Abstract
Malignant melanoma is a significant public health problem; according to 2013 Surveillance, Epidemiology, and End Results data, its average incidence rate rose 2.6% each year for the last decade, and it is now the fifth most common cancer diagnosis in the United States. The rising incidence and historical poor response to chemotherapy have led to intense investigation of novel treatments for melanoma, including therapies to improve the immune-mediated destruction of cancer cells. Among the hallmarks of malignancy is the ability to evade this process: while early stages of tumor growth can induce functional CD8+ T-cell responses, cancer cells become increasingly embedded in an immune-suppressive tumor stroma. In the tumor microenvironment, T-cell proliferation and effector function are impaired due to engagement of T-cell programmed death receptor 1 (PD-1) with programmed death receptor ligand (PD-L1) expressed by cancer cells and antigen-presenting cells, which blocks T-cell-mediated cytotoxicity. This receptor-ligand engagement thereby inhibits immunity, allows the tumor to continue to grow, and contributes to the phenomenon of ‘T-cell exhaustion’. One immunotherapy strategy currently under investigation is inhibition of the interaction between PD-L1 and PD-1, thereby overcoming a critical immune checkpoint to facilitate destruction of cancer cells. In this review we discuss the preclinical rationale for PD-1 pathway inhibition in cancer, recent results of clinical trials targeting PD-1 and PD-L1, and evaluate its potential as a future anticancer therapy.
Keywords: immunotherapy, melanoma, programmed death receptor 1, programmed death receptor ligand
Introduction
Melanoma is a growing problem in oncology, with approximately 200,000 new cases and 65,000 deaths each year worldwide [Garbe et al. 2010; World Health Organization 2013]. In the United States, it is estimated that there will be 76,690 new cases of melanoma and 9,480 deaths from this disease [National Cancer Institute, 2013]. In its early stages melanoma can be cured by surgical resection, but once it has progressed to the metastatic stage it remains an incurable disease, with a 5-year survival rate of only 16%. The finding of somatic mutations in the BRAF oncogene paved the way for the introduction of BRAF inhibitors as a standard treatment in patients with locally advanced or metastatic melanoma [Flaherty et al. 2010]. While clinical responses to BRAF inhibition may be dramatic, they are generally short lived [Salama and Flaherty 2013], leading to an interest in investigation of immunomodulatory agents. Cytotoxic T-lymphocyte antigen 4 (CTLA-4), an inhibitory receptor expressed by tumor-infiltrating T cells and regulatory T cells, was successfully targeted with the development of the human monoclonal antibody ipilimumab. A pivotal trial of ipilimumab in metastatic melanoma demonstrated a median overall survival (OS) of 10 months compared with 6.4 months among patients in the control group [hazard ratio (HR) for death 0.68; p < 0.001] [Hodi et al. 2010], and the drug gained US Food and Drug Administration approval in 2011. Long-term survival outcomes in patients with metastatic melanoma treated in phase II trials (at either 3 mg/kg or 10 mg/kg of ipilimumab) showed survival rates reaching a plateau beginning at year 3, with a proportion of patients (12.3–49.5%) still alive 5 years after the start of treatment [Lebbé et al. 2013]. Recently, pooled data from 4846 patients who received ipilimumab within a clinical study or expanded access program were analyzed to provide an estimation of long-term survival: a plateau in survival began approximately 3 years after treatment for 21% of patients, and in some continued for up to 10 years. The durability of long-term survival did not appear to be impacted by prior therapy, dose or treatment regimen [Schadendorf et al. 2013]. This success has catalyzed extensive investigation into the potential for inhibitors of other immune-suppressive pathways to induce clinical benefit in advanced cancer, including anti-programmed death receptor 1 (PD-1) and programmed death receptor ligand (PD-L1) directed therapies.
Tumor and T-cell interaction
One of the key mechanisms by which malignant cells suppress antitumor immunity lies in the interaction between tumor cells and infiltrating T cells; despite expression of numerous antigens, tumor evasion of host immunity occurs, and a number of tumor-associated immune inhibitory mechanisms have been identified in the last decade [Fridman et al. 2012]. They include expression of the ligand PD-L1 by tumor cells, which can engage the inhibitory receptor PD-1 on activated T cells; increased levels of the tryptophan-catabolizing enzyme indoleamine-2,3-dioxygenase (IDO), which starves T cells of essential tryptophan; tumor infiltration with various subtypes of FoxP3+ regulatory T cells (Tregs), which mediate extrinsic T-cell suppression; and defective interleukin-2 (IL-2) production, driven in part through early growth response protein 2 (EGR2), causing T-cell anergy [Jandus et al. 2008; Mellor et al. 2001; Zheng et al. 2012; Curiel et al. 2004]. These suppressive mechanisms have been demonstrated in solid tumor models in rodents, including melanoma, where increased expression of PD-L1, IDO, and FoxP3+ Tregs is seen in the tumor microenvironment [Spranger et al. 2013].
