Abstract
Growth-promoting Sphingomonas paucimobilis ZJSH1, associated with Dendrobium officinale, a traditional Chinese medicinal plant, was characterized. At 90 days post-inoculation, strain ZJSH1 significantly promoted the growth of D. officinale seedlings, with increases of stems by 8.6% and fresh weight by 7.5%. Interestingly, the polysaccharide content extracted from the inoculated seedlings was 0.6% higher than that of the control. Similar growth promotion was observed with the transplants inoculated with strain ZJSH1. The mechanism of growth promotion was attributed to a combination of phytohormones and nitrogen fixation. Strain ZJSH1 was found using the Kjeldahl method to have a nitrogen fixation activity of 1.15 mg l−1, which was confirmed by sequencing of the nifH gene. Using high-performance liquid chromatography-mass spectrometry, strain ZJSH1 was found to produce various phytohormones, including salicylic acid (SA), indole-3-acetic acid (IAA), Zeatin and abscisic acid (ABA). The growth curve showed that strain ZJSH1 grew well in the seedlings, especially in the roots. Accordingly, much higher contents of SA, ABA, IAA and c-ZR were detected in the inoculated seedlings, which may play roles as both phytohormones and ‘Systemic Acquired Resistance’ drivers. Nitrogen fixation and secretion of plant growth regulators (SA, IAA, Zeatin and ABA) endow S. paucimobilis ZJSH1 with growth-promoting properties, which provides a potential for application in the commercial growth of D. officinale.
Introduction
Dendrobium officinale wall. ex Lindl. is a valuable traditional Chinese medicinal orchid (Anon, 1999). As the major officinal part, the stem of D. officinale is rich in active compounds, especially polysaccharides (Chen and Guo, 2000). It has been used as the basis for a tonic in Chinese medicine due to possessing immuno-stimulating, anti-tumor and anti-mutagenic activities (Wang et al., 2010). Therefore, the demand for D. officinale has constantly been increasing, and has resulted in over-exploitation and depletion of this wild plant resource. However, D. officinale is usually distributed as a saxicolous epiphyte specie, found at heights ranging from 100∼3000 m, mainly in mountainous regions in Yunnan, Guangxi, Guizhou, Fujian and Zhejiang provinces of China (Lai et al., 2006). It usually requires 3–5 years of growth to reach the state of maturity at which it can be effectively utilized as a drug (Waterman and Bidartondo, 2008). Therefore, the supply of wild plants has fallen far short of demand, and artificial cultivation has become the main source of this plant for commercial medicinal use.
At present, D. officinale is widely cultivated in the south of China, with a steadily occurring increase in planting area. Because of the small seeds without endosperm characteristic of this plant (Smresiu and Currah, 1989), the supply of seedlings almost completely depends on the tissue culture rather than on seeds (Zhou et al., 2012). It normally takes 1 or 2 years to culture the seedlings, and 3 to 5 years for the plantlets to grow, before harvest. A long growth period is accordingly needed for the collection of D. officinale as a raw material for medicinal use, which inevitably induces a high price, of over US$1000 kg−1 dried stem (Zheng, 2011). Furthermore, the active components, e.g. polysaccharides, are usually found in lesser amounts in the cultivated materials than in the wild counterpart, which makes the former of lower efficacy as a medicine (Si et al., 2013). Therefore, it is important to promote the growth of robust seedlings to help improve the survival of the seedlings, and thus promote the growth of the transplants.
Fungi are reported to play a critical role on the growth of D. officinale. First, the germination of D. officinale benefits from the nutrition of fungi. The seeds do not germinate well without the symbiosis of fungi (Smresiu and Currah, 1989). Second, fungi are the main source of essential nutrients for the growth of D. officinale (Waterman and Bidartondo, 2008), which commonly attaches to cliffs usually with small amounts of available nutrients. The plant root cells are usually penetrated by fungal hyphae, and coil-like pelotons are formed within the cortical cells (Clements, 1988). In addition, some fungi can produce antibiotics and plant growth regulators, such as auxins, gibberellic acids, indoleacetic acid, abscisic acid, zeatin and zeatin riboside (Zhang et al., 2013).
To the present time, a wide range of fungi, including 38 genera, 11 families, 10 orders, five classes and three subdivisions, have been isolated from D. officinale (Li et al., 2012b. These fungi, such as Mycena, Rhizoctonia, Epulorhiza, Alternaria, Cephalosporium, Acremonium, Fusarium, Colletotrichum, Chaetomium and Xylaria, were found to be beneficial to the germination or growth of D. officinale (Guo et al., 2000). Many studies have shown that the role of non-mycorrhizal Dendrobium fungi in orchid root colonization can stimulate the growth of the host plant (Hou and Guo, 2009).
However, few studies have been reported on the effects of bacteria on D. officinale. This is rather surprising, since plants are reported to be closely associated with prokaryotes. Most genera of alpha, beta and gamma Proteobacteria and some members of Firmicutes, Bacteriodetes and Acinobacteria have been isolated from plants, and they have been recognized to have considerable favorable impact on plant growth (Ezra et al., 2004; Lee et al., 2004). Bacteria are also effective agents for stimulating secondary metabolism (Ali et al., 2012) and for improving or producing functional components. With regard to Orchidaceae, some bacteria were reported to improve seed germination of Dendrobium moschatum (Tsavkelova et al., 2007). In our previous work (Yu et al., 2013), we detected various bacteria in D. officinale, with the dominant bacteria genus being Burkholderia. Some of these bacteria commonly have the function of nitrogen fixation and thus may potentially play important roles in D. officinale. Moreover, bacteria in Orchidaceae are primarily concerned with antagonistic action towards plant diseases. Wang and Mo (2009) isolated seven antagonistic bacteria from some wild orchid varieties from Hainan, China. Tsavkelova and colleagues (2001) obtained four bacteria from D. moschatum which were all characterized as pathogens. Bacteria with other functions have rarely been reported in association with orchid plants.
