi: 10.1104/pp.111.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer WD, Mathesius U. Teplitski M. Eukaryotes deal with bacterial quorum sensing. ASM News. 2005;71:129–135. [Google Scholar]
- Deepak S, Shailasree S, Kini RK, Muck A, Mithofer A. Shetty SH. Hydroxyproline-rich glycoproteins and plant defence. J Phytopathol. 2010;158:585–593. [Google Scholar]
- Dempsey DA. Klessig DF. SOS – too many signals for systemic acquired resistance? Trends Plant Sci. 2012;17:538–545. doi: 10.1016/j.tplants.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Fu ZQ. Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- Fuqua WC. Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner S, Schmid M, Durr C, Schuhegger R, Steidle A, Hutzler P, et al. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol. 2006;56:188–194. doi: 10.1111/j.1574-6941.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- Garcia AV, Charrier A, Schikora A, Bigeard J, Pateyron S, de Tauzia-Moreau ML, et al. Salmonella enterica flagellin is recognized via FLS2 and activates PAMP-triggered immunity in Arabidopsis thaliana. Mol Plant. 2014;7:657–674. doi: 10.1093/mp/sst145. [DOI] [PubMed] [Google Scholar]
- Golberg D, Kroupitski Y, Belausov E, Pinto R. Sela S. Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs. Int J Food Microbiol. 2011;145:250–257. doi: 10.1016/j.ijfoodmicro.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol. 2007;45:101–127. doi: 10.1146/annurev.phyto.45.062806.094325. [DOI] [PubMed] [Google Scholar]
- Kaplan HB. Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D. Sela S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol. 2009;75:6076–6086. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Bian Z, Jia Z, Zhao Q. Song S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-Acyl-Homoserine lactones, the bacterial quorum-sensing signals. Mol Plant Microbe Interact. 2012;25:677–683. doi: 10.1094/MPMI-10-11-0274. [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A. Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17:9–15. doi: 10.1016/j.tplants.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG. Bauer WD. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA. 2003;100:1444–1449. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Altier C. Martin GB. Salmonella colonization activates the plant immune system and benefits from association with plant pathogenic bacteria. Environ Microbiol. 2013;15:2418–2430. doi: 10.1111/1462-2920.12113. [DOI] [PubMed] [Google Scholar]
- Miao C, Liu F, Zhao Q, Jia Z. Song S. A proteomic analysis of Arabidopsis thaliana seedling responses to 3-oxo-octanoyl-homoserine lactone, a bacterial quorum-sensing signal. Biochem Biophys Res Commun. 2012;427:293–298. doi: 10.1016/j.bbrc.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Foolad MR, Nowakowska M. Kozik EU. Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis. 2011;96:4–17. doi: 10.1094/PDIS-05-11-0458. [DOI] [PubMed] [Google Scholar]
- Ortiz-Castro R, Martinez-Trujillo M. Lopez-Bucio J. N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ. 2008;31:1497–1509. doi: 10.1111/j.1365-3040.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- Pang T, Bhutta ZA, Finlay BB. Altwegg M. Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 1995;3:253–255. doi: 10.1016/s0966-842x(00)88937-4. [DOI] [PubMed] [Google Scholar]
- Pang YD, Liu XG, Ma YX, Chernin L, Berg G. Gao KX. Induction of systemic resistance, root colonisation and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur J Plant Pathol. 2009;124:261–268. [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, van Wees SCM. Bakker PAHM. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A, et al. Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta. 2008;229:73–85. doi: 10.1007/s00425-008-0811-4. [DOI] [PubMed] [Google Scholar]
- Rangel JM, Sparling PH, Crowe C, Griffin PM. Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C-M, Hu C-H, Reddy MS. Kloepper JW. Different signaling pathways of induced resistance by rhizobacteria in Arabidopsis thaliana against two pathovars of Pseudomonas syringae. New Phytol. 2003;160:413–420. doi: 10.1046/j.1469-8137.2003.00883.x. [DOI] [PubMed] [Google Scholar]
- Schenk ST, Stein E, Kogel KH. Schikora A. Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal Behav. 2012;7:178–181. doi: 10.4161/psb.18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk ST, Hernandez-Reyes C, Samans B, Stein E, Neumann C, Schikora M, et al. N-Acyl-Homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell. 2014;26:2708–2723. doi: 10.1105/tpc.114.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Carreri A, Charpentier E. Hirt H. The dark side of the salad: Salmonella typhimurium overcomes the innate immune response of Arabidopsis thaliana and shows an endopathogenic lifestyle. PLoS ONE. 2008;3:e2279. doi: 10.1371/journal.pone.0002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A. Kogel KH. N-acyl-homoserine lactone confers resistance towards biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011a;157:1407–1418. doi: 10.1104/pp.111.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Virlogeux-Payant I, Bueso E, Garcia AV, Nilau T, Charrier A, et al. Conservation of Salmonella infection mechanisms in plants and animals. PLoS ONE. 2011b;6:e24112. doi: 10.1371/journal.pone.0024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora M, Neupane B, Madhogaria S, Koch W, Cremers D, Hirt H, et al. An image classification approach to analyze the suppression of plant immunity by the human pathogen Salmonella Typhimurium. BMC Bioinformatics. 2012;13:171. doi: 10.1186/1471-2105-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Shah J. Zeier J. Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci. 2013;4:30. doi: 10.3389/fpls.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirron N. Yaron S. Active suppression of early immune response in tobacco by the human pathogen Salmonella Typhimurium. PLoS ONE. 2011;6:e18855. doi: 10.1371/journal.pone.0018855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M, Eberhard A, Gronquist MR, Gao M, Robinson JB. Bauer WD. Chemical identification of N-acyl homoserine lactone quorum-sensing signals produced by Sinorhizobium meliloti strains in defined medium. Arch Microbiol. 2003;180:494–497. doi: 10.1007/s00203-003-0612-x. [DOI] [PubMed] [Google Scholar]
- Van der Ent S, Verhagen BW, Van Doorn R, Bakker D, Verlaan MG, Pel MJ, et al. MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol. 2008;146:1293–1304. doi: 10.1104/pp.107.113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wees SC, Van der Ent S. Pieterse CM. Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Voigt CA. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front Plant Sci. 2014;5:168. doi: 10.3389/fpls.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkani AA, Stein E, Rohrich CR, Schikora M, Evguenieva-Hackenberg E, Degenkolb T, et al. Homoserine lactones influence the reaction of plants to rhizobia. Int J Mol Sci. 2013;14:17122–17146. doi: 10.3390/ijms140817122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of primers used in quantitative RT-PCR. Annealing temperature for all primers was set at 60°C.