PD-1 and PD-L1 in tumor survival
An intense focus in immunotherapy has been on PD-1, a 55 kD type I transmembrane protein with two known ligands: PD-L1 (B7-H1/CD274) and PD-L2 (B7-DC/CD273) [Freeman et al. 2000]. When bound to PD-1 on T cells, both PD-L1 and PD-L2 have been shown to downregulate T-cell activation in both murine and human models [Latchman et al. 2001; Carter et al. 2002; Nishimura and Honjo, 2001]. PD-1-deficient mice develop autoimmune phenomena, including dilated cardiomyopathy [Nishimura et al. 2001] and a lupus-like syndrome with arthritis and nephritis [Nishimura et al. 1999]. Additional mouse models have showed that antibody-mediated abrogation of PD-1 led to encephalomyelitis [Salama et al. 2003] and graft-versus-host disease [Blazar et al. 2003], suggesting that its blockade has the potential to activate autoinflammatory T-cell responses. Importantly, inhibition of PD-1 activity also enhances antitumor immune responses [Iwai et al. 2002, 2004; Hirano et al. 2005].
PD-L1 can be found on neutrophils [Bowers et al. 2014], antigen-presenting cells (macrophages and dendritic cells), myeloid-derived suppressor cells [Gabrilovich and Nagaraj, 2009], and activated T cells. It is also found on a number of solid tumors, including non-small cell lung cancer (NSCLC) [Konishi et al. 2004], renal cell carcinoma [Thompson et al. 2004, 2005a, 2005b], esophageal cancer [Ohigashi et al. 2005], and oral squamous cell carcinoma [Strome et al. 2003; Tsushima et al. 2006]. In melanoma, PD-L1 is expressed both in melanoma and also on stromal cells, and its expression increases with disease progression [Taube et al. 2012].
PD-L1 binding enhances apoptosis of activated tumor-specific T cells in vitro [Dong et al. 2002], and may protect tumor cells from the induction of apoptosis by effector T cells [Blank et al. 2004]. Retrospective analyses in several human tumor types suggest that tumor expression of PD-L1 may permit immune evasion by tumors; in renal cell carcinoma, high surface expression of PD-L1 on tumor cells is related to tumor aggressiveness. Patients with high tumor or lymphocyte PD-L1 levels were 4.5 times more likely to die from their cancer than those exhibiting low levels of PD-L1 [Thompson et al. 2004]. In a multivariate analysis, high expression of PD-L1 in melanoma was independently associated with a worse OS rate compared with low expression levels [Hino et al. 2010], conferring an adaptive resistance to immune attack and release of interferon γ by tumor-infiltrating lymphocytes (TILs) [Pardoll, 2012]. Tumor overexpression of PD-L1 may explain how melanomas escape immune destruction, with preclinical data leading to the suggestion that therapies directed at this pathway may benefit patients.
Various experimental systems have been tested to design strategies to block the PD-1/PD-L1 interaction, including DNA vaccination against the extracellular region of PD-1 [He et al. 2004], genetic ablation of the PD-1 gene [Curiel et al. 2003], and recombinant antibodies directed at the extracellular region of PD-1 and PD-L1 [Hirano et al. 2005]. Of these, monoclonal antibodies have been the most studied in patients, with results of recent clinical trials of PD-1 and PD-L1 antibodies discussed below.