Based on the above studies, in the present study we focus on the function of plant-associated bacteria on the growth of seedlings and transplants of D. officinale. In our previous research, we isolated 21 bacterial endophytes from surface-sterilized wild D. officinale from Qingyuan in Zhejiang Province (data not shown). Among these 21 endophytes, strain ZJSH1 was found to have the potential of growth-promoting activity in preliminary experiments. Herein, the bacterium ZJSH1 will be phenotypically and genotypically characterized, and its effect and mechanism of growth promotion will be investigated.
Results
Phenotypic and Genotypic characterization of strain ZJSH1 as Sphingomonas paucimobilis.
Strain ZJSH1 was phenotypically and genotypically identified. Cultured on nutrient agar (NA) at 28°C for 24 h, the colonies were yellow and smooth edged (Fig. S1B). The cells were aerobic Gram-negative short rods without capsules or spores, and motile with one polar flagellum (Fig. S1C). Tests for oxidase, urease, catalase and for utilization of citrate, fructose and mannose were positive (Table S1). The other enzyme tests, such as for proteinase, lecithinase and amylase, were negative. It is apparent that strain ZJSH1 has phenotypic characteristics in common with S. paucimobilis ATCC 31461 (Li and Zhang, 2000).
To further characterize strain ZJSH1, the 16S rRNA gene was amplified and sequenced. Amplification by polymerase chain reaction resulted in a 1450 bp fragment which shared 100% similarity with the 16S rRNA gene of S. paucimobilis GIFU2395T (D16144). Phylogenetic analysis revealed that ZJSH1 clustered closely with S. paucimobilis and formed a subgroup phylogenetically distinct from other Sphingomonas species. The phylogenetic tree (Fig. S2) was constructed from the nucleotide sequence and 18 other homologous sequences, using the neighbor-joining method. The 16S rRNA gene's nucleotide sequence of ZJSH1 was submitted to GenBank with accession number KC017473.
Growth promotion and polysaccharide accumulation of D. officinale
According to our preliminary experiments, the optimum inoculum concentration of strain ZJSH1 was 104 CFU ml−1 cells. The weights of the fresh seedlings, and the lengths of the roots and the stems, were recorded at 90 days post-inoculation (Fig. 1). As shown in Fig. 1A, the seedlings inoculated with ZJSH1 had higher weights and longer stems and roots than the control seedlings. Only one bacterium, ZJSH1, was then re-isolated from the leaves of the inoculated seedlings of D. officinale, demonstrating that the growth promotion was induced by strain ZJSH1.These results indicate that strain ZJSH1 was able to promote the growth of the D. officinale seedlings.
Fig 1.

Effects of strain ZJSH1 on growth-promoting of tissue culture seedlings (A) and on polysaccharide accumulation (B).A. Increasing ratio of treatment versus control.B. Polysaccharide content of D. officinale seedlings (*P < 0.05; **P < 0.01; CK: control).
The effects of strain ZJSH1 on the growth of the transplants are shown in Fig. 2. According to Fig. 2, it may be seen that at 10 months post-inoculation, the transplants inoculated before transplanting had much higher weights, longer stems and longer roots than both the control group and the group that was inoculated during transplanting. Significantly, the average root length was about 52% greater than that of the control, the average stem length was about 81% greater, and the fresh weight was about 77% greater than that of the control. Inoculation during transplanting also significantly promoted the growth of the seedlings compared with the control. It is evident that inoculation before transplanting had more significant effects on the growth of D. officinale, indicating that the earlier inoculation took place, the greater the extent of growth promotion.
Fig 2.
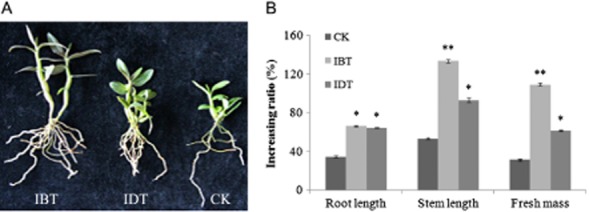
Growth-promoting effect of strain ZJSH1 on the transplants in pot.A. The plants showing stem and root.B. Increasing ratio 10 months post-transplanting versus pre-transplanting (*P < 0.05; **P < 0.01; IBT: inoculation before transplantation; IDT: inoculation during transplantation; CK: control).
The polysaccharides were extracted from the D. officinale tissue culture seedlings at 90 days post-inoculation with strain ZJSH1. As shown in Fig. 1B, 4.154 g crude polysaccharide was extracted from 50 g of inoculated D. officinale, with a polysaccharide content of 8.3%, while 3.852 g crude polysaccharide was obtained from the control, with a polysaccharide content of 7.7%. However, no polysaccharide was extracted from the fermentation broth of strain ZJSH1 (data not shown), indicating that the polysaccharide originated from the seedlings. It is thus evident that the inoculation of strain ZJSH1 is beneficial for the accumulation of polysaccharides in D. officinale seedlings.