Anti-PD-1: clinical outcome data
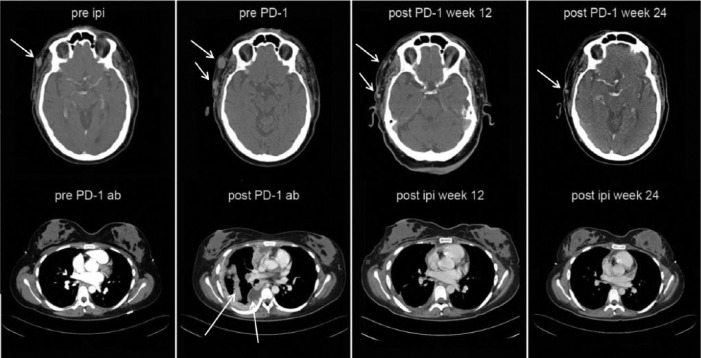
A significant amount of clinical experience has been gained with PD-1 blockade using nivolumab, formerly BMS-93658/MDX-1106. The first-in-human phase I trial of this fully human immunoglobulin (Ig)-G4 monoclonal antibody employed single dosing in patients with treatment-refractory metastatic solid tumors (melanoma, renal cell carcinoma, colorectal cancer and NSCLC), allowing detailed analysis of pharmacokinetics and pharmacodynamics over a wide dose range [Brahmer et al. 2010]. PD-1 receptor occupancy by nivolumab was prolonged well beyond the measured serum half life of 12–20 days, indicating the biological durability of this high-affinity monoclonal antibody. A follow-up trial of repeated biweekly nivolumab showed durable complete or partial tumor regressions in approximately one-third of patients with advanced melanoma and kidney cancer, with confirmed activity against NSCLC [Sznol et al. 2010]. Grade 1 or 2 drug-related adverse events included fatigue (56.3%), nausea (25%), diarrhea, xerostomia, and pruritus (18.8% each); no grade 3 or 4 adverse events were reported. In that trial, 6 of 16 evaluable patients (37.5%) had objective tumor responses. Examples of objective tumor response to PD-1 antibody therapy after failing ipilimumab, and response to ipilimumab after progression on PD-1 antibody, can be seen in Figure 1.
Figure 1.
Patient response to programmed death receptor 1 (PD-1) antibody after ipilimumab (top row), and response to ipilimumab after PD-1 antibody in another patient (bottom row).
In a phase I/II trial of nivolumab published in 2012 [Topalian et al. 2012a], a total of 296 patients were treated, with cohorts including melanoma, NSCLC, renal cell cancer, castration-resistant prostate cancer, and colorectal cancer. Among 236 patients in whom response could be evaluated, objective responses (complete or partial) were observed in patients with NSCLC (18%), melanoma (28%), and renal-cell cancer (27%), with 20 of 31 responses lasting 1 year or more. Common treatment-related adverse events included fatigue, rash, diarrhea, pruritus, decreased appetite, and nausea; grade 3 or 4 treatment-related adverse events were observed in 41 of 296 patients (14%) and included pneumonitis, colitis, hepatitis, hypophysitis, and thyroiditis, with three deaths from pulmonary toxicity. A maximum tolerated dose was not defined at doses up to 10 mg/kg. In a follow-up study of 107 patients with metastatic melanoma within that group administered nivolumab every 2 weeks for up to 96 weeks, survival, safety and antitumor responses were examined. Median OS was 16.8 months across doses and 20.3 months at the 3 mg/kg dose, with 62%, 44%, and 40% of patients alive at 1, 2, and 3 years, respectively [Sznol et al. 2013]. Among 33 patients with objective tumor regressions (31%), the Kaplan–Meier estimated median response duration was 2 years [Topalian et al. 2014].
MK-3475 is another PD-1 blocking humanized IgG4 monoclonal antibody that has demonstrated clinical efficacy in patients with advanced and metastatic melanoma and NSCLC [Garon et al. 2014]. The results of a phase I/II clinical trial of MK-3475 in 135 patients with advanced melanoma showed a confirmed RECIST 1.1 response rate of 38% across all dose cohorts [Hamid et al. 2013a], with the highest confirmed response rate observed in the cohort that received 10 mg/kg every 2 weeks [52%; 95% confidence interval (CI) 38–66]. The response rate did not differ significantly between patients who had received prior ipilimumab treatment and those who had not [overall response rate (ORR) 38%, 95% CI 23–55 and 37%, 95% CI 26–49, respectively]. Responses were durable in the majority of patients (median follow up, 11 months among patients with a response); 81% of the patients who had a response (42 of 52) were still receiving treatment at the time of analysis. The median progression-free survival (PFS) among 135 patients was greater than 7 months. Constitutional symptoms (fatigue, fever, chills, myalgias, headaches) were reported frequently but were of low grade in more than 95% of the cases. Inflammatory phenomena including diarrhea (20% of patients), hypothyroidism (8% of patients), and pneumonitis (4% of patients) were also grade 1 or 2, and responded well to glucocorticoids. Recently presented data on 365 evaluable patients treated with MK-3475 showed an ORR of 40% (95% CI 32–48%) in ipilimumab-naïve patients, and ORR of 28% (95% CI 22–35%) in patients previously treated with ipilimumab [Ribas et al. 2014]. Median PFS was 24 weeks and 23 weeks, respectively, with demonstrated activity in all major subgroups irrespective of number and type of prior therapy. Overall, 12% of patients experienced drug-related grade 3/4 adverse events and 4% discontinued due to a drug-related adverse event. There were no drug-related deaths.
A third investigational compound (CT-011), which was originally generated as a monoclonal antibody against B-lymphoblastoid cells, was found to recognize PD-1 [Berger et al. 2008]. A phase I clinical trial of CT-011 was conducted in 17 patients with different hematologic malignancies [acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma (NHL), Hodgkin’s lymphoma (HL), or multiple myeloma (MM)] at an advanced stage of disease and following chemotherapy or stem cell transplantation. The study showed the antibody to be safe and well tolerated, with no single maximum tolerated dose defined, and clinical benefit observed in 33% of patients. Mean survival time in the study was 25 ± 27 weeks, ranging from 1.7 to over 77 weeks, with one sustained remission of 68 weeks in a patient with follicular lymphoma. One minimal response was observed in a patient with AML, and four patients have shown stable disease (HL, CLL, and MM). CT-011 is currently undergoing further testing in clinical trials for patients with MM [Benson et al. 2010] and in combination with rituximab for relapsed follicular lymphoma [Westin et al. 2010].
Trials of AMP-224, a recombinant protein fusing the extracellular domain of human B7-DC/PD-L2 to IgG1, are also underway for treatment-refractory metastatic cancers. A phase I study of 26 patients with relapsed or refractory solid tumors, including 18 with melanoma, showed promising initial evidence of clinical activity and no evidence of pneumonitis or gastrointestinal toxicities [Infante et al. 2013].
Anti-PD-L1: clinical outcome data
A high-affinity, fully human, anti-PD-L1 IgG4 monoclonal antibody that blocks the binding of PD-L1 to both PD-1 and CD80 (MDX-1105/BMS-936559) has been tested in patients with advanced solid tumors, with preliminary evidence of clinical activity against melanoma, kidney cancer, and NSCLC [Topalian et al. 2012b]. In a study of BMS-936559 published in 2012, 207 patients received treatment (75 with NSCLC, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer). Efficacy was analyzed in 160 patients in whom a response could be evaluated, with objective responses (confirmed complete or partial responses) observed in patients with melanoma, NSCLC, renal-cell cancer, and ovarian cancer [Brahmer et al. 2012].
There were nine objective responses among 52 patients with melanoma receiving 1 mg/kg, 3 mg/kg, and 10 mg/kg doses (response rates of 6%, 29%, and 19% respectively) with three patients achieving complete responses and 27% with stable disease lasting at least 24 weeks. Adverse events of any grade were reported in 188 of 207 patients (91%), with the most common drug-related adverse events being fatigue, infusion reactions, diarrhea, arthralgia, rash, nausea, pruritus, and headache. Most events were low grade, with treatment-related grade 3 or 4 events noted in 19 of 207 (9%). Drug-related immune-related adverse events were observed in 81 of 207 patients (39%) and included rash, hypothyroidism, hepatitis, and one case each of sarcoidosis, endophthalmitis, diabetes mellitus, and myasthenia gravis; these events were managed with glucocorticoids, treatment interruption, or trial discontinuation. Of the nine patients treated with high-dose glucocorticoids, four maintained disease control.
A chimeric IgG1 anti-PD-L1 antibody, MPDL3280A, has shown excellent clinical activity in patients with metastatic melanoma. In a phase I trial, 45 patients with metastatic melanoma (64% of whom had received prior systemic therapy) were treated with doses of 1–20 mg/kg; of patients evaluable for efficacy, there was an ORR of 26% (9/35) and a 24-week PFS of 35% [Hamid et al. 2013b]. Several additional patients had delayed antitumor activity after apparent radiographic progression. Grade 3/4 adverse events included hyperglycemia (7%), elevated alanine aminotransferase (ALT) (7%), and elevated aspartate aminotransferase (AST) (4%), with no high-grade pneumonitis reported. In patients with NSCLC, MPDL3280A showed an ORR of 24% (9/37) with a 24-week PFS of 48% [Spigel et al. 2013]. Based on the results of these phase I studies, a phase II study of MPDL3280A in patients with NSCLC is underway [ClinicalTrials.gov identifier: NCT01846416]. MPDL3280A is also being explored in combination with vemurafenib in advanced melanoma [ClinicalTrials.gov identifier: NCT01656642], combined with bevacizumab in renal cell carcinoma [ClinicalTrials.gov identifier: NCT01984242] and with bevacizumab, with or without chemotherapy, in advanced solid tumors [ClinicalTrials.gov identifier: NCT01633970].