Phytohormone production and nitrogen fixation play a vital role in the growth-promoting properties of D. officinale
Phytohormones production by strain ZJSH1
The major ingredients in the supernatant of the fermentation broth were further analysed using the LC-MS/MS method described below. As shown in Table 1, 11 types of phytohormones in total were detected in the 2-d culture of strain ZJSH1. Salicylic acid (SA) had the highest concentration of 60.59 ng ml−1, and indole-3-acetic acid (IAA) followed with 11.75 ng ml−1. Six isomers of zeatin were detected, and their overall total concentration was 5.78 ng·ml−1 with c-Z and DZ7G as the major isomers present. In addition, abscisic acid (ABA) and N6-isopentenyladenine (Ip and iP7G) were also detected in the fermentation broth. Therefore, strain ZJSH1 appears to produce relatively abundant types of phytohormones, which could be the main reason that it effectively promotes the growth of D. officinale.
Table 1.
Phytohormones produced by endophyte ZJSH1 in fermentation broth
| Item | ZJSH1 (ng ml−1) |
|---|---|
| SA (salicylic acid) | 60.59 ± 4.56 |
| IAA (indole-3-acetic acid) | 11.75 ± 0.90 |
| c-Z (cis-zeatin) | 2.18 ± 0.11 |
| DZ7G (dihydrozeatin-7-glucoside) | 2.11 ± 0.16 |
| Z7G (trans-zeatin-7-glucoside) | 0.94 ± 0.06 |
| t-Z (trans-zeatin) | 0.31 ± 0.04 |
| c-ZR (cis-zeatin riboside) | 0.24 ± 0.01 |
| ZOG (trans-zeatin-o-glucoside) | 0.02 ± 0.005 |
| ABA (abscisic acid) | 1.81 ± 0.06 |
| iP (N6-isopentenyladenine) | 0.89 ± 0.07 |
| iP7G (N6-isopentenyladenine-7-glucoside) | 1.21 ± 0.06 |
Phytohormone production in the inoculated plants
To determine the exact contribution of strain ZJSH1 on the phytohormone production in the inoculated plants, the phytohormone concentration was analysed in the inoculated seedlings. First, the population dynamics of strain ZJSH1 in the seedlings were investigated. As shown in Fig. 3A, the highest population was found within and on the surface of the roots, followed by the stems, and then the leaf samples. During the 90 days growth period of the study, the population of strain ZJSH1 increased continuously during 45–60 days, with a constant population then maintained for the remaining time. The population remained at about 104 CFU g−1 in roots, at about 103 CFU g−1 in stems and at about 102 CFU g−1 in leaves. Therefore, the phytohormone content was accordingly determined at the 30, 60 and 90 days post-inoculation periods (Table S2).
Fig 3.

Reproduction dynamics of strain ZJSH1 (A) and their effect on the contents of the four main phytohormones in the culture seedlings (B) (CK: control).
As shown in Fig. 3B, the same phytohormones were detected in plants as in bacterial fermentation broth, and four types of phytohormones were found at levels in the inoculated plants which were higher than in the control groups. Among the four, SA was the phytohormone with the highest concentration, and its level increased over the 90 day period. The SA content was almost twice as high in the inoculated seedlings compared with the controls. Abscisic acid was the second most significant phytohormone, with higher increasing rate detected over the entire 90 days, which was different from that observed for the corresponding content in the supernatant of the fermentation broth. The IAA content showed a rapid increase during the 30–60 day period, with a peak value of 15.50 ng g−1, after which it slowly declined over time. The c-ZR content continually increased with higher rate in the later growth term.
Nitrogen fixation and phosphate solubilization
Nitrogen fixation and phosphate solubilization are two of the most common mechanisms by which bacteria promote growth in their host. Accordingly, the nitrogen fixation and phosphate solubilization activities were investigated for strain ZJSH1. As shown in Fig. S1A, strain ZJSH1 grew well on nitrogen-free agar plates. In nitrogen-free liquid medium, the turbidity was 1.316 (OD595) compared with that of 1.709 in liquid NA, and the total nitrogen content increased to 1.15 mg L−1 after culturing for 3 days. The nitrogen fixation-related gene nifH was sequenced with accession number KF182367.1, and the resulting sequence shared 99% homology with that of Stenotrophomonas maltophilia KNUC170 (GenBank: DQ431165.1). Therefore, the cloning results further confirmed that strain ZJSH1 possessed the activity of nitrogen fixation.
Pikovskaya's agar plate was used to test the phosphate solubilization of ZJSH1. After culturing at 28°C for 3–5 days, no soluble zones were formed, indicating that strain ZJSH1 had no phosphate solubilization activity.
Discussion
Dendrobium officinale, one species of the second largest genus Dendrobium in the family Orchidaceae, is a valuable traditional Chinese medicinal herb which has been used in China for over 1000 years. Microbes play a vital role in the germination and growth of D. officinale, and fungi are commonly the first type of microbe to be considered and studied regarding their function on the plants of Orchidaceae. Bacteria, however, are seldom considered in this role. Herein, one particular bacterium, ZJSH1, showed significant growth promotion on both culture seedlings and transplants of D. officinale.
The growth-promoting strain ZJSH1 was phenotypically and phylotypically identified as Sphingomonas paucimobilis. Sphingomonas sp. has a close relationship with the growth of plants. Takeuchi and colleagues (1995) found that Sphingomonas sp. could promote the absorption of mineral ions in plants. Sessitsch and colleagues (2004) found that Sphingomonas sp. could synthesize siderophores, which promote the absorption of iron ions in plants. Tsavkelova and colleagues (2007) extended beyond individual strains as inoculants and reported an increase in the germination of orchid seeds (Dendrobium moschatum) inoculated with Sphingomonas sp. and IAA-producing Mycobacterium sp. However, S. paucimobilis has seldom been studied as a plant-associated bacterium, and to the present time, it has been found to exist in only a few plants, such as potato and Petunia hybrida (Garbeva et al., 2001; Miyazaki et al., 2010). It was recently discovered to be a novel microorganism for biodegrading aromatic compounds, which endows it with potential value in environmental conservation and industrial production (Bending et al., 2003). To our knowledge, this is the first time that S. paucimobilis has been reported as being a potential plant growth-promoting bacteria (PGPB) in D. officinale.
Plant growth promoting bacteria are widely used to improve plant growth. Plant growth-promoting bacteria play vital roles through many ways, including facilitating resource acquisition, by providing plants with resources/nutrients that they lack, such as nitrogen (Iniguez et al., 2004) and phosphorus (Kuklinsky-Sobral et al., 2004), or by improving the competition for nutrients and niches (Sturz et al., 2000). They may also secrete phytohormones (Tsavkelova et al., 2006), prevent the proliferation of phytopathogens through inducing systemic resistance or modulate the effects of environmental stress (manifest as heavy metals such as Ca2+ and Ni2+, salt and drought) or synthesize various antibiotics, lytic enzymes, siderophores, ACC deaminase (Glick, 2012) and detoxification enzymes (Sturz and Christie, 2003).
The growth-promoting mechanism of strain ZJSH1 was determined to be due to a combination of phytohormone excretion and nitrogen fixation. The major phytohormones produced by strain ZJSH1 were SA, IAA, Zeatin and ABA. These phytohormones can be classified into two types according to their function: growth and regulatory hormones (SA, IAA and Zeatin) and stress resistance hormones (SA and ABA). Salicylic acid, a phenolic derivative, is considered to be a potent plant hormone because of its diverse regulatory roles in plants. It has direct involvement in plant growth, thermogenesis, flower induction and uptake of ions (Pérez-Jiménez et al., 2013). Enhancement of the levels of chlorophyll and carotenoid pigments and photosynthetic rate, as well as modifying the activity of some of the important enzymes, are other roles assigned to SA, all of which enhance plant growth and yield (Hayat et al., 2007). Indole-3-acetic acid, the physiologically most active phytohormone in plants, usually functions as an important signalling molecule in the regulation of plant development, including organogenesis, tropic responses, cellular responses such as cell expansion, division and differentiation and gene regulation (Ryu and Patten, 2008). Zeatin is a member of the plant growth hormone family known as cytokinins, promoting cell division and growth of lateral buds and stimulating cell division to produce bushier plants (Patel et al., 2012). These phytohormones may play significant roles on the growth promotion of the seedlings and transplants of D. officinale.
Plant growth-promoting bacteria also activate plant defense, resulting in systemic protection against plant pathogens, a phenomenon termed induced systemic resistance (ISR). Some PGPB trigger an SA-dependent signalling pathway by producing nanogram amounts of SA in the rhizosphere (De Meyer and Höfte, 1997). However, the majority of PGPB that activate ISR appear to do so via a SA-independent pathway involving jasmonate and ethylene signals (Pieterse et al., 1998). Considerable interest has been generated by the ability of SA to produce protective effects in plants under the actions of biotic stress (Maurhofer et al., 1998) and abiotic stresses such as drought, chilling, heavy metal toxicity and osmotic stress (Rivas-San Vicente and Plasencia, 2011). The important role of SA in such protective actions probably determines its ability to induce the expression of genes coding not only for pathogenesis-related protein but also for genes of extensin in Arabidopsis plants (Georgios et al., 1999). In addition, ABA is understood to mainly contribute to the plant's stress response (Perrig et al., 2007). In dry soil, ABA can be transported to the shoots to restrict leaf growth and limit water loss (Dodd, 2005). The stress resistance of these phytohormones is important for the growth of artificial D. officinale since the corresponding wild plants favour moist and shady mountainous environments (Lai et al., 2006). Such moist and shady environmental conditions are usually not easy to control sufficiently well for artificial plant growth, even under tissue-culturing condition, which places these plants under stress and consequently influences their growth. Therefore, these stress-related phytohormones produced by strain ZJSH1 might protect D. officinale from various stresses; however, their effect on the growth needs further confirmation.
The results for the inoculated tissue culture seedlings of the present study showed that strain ZJSH1 became endophytically established mainly in roots from the surface base of seedlings, and then transferred to the stems and leaves, and subsequently the contents of SA, IAA and Zeatin increased. These phytohormones thus may play a significant role on the growth or stress response of the culture seedlings or transplants of D. officinale, either singly or in combination through possible synergistic effects. The process through which the phytohormones react on the plants of D. officinale requires further detailed study.
Dendrobium officinale usually grows on nutrient-poor cliffs on mountains (Lai et al., 2006). It grows slowly and does not grow well without the presence of microbes (Waterman and Bidartondo, 2008). These facts could explain the growth-promoting effect of strain ZJSH1. Until now, there have been few reports concerning the study of strains which have the ability of nitrogen fixation in orchid plants, and especially in Dendrobium. Only associative cyanobacteria and some strains of Pseudomonas and Bacillus were isolated from the orchids Calanthe vestita var. rubro-oculata and D. moschatum, and some of these were assessed as having nitrogen fixation activity (Tsavkelova et al., 2001). In the present study, strain ZJSH1 has the activity of nitrogen fixation which would assist nitrogen absorption in D. officinale, and the nitrogen fixation-related gene was cloned and the sequence had 99% similarity with the nifH gene of Stenotrophomonas maltophilia KNUC170 (GenBank: DQ431165.1). The present study is the first in which Sphingomonas sp. has been found to have nitrogen fixation activity. The extent to which strain ZJSH1 contributes to the providing of nitrogen for the growth of D. officinale, and its distribution in D. officinale need to be elucidated through further research.