MEDI4736 is another PD-L1 inhibitor that has shown promising early activity in NSCLC; interim results of a phase I trial reported no colitis or pneumonitis of any grade, with several durable remissions [Khleif et al. 2013]. MEDI4736 is also currently under investigation in combination with dabrafenib (a BRAF inhibitor) plus trametinib (a MEK inhibitor) or with trametinib alone in subjects with metastatic or unresectable melanoma [ClinicalTrials.gov identifier: NCT02027961].
PD-L1 expression as a predictor of tumor response
An association between PD-L1 expression and tumor response has yet to be fully validated, but is being closely examined based on preclinical and clinical data. A recent immunohistochemical analysis of the tumor microenvironment in 41 patients treated with anti PD-1 antibody showed that PD-L1 expression was geographically associated with infiltrating immune cells (p < 0.001), and that PD-L1 and PD-L2 expression were closely associated with response to anti-PD-1 therapy [Taube et al. 2014]. Interestingly, PD-L1+ cell lines isolated from 83 patients with melanoma treated at a single institution demonstrated enhanced invasion and growth in immunocompromised mouse models [Brusa et al. 2014], suggesting that PD-L1 expression may be associated with a more aggressive phenotype.
In the study of MPDL3280A in NSCLC, a correlation between PD-L1 status and clinical efficacy was demonstrated, in that patients who were PD-L1+ showed an ORR of 100% (4/4) and a progressive disease (PD) rate of 0%, while patients who were PD-L1– showed an ORR of 15% (4/26) and a PD rate of 58%. In a phase Ib study of MK3475 in 71 patients with advanced melanoma, those with PD-L1 expressing tumors (n = 53) had an ORR of 53% (95% CI 38–61%) compared with 13% in nonexpressing tumors (95% CI 4–31%), and also had an improved PFS (10.6 months compared with 2.9 months) [Daud et al. 2014]. In a subset analysis of patients with melanoma treated with nivolumab, higher ORR, longer PFS, and longer OS were seen in patients who were PD-L1+ [Grosso et al. 2013], with subset analysis in NSCLC still ongoing. While these data have yet to be validated in larger patient cohorts, correlation remains under study, including testing subjects prospectively for both baseline and post-treatment tumor PD-L1 expression.
Combination therapy utilizing PD-1 blockade
Combination treatment strategies utilizing PD-1 blockade are also currently under investigation. A trial of patients receiving either concurrent or sequenced nivolumab and ipilimumab showed an ORR of 40% in the concurrent group, with evidence of clinical activity observed in 65% of patients [Wolchok et al. 2013]. Ten percent of patients in the concurrent therapy cohort had complete responses, and 31% had significant reduction of tumor burden according to World Health Organization response criteria. Responses were ongoing in 19 of 21 patients, with the duration ranging from 6.1 to 72.1 weeks. At the maximum doses that were associated with an acceptable level of adverse events (nivolumab 1 mg/kg with ipilimumab 3 mg/kg), grade 3 or 4 therapy-related adverse events occurred in 53% of patients in the concurrent-regimen group, but were qualitatively similar to those previously observed with anti-PD-1 monotherapy and were generally reversible. Recently presented data on 53 patients with melanoma and up to three prior therapies, treated with concurrent nivolumab and ipilimumab (every 3 weeks for four doses) followed by nivolumab every 2 weeks for an additional 96 weeks showed 1-year and 2-year OS rates of 82% and 75% respectively. By week 36, 42% demonstrated at least 80% tumor reduction, with median duration of response not reached. Grade 3/4 adverse events were seen in 62% of patients, and most commonly included elevated lipase and transaminases (15% and 14% respectively). Twenty-three percent of patients discontinued therapy due to treatment-related adverse events. These preliminary data suggest combined therapy demonstrates encouraging survival outcomes and a considerable, but manageable, safety profile [Sznol et al. 2014].
Nivolumab has also been tested with or without a peptide vaccine in patients with treatment-refractory melanoma [Weber et al. 2013]; 90 patients with either ipilimumab-naïve or ipilimumab-refractory stage III or IV melanoma received nivolumab at 1, 3, or 10 mg/kg every 2 weeks for 24 weeks, then every 12 weeks for up to 2 years, with or without a multipeptide vaccine. Treatment was well tolerated, and in a cohort of 20 patients who had prior grade 3/4 adverse events with ipilimumab, the dose-limiting toxicity was not reproduced. The ORR for patients who were ipilimumab refractory and naive was 25%, with induced responses lasting up to 140 weeks.