Experimental procedures
Materials
The plant-associated bacterium ZJSH1, isolated from surface-sterilized roots of D. officinale by our laboratory and stored as No: M 2010041 in the China Center for Type Culture Collection (CCTCC), was routinely cultured on NA at 28°C. The sterile tissue culture seedlings of D. officinale were prepared in our laboratory according to the protocol described by Li and colleagues (2012a).
Characterization of strain ZJSH1
Phenotypic characteristics
Strain ZJSH1 was inoculated and cultured on NA for 24 h at 28°C, and phenotypical analysis was conducted by analyzing its morphology, gram staining and biochemical characteristics. Cell morphology was examined using a light microscope following Gram staining. Microscopic cell morphology and flagella of strain ZJSH1 were observed by transmission electron microscopy (JEM 1200 EX) after negative staining with 1% uranyl acetate. Physiological tests, including aerobic growth, Methyl Red and Voges–Proskauer reactions, production of acid from carbohydrates, utilization of organic acids, NaCl tolerance, gelatin liquefaction and enzyme activities, were performed by the methods of Claus and Berkeley (1986).
Genotypic characterization and phylogenetic analysis
For genotypic characterization, genomic DNA extraction, amplification and sequencing of 16S rRNA and nifH genes were performed as previously described (Hu et al., 2009). The sequencing was carried out by Shanghai Invitrogen Biotechnology Company, Limited (Shanghai, China). The nucleotide sequences of 16S rRNA and nifH genes were aligned with representatives from similar genera deposited in the GenBank, EMBL and DDBJ databases using the program MEGA version 4.1. The phylogenetic trees based on the 16S rRNA gene were constructed by three methods: the neighbor-joining (mega version 3.1), maximum parsimony and maximum likelihood (Phylip version 3.65) (Saitou and Nei, 1987) methods. Bootstrap re-sampling analysis was performed to estimate the confidence of the tree topologies.
Growth-promoting effect of strain ZJSH1 on D. officinale
To evaluate the growth-promoting effect on D. officinale, strain ZJSH1 was inoculated on the tissue culture seedlings and transplants of D. officinale.
The inoculum was cultured by transferring strain ZJSH1 to a 250 ml Erlenmeyer flask containing 50 ml nutrient broth, followed by incubation at 28°C on a shaker (220 r.p.m.) for 2 days. After the incubation period, the culture was centrifuged and diluted into 104 CFU ml−1 using double-distilled (dd) H2O. A 50-μL bacterial suspension was inoculated on the base of each of the tissue culture seedlings of D. officinale, which were 45 days old, while the control was treated only with 50 μL dd H2O instead. The tissue culture seedlings were grown in a greenhouse under conditions of 25°C constant temperature with a 12 h sustained photoperiod. After 90 days of inoculation, the growth (height and fresh weight) and quality (content of polysaccharides) of the seedlings were determined. Fifteen replicates were used for each treatment, and the experiment was repeated in triplicate.
Some of the tissue culture seedlings above were used for transplanting. Before transplanting, all the seedlings were rinsed with sterilized tap water to remove the medium, and air-dried for 12 h indoors, which helps to remove some water and thus raise survival rate. The seedlings previously inoculated with strain ZJSH1 were used as the inoculation treatment for potted culturing before transplanting. The seedlings without inoculation of ZJSH1 were divided into two portions before being transplanted. The first portion was used for seedling inoculation through soaking in bacterial suspension (104 CFU ml−1) for 10 min, prepared as inoculation treatment during transplanting. The second portion was used for the non-inoculated seedlings as a control. Each treatment was replicated in triplicate, and each replicate consisted of 10 pots. The cracked bark of pine trees was used as the cultivation medium for the potted culture experiments. All the plants were sprayed with water every day. The effect of the bacteria on the pot-cultured D. officinale was investigated by measuring the roots, stems and fresh weights of all groups.
Polysaccharide content
As the major active ingredient, polysaccharide content is usually determined to evaluate the quality of D. officinale seedlings. The water extraction and alcohol precipitation method (Luo et al., 2009) was adopted to extract the polysaccharides of the inoculated and non-inoculated 90-day-old tissue culture seedlings of D. officinale. Prior to extraction, the stems were thoroughly washed using tap water, dried at 60°C for 5 h and ground with liquid nitrogen. After grinding, 50 g of the powder were treated with 80% ethanol for 1 h, and then filtered. After filtering, the residue was further extracted with 1000 ml distilled water. All extracts were combined and then concentrated to 100 ml under low pressure. The extracts were precipitated with 400 ml of 95% ethanol, and the mixtures were kept overnight at 0°C. Finally, the precipitate was washed successively with ethanol and acetone (each for five times), and recovered by freeze-drying, which produced a crude polysaccharide sample.
Growth promotion mechanism analysis
Phytohormones produced by ZJSH1 in medium and in plants
Phytohormones are usually involved in promoting plant growth. The contents of phytohormones in the fermentation broth and in the inoculated seedlings were analysed. To detect the production of phytohormones, strain ZJSH1 was inoculated into a flask with nutrient broth and then cultured at 28°C with shaking at 220 r.p.m. for 2 days. After centrifugation to remove the cells, the supernatant was decanted and used for analysis of phytohormones.
To detect the contents of phytohormones in the inoculated seedlings, a 50 mg sample of stem was taken from the inoculated plants, and frozen with liquid nitrogen. The plant tissues were then ground into powder, to which 50 μl of the internal standard working solution were added. The microscale extraction and centrifugation process was performed using the method described by Pan and colleagues (2010). The supernatant was used for phytohormone analysis by LC-MS/MS.
HPLC conditions
High-performance liquid chromatography analysis was performed using a Surveyor HPLC System (Thermo Scientific, San Jose, CA). Chromatographic separation was carried out using a 100 mm × 2.1 mm inner diameter LC column (ZORBAX Extend-C18, 1.8 μm particle size, Agilent Technologies, Lawrence, USA,). The binary solvent system consisted of water with 5 mmol L−1 formic acid (A) and methanol (B) as mobile phase. The column temperature was maintained at 40°C, and the flow rate was 150 μl min-1. The injection volume was 2 μl.
Mass spectrometry analysis
Mass spectrometry (MS) analysis was carried out on a TSQ Quantum Access MAX triple stage quadrupole mass spectrometer with a heated electrospray ionization (HESI) probe (Thermo Scientific, San Jose, CA). The MS conditions were as follows: ion source polarity: negative or positive; spray voltage: −2200 V or 2800 V; vaporizer temperature: 300°C; skimmer offset: 3 V; sheath gas pressure (N2): 30 (arbitrary units); auxiliary gas pressure (N2): 5 (arbitrary units); collision pressure: 1.2 m Torr; ion transfer tube temperature: 300°C; scan type: selective reaction monitoring.
In order to elucidate the exact contribution of endophyte on the effect of phytohormones in plants, the growth dynamics of inoculated bacteria were investigated. Therefore, endophytic bacteria were simultaneously isolated from the roots, stems and leaves of the inoculated tissue culture seedlings of D. officinale every other week. After washing the seedlings using sterile tap water, 1 g each of root, stem and leaf samples was pulverized and diluted in dd H2O, and spread on the NA medium. The resulting bacterial populations were calculated using the plate counting method.
Phosphate solubilization
The phosphate-solubilizing activity of strain ZJSH1 was determined by measuring the size of the transparent zone formed by solubilization of insoluble phosphate on Pikovskaya's agar plates [1% C6H12O6, 0.5% Ca3(PO4)2, 0.05% (NH4)2SO4, 0.02% NaCl, 0.01% MgSO4.7H2O, 0.02% KCl, 0.05% yeast extract, 0.0002% MnSO4.H2O, 0.0002% FeSO4.7H2O, pH 7.2]. The zone of clearance around the colony was observed at 3–5 days post-inoculation.
Nitrogen fixation
To estimate the nitrogen fixation ability, strain ZJSH1 was qualitatively and quantitatively analysed. For the qualitative test, strain ZJSH1 was both inoculated and cultured for 3–5 days in a liquid nitrogen-free medium on the same plate. The nitrogen fixation ability was evaluated by the growth, the colony diameter sizes on the plate and OD595nm in liquid medium. For the quantitative test, strain ZJSH1 was incubated in a flask with nitrogen-free liquid medium and then cultured at 28°C with shaking at 220 r.p.m. for 2 days. The nitrogen fixation ability was quantitatively evaluated by the increase of total nitrogen which was measured using the Micro-Kjeldahl method (Allen, 1931). The nitrogen-free medium consisted of 1% mannitol, 0.02% KH2PO4, 0.02% MgSO4·7H2O, 0.02% NaCl, 0.5% CaCO3, 0.01% CaSO4·2H2O, pH 6.8–7.0. To confirm the nitrogen fixation ability, the gene encoding nitrogenase was further cloned using the primers PolF (5′-TGCGAYCCSAARGCBGACTC-3′) and PolR (5′-ATSGCCATCATYTCRCCGGA-3′) described by Doreen and colleagues (2012).
Statistical analysis
The experimental data were statistically analysed by a t-test using the program dps 7.05 (Tang and Feng, 2007). Values are given as means ± SD for each sample, and differences were considered to be significant at the P < 0.05 level.
Conclusions
The growth-promoting bacterium ZJSH1 of D. officinale, with the ability to fix nitrogen and to excrete plant growth regulators such as SA, IAA, Zeatin and ABA, was investigated in this study and identified as S. paucimobilis. This growth-promoting strain may provide a more environmentally safe and efficient alternative to solving the problems existing in the artificial planting and cultivation of D. officinale.
Acknowledgments
We are grateful to Dr. Greg Duns for his valuable comments on the manuscript.
Conflict of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. The growth of strain ZJSH1 on nitrogen-free agar plate (A) and morphologies of colonies (B) and cells (C) of strain ZJSH1.
Fig. S2. Phylogenetic tree showing the relationship between strain ZJSH1 and its closely related strains. The tree was based on alignment of 16S rRNA gene sequences and was constructed using the Neighbor Joining method (mega version 4.1). The sequence of Escherichia coli was used as an out-group. Stability of the tree was assessed by 1000 bootstrap replications with Felsenstein confidence limits.
Table S1. Phenotypic characteristics of endophyte ZJSH1 compared with Sphingomonas paucimobilis ATCC 31461.
Table S2. Auxin detected in the seedlings inoculated with or without endophyte ZJSH1.