Additional combination and sequential therapies with PD-1 blockade are currently being tested, including IL-2 and nivolumab for metastatic clear cell renal carcinoma [Brayer and Fishman, 2014], nivolumab combined with ipilimumab versus bevacizumab in recurrent glioblastoma multiforme [ClinicalTrials.gov identifier: NCT02017717], and a phase III trial of nivolumab plus ipilimumab versus ipilimumab alone in previously untreated advanced melanoma [ClinicalTrials.gov identifier: NCT01844505]. Nivolumab has also recently shown clinical activity in NSCLC [Gettinger et al. 2014], metastatic renal cell carcinoma [Amin et al. 2014], and platinum-resistant ovarian carcinoma [Hamanishi et al. 2014].
The future of immunotherapy
Immunotherapy has generated great excitement in oncology, with current agents demonstrating clinical responses of long duration, antitumor activity long after drug discontinuation, and favorable toxicity profiles. As discussed here, clinical trials have demonstrated that PD-1 and PD-L-1 blockade results in impressive PFS and OS in metastatic melanoma, making it an important treatment option for this lethal cancer. Indeed, the concept of melanoma being an ‘incurable’ disease is increasingly challenged by long-term survival outcomes seen with immunomodulatory agents, in particular the plateau observed of 2- and 3-year survival seen in patients with metastatic melanoma treated with anti-PD1 therapy [Hodi et al. 2014]. Such advances are due to important discoveries made in the fields of immunology and molecular biology, and future therapies will depend upon successful clinical trial translation of ongoing developments. Recently, a combination of anti-CTLA4, anti-PD-L1, and IDO inhibition in vivo showed a synergistic diminution of tumor growth and proliferation of tumor-infiltrating CD8+ T cells [Spranger et al. 2014], knowledge that may contribute to a growing therapeutic armamentarium. Development of other combinations, including TILs and targeting of Tregs, also has potential for future treatments. Much interest has been shown in this rapidly moving field of immunotherapeutic drug development, with the potential for lasting and profound clinical responses in a variety of cancers. As it stands, immune checkpoint inhibition is an important therapeutic advance in oncology, with a very promising future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Weber has been a paid consultant for Bristol-Myers Squibb, Merck and Genentech. Dr Freeman-Keller has no conflicts of interest to disclose.
Contributor Information
Morganna Freeman-Keller, Graduate Medical Education, Moffitt Cancer Center, Tampa, FL, USA.
Jeffrey S. Weber, Cutaneous Oncology, Moffitt Cancer Center, 12902 Magnolia Drive, Mailstop SRB 2, Tampa, FL 33162, USA
References
- Amin A., Plimack E., Infante J., Ernstoff M., Rini B., McDermott D., et al. (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC).J Clin Oncol 32(5 Suppl.): abstract 5010. [Google Scholar]
- Benson D., Bakan C., Mishra A., Hofmeister C., Efebera Y., Becknell B., et al. (2010) The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R., Rotem-Yehudar R., Slama G., Landes S., Kneller A., Leiba M., et al. (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- Blank C., Brown I., Peterson A., Spiotto M., Iwai Y., Honjo T., et al. (2004) PD-L1/B7-H1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64: 1140–1145. [DOI] [PubMed] [Google Scholar]
- Blazar B., Carreno B., Panoskaltsis-Mortari A., Carter L., Iwai Y., Yagita H., et al. (2003) Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma dependent mechanism. J Immunol 171: 1272–1277. [DOI] [PubMed] [Google Scholar]
- Bowers N., Helton E., Huijbregts R., Goepfert P., Heath S., Zdenek H. (2014) Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathogens 10: e1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Drake C., Wollner I., Powderly J., Picus J., Sharfman W., et al. (2010) Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayer J., Fishman M. (2014) Regression of metastatic clear cell kidney cancer with interleukin-2 treatment following nivolumab. J Immunother 37: 187–191. [DOI] [PubMed] [Google Scholar]
- Brusa D., Massi D., Merelli B., Ciano M., Audrito V., Serra S., et al. (2014) PD-L1 expression identifies a subpopulation of melanoma cells characterized by enhanced invasiveness and aggressiveness. Poster presented at American Association for Cancer Research 2014 Annual Meeting, 9 April, San Diego, CA, USA: abstract: CT5604. [Google Scholar]
- Carter L., Fouser L., Jussif J., Fitz L., Deng B., Wood C., et al. (2002) PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol 32: 634–643. [DOI] [PubMed] [Google Scholar]
- Curiel T., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., et al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949. [DOI] [PubMed] [Google Scholar]
- Curiel T., Wei S., Dong H., Alvarez X., Cheng P., Mottram P., et al. (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9: 562–567. [DOI] [PubMed] [Google Scholar]