References
- Ali S, Charles TC. Glick BR. Delay of flower senescence by bacterial endophytes expressing ACC deaminase. J Appl Microbiol. 2012;5:1139–1144. doi: 10.1111/j.1365-2672.2012.05409.x. [DOI] [PubMed] [Google Scholar]
- Allen WF. A micro-Kjeldahl method for nitrogen determination. J Am Oil Chem Soc. 1931;10:391–397. [Google Scholar]
- Anon . Dendrobium candidum wall. ex Lindl. In: Hu SM, editor. Chung-Hua-Ben-Tsao. Shanghai, China: Shanghai Science Technology Press; 1999. pp. 705–711. in Chinese. [Google Scholar]
- Bending GD, Lincoln SD, Sørensen SR, Morgan JA, Aamand J. Walker A. In-field spatial variability in the degradation of the phenyl-urea herbicide isoproturon is the result of interactions between degradative Sphingomonas spp. and soil pH. Appl Environ Microbiol. 2003;69:827–834. doi: 10.1128/AEM.69.2.827-834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM. Guo SX. Advances in the research of constituents and pharmacology of Dendrobium. Nat Prod Res Dev. 2000;13:70–75. [Google Scholar]
- Claus D. Berkeley CW. The genus Bacillus. In: Sneath PHA, Mair NS, Sharp ME, Holt JG, editors; Bergey's Manual of Systematic Bacteriology. Vol. 2. Baltimore, USA: Williams and Wilkins; 1986. pp. 1105–1139. [Google Scholar]
- Clements MA. Orchid mycorrhizal associations. Lindleyana. 1988;3:73–86. [Google Scholar]
- De Meyer G. Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- Dodd IC. Root-to-shoot signalling, assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant Soil. 2005;274:251–270. [Google Scholar]
- Doreen F, Barbara P, Michael S, Jean L, Simões A, Veronica MR, et al. Molecular characterisation of the diazotrophic bacterial community in uninoculated and inoculated field-grown sugarcane (Saccharum sp.) Plant Soil. 2012;356:83–99. [Google Scholar]
- Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JB, et al. Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiology. 2004;150:785–793. doi: 10.1099/mic.0.26645-0. [DOI] [PubMed] [Google Scholar]
- Garbeva P, Van Overbeek LS, Van Vuurde JWL. Van Elsas JD. Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA based PCR fragments. Microb Ecol. 2001;41:369–383. doi: 10.1007/s002480000096. [DOI] [PubMed] [Google Scholar]
- Merkouropoulos G, Barnett DC. Shirsat AH. The Arabidopsis extensin gene is developmentally regulated, is induced by wounding, methyl jasmonate, abscisic and salicylic acid, and codes for a protein with unusual motifs. Planta. 1999;208:212–219. doi: 10.1007/s004250050552. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Chen XM, Yu XM. Fan L. Investigation on the isolation of mycorrhizal fungi from Anoectochilus roxburghii and its biological activity. Chin Pharm J. 2000;35:443–445. (in Chinese) [Google Scholar]
- Hayat S, Ali B, Ahmad A. Salicylic acid: biosynthesis, metabolism and physiological role in plants. In: Hayat S, Ahmad A, editors. Salicylic acid: A Plant Hormone. Dordrecht, Netherlands: Springer; 2007. pp. 1–14. [Google Scholar]
- Hou XQ. Guo SX. Interaction between a dark septate endophytic isolate from Dendrobium sp. and roots of D. nobile seedlings. J Integr Plant Biol. 2009;51:374–381. doi: 10.1111/j.1744-7909.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Hu XF, Fang QL, Li SX, Wu JG. Chen JS. Isolation and characterization of endophytic and rhizosphere bacterial antagonists of soft-rot pathogen from Pinellia ternata. FEMS Microbiol Lett. 2009;295:10–16. doi: 10.1111/j.1574-6968.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- Iniguez AL, Dong Y. Triplett EW. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol Plant Microbe Interact. 2004;17:1078–1085. doi: 10.1094/MPMI.2004.17.10.1078. [DOI] [PubMed] [Google Scholar]
- Kuklinsky-Sobral J, Araújo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA. Azevedo JL. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol. 2004;6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Lai YH, Wang SY. Xiao FH. The major ecological factors in Dendrobium resources distribution areas in China. Chin Agric Sci Bull. 2006;22:397–400. (in Chinese) [Google Scholar]
- Lee S, Flores-Encarnación M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE. Kennedy C. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in Cytochrome c biogenesis genes. J Bacteriol. 2004;186:5384–5391. doi: 10.1128/JB.186.16.5384-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Zan YY. Yang B. Tissue culture of Dendrobium officinale Kimura et Migo. Subtrop Plant Sci. 2012a;41:76–77. [Google Scholar]
- Li SL. Zhang HC. Isolation and identification of Sphingomonas paucimobilis. Inspec Quarant Sci. 2000;1:12–15. 43. [Google Scholar]
- Li SQ, Yi Y, Zhang CB. Sun JS. Research progress of the diversity and symbiotic mechanism of endophytic fungi from Dendrobium. North Hortic. 2012b;8:191–194. (in Chinese) [Google Scholar]
- Luo AX, He XJ, Zhou SD, Fan YJ, He T. Chun Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile lindl. extracts. Int J Biol Macromol. 2009;45:359–363. doi: 10.1016/j.ijbiomac.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D. Defago G. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of system resistance in tobacco against tobacco necrosis virus. Phytopathology. 1998;88:678–684. doi: 10.1094/PHYTO.1998.88.7.678. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Tan BH. Errington SG. Eradication of endophytic bacteria via treatment for axillary buds of Petunia hybrida using Plant Preservative Mixture (PPM TM) Plant Cell Tiss Organ Cult. 2010;102:365–372. [Google Scholar]
- Pan X, Welti R. Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc. 2010;6:986–992. doi: 10.1038/nprot.2010.37. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez M, Cantero-Navarro E, Acosta M. Cos-Terrer J. Relationships between endogenous hormonal content and direct somatic embryogenesis in Prunus persica L. Batsch cotyledons. Plant Growth Regul. 2013;9:1–6. [Google Scholar]
- Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassán FD. Luna MV. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol. 2007;5:1143–1150. doi: 10.1007/s00253-007-0909-9. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, van Wees SCM, van Pelt JA, Knoester M, Laan R, Gerrits H, et al. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PP, Rakhashiya PM, Chudasama KS, Thaker VS. Thaker VS. Isolation, purification and estimation of zeatin from Corynebacterium aurimucosum. Eur J Exp Biol. 2012;1:1–8. [Google Scholar]
- Rivas-San Vicente M. Plasencia J. Salicylic acid beyond defence, its role in plant growth and development. J Exp Bot. 2011;10:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- Ryu R. Patten CL. Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in Enterobacter cloacae UW5. J Bacteriol. 2008;190:7200–7208. doi: 10.1128/JB.00804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N. Nei M. The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sessitsch A, Reiter B. Berg G. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol. 2004;4:239–249. doi: 10.1139/w03-118. [DOI] [PubMed] [Google Scholar]
- Si JP, He WB. Yu QX. Progress and countermeasures of Dendrobium officinale breeding. China J Chin Mater Med. 2013;4:475–480. (in Chinese) [PubMed] [Google Scholar]
- Smresiu EA. Currah RS. Symbiotic germination of seed of terrestrial orchids of North America and Europe. Lindleyana. 1989;1:6–15. [Google Scholar]
- Sturz AV. Christie BR. Beneficial microbial allelopathies in the root zone, the management of soil quality and plant disease with rhizobacteria. Soil Till Res. 2003;72:107–123. [Google Scholar]
- Sturz AV, Christie BR. Nowak J. Bacterial endophytes, potential role in developing sustainable systems of crop production. Crit Rev Plant Sci. 2000;19:1–30. [Google Scholar]
- Takeuchi M, Sakane T, Yanagi M, Yamasato K, Hamana K. Yokota A. Taxonomic study of bacteria isolated from plants, proposal of Sphingomonas rosasp. nov. Sphingomonas asaccharolytica. nov. and Sphingomonas mali sp. nov. Int J Syst Bacteriol. 1995;45:334–341. doi: 10.1099/00207713-45-2-334. [DOI] [PubMed] [Google Scholar]
- Tang Q. Feng M. DPS Data Processing System: Experimental Design, Statistical Analysis and Data Mining. Beijing, China: Science Press; 2007. in Chinese. [DOI] [PubMed] [Google Scholar]
- Tsavkelova EA, Cherdyntseva TA, Lobakova ES, Kolomeitseva GL. Netrusov AI. Microbiota of the orchid rhizoplane. Microbiology. 2001;70:492–497. [PubMed] [Google Scholar]
- Tsavkelova EA, Klimova SIU, Cherdyntseva TA. Netrusov AI. Microbial producers of plant growth stimulators and their practical use, a review. Appl Biochem Microbiol. 2006;2:117–126. [PubMed] [Google Scholar]
- Tsavkelova EA, Cherdyntseva TA, Klimova SY, Shestakov AI, Botina SG. Netrusov AI. Orchid-associated bacteria produce indole-3-acetic acid, promote seed germination, and increase their microbial yield in response to exogenous auxin. Arch Microbiol. 2007;188:655–664. doi: 10.1007/s00203-007-0286-x. [DOI] [PubMed] [Google Scholar]
- Wang F. Mo R. Investigation and analysis of the endophyte bacteria of wild orchids in Hainan province. J Anhui Agric Sci. 2009;16:7330–7331. 7340 (in Chinese) [Google Scholar]
- Wang JH, Luo JP, Zha XQ. Feng BJ. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohyd Polym. 2010;361:114–118. [Google Scholar]
- Waterman RJ. Bidartondo MI. Deception above, deception below, linking pollination and mycorrhizal biology of orchids. J Exp Bot. 2008;59:1085–1096. doi: 10.1093/jxb/erm366. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhou XF, Yang SJ, Liu WH. Hu XF. Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in Dendrobium officinale using nested PCR-DGGE. Appl Microbiol Biotechnol. 2013;97:9825–9836. doi: 10.1007/s00253-013-5294-y. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Jennings A, Barlow PW. Forde BG. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. The development and thinking of D. officinale about imitating wild planting in Yueqing. Subtrop Crop Commun Zhejiang. 2011;33:34–38. (in Chinese) [Google Scholar]
- Zhou YQ, Yu LY. Tan XM. Advances in tissue culture of Dendrobium candidum Wall. ex Lindl. Chin J Ethnomed Ethnopharm. 2012;1:48–49. (in Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The growth of strain ZJSH1 on nitrogen-free agar plate (A) and morphologies of colonies (B) and cells (C) of strain ZJSH1.
Fig. S2. Phylogenetic tree showing the relationship between strain ZJSH1 and its closely related strains. The tree was based on alignment of 16S rRNA gene sequences and was constructed using the Neighbor Joining method (mega version 4.1). The sequence of Escherichia coli was used as an out-group. Stability of the tree was assessed by 1000 bootstrap replications with Felsenstein confidence limits.
Table S1. Phenotypic characteristics of endophyte ZJSH1 compared with Sphingomonas paucimobilis ATCC 31461.
Table S2. Auxin detected in the seedlings inoculated with or without endophyte ZJSH1.