- Daud A., Hamid O., Ribas A., Hodi F., Hwu W., Kefford R., et al. (2014) Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma: correlation of tumor PD-L1 expression with outcome. Poster presented at American Association for Cancer Research 2014 Annual Meeting, 9 April, San Diego, CA, USA: abstract: CT104. [Google Scholar]
- Dong H., Strome S., Salomao D., Tamura H., Hirano F., Flies D., et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. [DOI] [PubMed] [Google Scholar]
- Flaherty K., Puzanov I., Kim K., Ribas A., McArthur G., Sosman J., et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G., Long A., Iwai Y., Bourque K., Chernova T., Nishimura H., et al. (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman W., Pages F., Sautes-Fridman C., Galon J. (2012)The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Spatz A., et al. (2010) Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer 46: 270–283. [DOI] [PubMed] [Google Scholar]
- Garon E., Balmanoukian A., Hamid O., Hui R., Gandhi L., Leighl N., et al. (2014) MK-3475 monotherapy for previously treated non-small cell lung cancer (NSCLC): Preliminary safety and clinical activity. Clin Cancer Res 20: abstract A20. [Google Scholar]
- Gettinger S., Shepherd F., Antonia S., Brahmer J., Man Chow L., Juergens R., et al. (2014) First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 32 (5 Suppl.): abstract 8024. [Google Scholar]
- Grosso J., Horak C., Inzunza D., Cardona D., Simon J., Gupta A., et al. (2013) Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 31: abstract CRA3016. [Google Scholar]
- Hamanishi J., Mandai M., Ikeda T., Minami M., Kawaguchi A., Matsumura N. (2014) Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. J Clin Oncol 32(5 Suppl.): abstract 5511. [DOI] [PubMed] [Google Scholar]
- Hamid O., Robert C., Daud A., Hodi F., Hwu W., Kefford R., et al. (2013a) Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O., Sosman J., Lawrence D., Sullivan R., Ibrahim N., Kluger H., et al. (2013b) Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma. J Clin Oncol 31: abstract CRA9010. [Google Scholar]
- He Y., Zhang G., Wang X., Zhang H., Yuan Y., Li D., et al. (2004) Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol 173: 4919–4928. [DOI] [PubMed] [Google Scholar]
- Hino R., Kabashima K., Kato Y., Yagi H., Nakamura M., Honjo T., et al. (2010) Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116: 1757–1766. [DOI] [PubMed] [Google Scholar]
- Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., et al. (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65: 1089–1096. [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F., Sznol M., Kluger H., McDermott D., Carvajal R., Lawrence D., et al. (2014) Long-term survival of ipilimumab-naive patients) with advanced melanoma treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. J Clin Oncol 32(Suppl. 5): abstract CRA9002. [Google Scholar]
- Infante J., Powderly J., Burris H., Kittaneh M., Grice J., Smothers J., et al. (2013) Clinical and pharmacodynamic (PD) results of a phase 1 trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J Clin Oncol 31: abstract CRA3044. [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-1 blockade. Proc Natl Acad Sci U S A 99: 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Terawaki S., Honjo T. (2004) PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol 17: 133–144. [DOI] [PubMed] [Google Scholar]
- Jandus C., Bioley G., Speiser D., Romero P. (2008) Selective accumulation of differentiated FOXP3(+) CD4 (+) T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother 57: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khleif S., Lutzky J., Segal N., et al. (2013) MEDI4736, an anti-PD-L1 antibody with modified Fc domain: preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors. Proceedings from the European Cancer Congress, 30 September, Amsterdam: abstract 802. [Google Scholar]
- Konishi J., Yamazaki K., Azuma M., Kinoshita I., Dosaka-Akita H., Nishimura M. (2004) B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10: 5094–5100. [DOI] [PubMed] [Google Scholar]
- Latchman Y., Wood C., Chernova T., Chaudhary D., Borde M., Chernova I., et al. (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2: 261–268. [DOI] [PubMed] [Google Scholar]
- Lebbé C., Weber J., Maio M., Neyns B., Harmankaya K., Hamid O., et al. (2013) Long-term survival in patients with metastatic melanoma who received ipilimumab in four phase II trials. J Clin Oncol 31: abstract CRA9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A., Sivakumar J., Chandler P., Smith K., Molina H., Mao D., et al. (2001) Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2: 64–68. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (2013) SEER 9 incidence & U.S. mortality 1975–2010, all races, both sexes. Available at: http://seer.cancer.gov/statfacts/html/melan.html (accessed 26 March 2014).
- Nishimura H., Honjo T. (2001) PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 22: 265–268. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Masato N., Hiroshi H., Nagahiro M., Tasuku H. (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141–151. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Okazaki T., Tanaka Y., Nakatani K., Hara M., Matsumori A., et al. (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291: 319–322. [DOI] [PubMed] [Google Scholar]
- Ohigashi Y., Sho M., Yamada Y., Tsurui Y., Hamada K., Ikeda N., et al. (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11: 2947–2953. [DOI] [PubMed] [Google Scholar]
- Pardoll D. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Hodi F., Kefford R., Hamid O., Daud A., Wolchok J., et al. (2014) Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients with melanoma. J Clin Oncol 32(5 Suppl.): abstract LBA9000. [Google Scholar]
- Salama A., Chitnis T., Imitola J., Ansari M., Akiba H., Tushima F., et al. (2003) Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 198: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A., Flaherty K. (2013) BRAF in melanoma: current strategies and future directions. Clinical Cancer Research 19: 4326–4334. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Hodi F., Robert C., Weber J., Margolin K., Hamid O., et al. (2013) Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. Eur J Cancer 49(Suppl. 2): abstract 24LBA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel D., Gettinger S., Horn L., Herbst R., Gandhi L., Gordon M., et al. (2013) Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 31: CRA8008 [Google Scholar]
- Spranger S., Koblish H., Horton B., Scherle P., Newton R., Gajewski T. (2014) Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J Immunother Cancer 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S., Spaapen R., Zha Y., Williams J., Meng Y., Ha T., et al. (2013) Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med 5: 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S., Dong H., Tamura H., Voss S., Flies D., Tamada K., et al. (2003) B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 63: 6501–6505. [PubMed] [Google Scholar]
- Sznol M., Kluger H., Callahan M., Postow M., Gordon R., Segal N., et al. (2014) Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma. J Clin Oncol 32(5 Suppl.): LBA9003. [Google Scholar]
- Sznol M., Kluger H., Hodi F., McDermott D., Carvajal R., Lawrence D., et al. (2013) Survival and long-term follow-up of safety and response in patients with advanced melanoma in a phase I trial of nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 31: CRA9006. [Google Scholar]
- Sznol M., Powderly J., Smith D., Brahmer J., Drake C., McDermott D., et al. (2010) Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies. J Clin Oncol 28: CRA2506. [Google Scholar]
- Taube J., Anders R., Young G., Xu H., Sharma R., McMiller T., et al. (2012) Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4: 30036–30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J., Klein A., Brahmer J., Xu H., Pan X., Kim J., et al. (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 8 April 8 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Gillett M., Cheville J., Lohse C., Dong H., Webster W., et al. (2004) Costimulatory B7-H1 in renal cell carcinoma subjects: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 101: 17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Gillett M., Cheville J., Lohse C., Dong H., Webster W., et al. (2005a) Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 104: 2084–2091. [DOI] [PubMed] [Google Scholar]
- Thompson R., Webster W., Cheville J., Lohse C., Dong H., Leibovich B., et al. (2005b) B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in subjects with renal cell carcinoma. Urology 66: 10–14. [DOI] [PubMed] [Google Scholar]
- Topalian S., Drake C., Pardoll D. (2012a) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012b) Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Sznol M., McDermott D., Kluger H., Carvajal R., Sharfman W., et al. (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Cin Oncol 3 March 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima F., Tanaka K., Otsuki N., Youngnak P., Iwai H., Omura K., et al. (2006) Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol 42: 268–274. [DOI] [PubMed] [Google Scholar]
- Weber J., Kudchadkar R., Yu B., Gallenstein D., Horak C., Inzunza H., et al. (2013) Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 34: 4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin J., Chu F., Foglietta M., Rotem-Yehudar R., Neelapu S. (2010) Phase II safety and efficacy study of CT-011, a humanized anti-PD-1 monoclonal antibody, in combination with rituximab in patients with relapsed follicular lymphoma. J Clin Oncol 28: TPS305. [Google Scholar]
- Wolchok J., Kluger H., Callahan M., Postow M., Rizvi N., Lesokhin A., et al. (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2013) Skin cancers. Available at: http://www.who.int/uv/faq/skincancer/en/index1.html (accessed 1 September, 2014).
- Zheng Y., Zha Y., Driessens G., Locke F., Gajewski T. (2012) Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med 209: 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]