Abstract
Advanced thyroid carcinoma is an infrequent tumor entity with limited treatment possibilities until recently. The extraordinary improvement in the comprehension of genetic and molecular alterations involving the RAS/RAF/mitogen-activated protein kinase and phosphatidylinositide 3-kinase/Akt/mammalian target of rapamycin signaling and interacting pathways that are involved in tumor survival, proliferation, differentiation, motility and angiogenesis have been the rationale for the development of new effective targeted therapies. Data coming from phase II clinical trials have confirmed the efficacy of those targeted agents against receptors in cell membrane and cytoplasmic molecules. Moreover, four of those investigational drugs, vandetanib, cabozantinib, sorafenib and lenvatinib, have reached a phase III clinical trial with favorable results in progression-free survival and overall survival in medullary thyroid carcinoma and differentiated thyroid carcinoma. Further analysis for an optimal approach has been conducted according to mutational profile and tumor subtypes. However, consistent results are still awaited and the research for adequate prognostic and predictive biomarkers is ongoing. The following report offers a comprehensive review from the rationale to the basis of targeted agents in the treatment of thyroid carcinoma. In addition, current and future therapeutic developments by the inhibition of further molecular targets are discussed in this setting.
Keywords: angiogenesis, phosphatidylinositide 3-kinase/Akt/mammalian target of rapamycin, RAS/RAF/mitogen-activated protein kinase, targeted agents, thyroid carcinoma
Introduction
Thyroid carcinoma (TC) is a rare tumor entity representing 1% of all oncological diagnoses [Tuttle et al. 2013]. The most frequent subtype is the differentiated TC (DTC) derived from epithelial cells. This first group includes papillary (PTC, 80%), follicular (FTC, 11%) and other less frequent histologic subtypes, such as Hürthle cells, insular, poorly differentiated TC (PDTC) and follicular variant of PTC or tall cell carcinoma. The second group is represented by medullary TC (MTC) derived from the calcitonin-producing parafollicular cells (C cells) of the thyroid gland and accounts for 5–10% of all TCs [Pusztaszeri et al. 2014]. Finally, the anaplastic TC (ATC) is a highly aggressive tumor present in only 2% of patients, followed by other subtypes even less frequently, such as lymphomas or sarcomas from the thyroid gland.
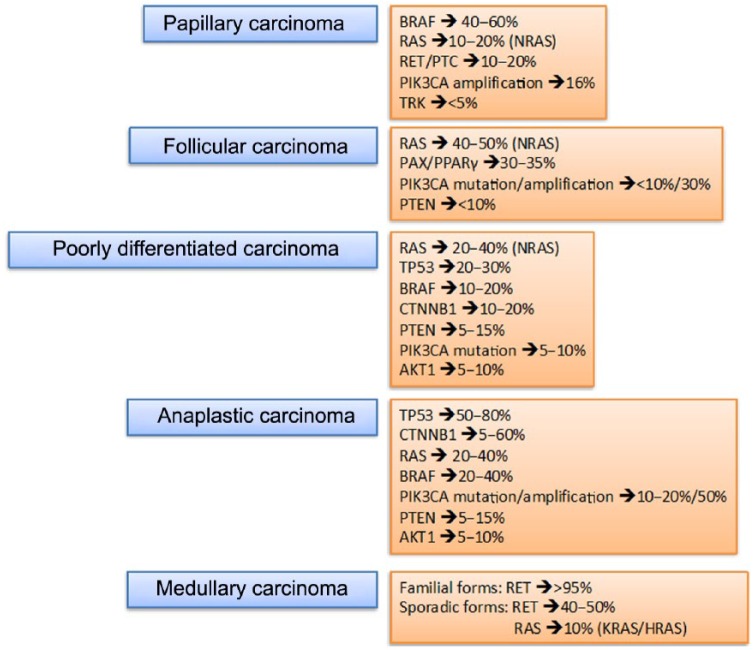
For the last 5–10 years, major serious efforts have been made to improve investigation into the molecular pathways and critical alterations involved in the tumorigenesis of TC and, in the latter, to increase the therapeutic possibilities for patients with TC based on targeted therapies [Xing et al. 2013] (Figure 1).
Figure 1.
Thyroid tumors and mutational profile. PTEN, phosphatase and tensin homolog.
BRAF, b-type rapidly accelerated fibrosarcoma;RAS, rat sarcoma; RET/PTC, rearranged during transfection/papillary thyroid carcinoma; PI3KCA, phosphatidylinositol 3-kinase oncogene; TRK, receptor thyrosine kinase; PAX8/PPAR, paired box 8/peroxisome proliferator-activated receptor gamma; PTEN, phosphatase and tensin homolog; TP53, tumor protein p53; CTNNB1, catenin (cadherin-associated protein beta 1); AKT1, v-akt murine thymoma viral oncogene homolog 1;RET, rearranged during transfection.
What have we learned recently about molecular processes of DTC and MTC?
Tumorigenesis of DTC
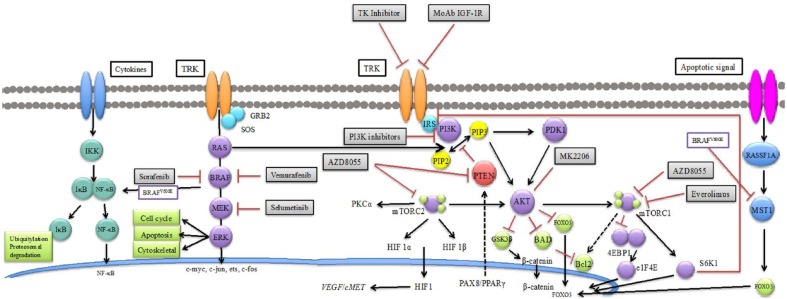
The main oncogenic pathways involved in initiation and progression of thyroid carcinogenesis are the RAS/RAF/mitogen-activated protein kinase (MAPK) and phosphatidylinositide 3-kinase (PI3K)/Akt pathways because of their relevance in survival, proliferation, differentiation and motility [Nikiforov and Nikiforova, 2011] (Figure 2). Progressive tumor dedifferentiation involves the sum of activated kinases or inactivated tumor suppressor genes. This tumor is, at last, less dependent on thyrotropin stimulation [Guerra et al. 2014]. Disorders such as RAS and BRAF point mutations or RET/PTC and AKP9/BRAF rearrangements have been identified in 70% of PTCs, in which overlapping mutations have been rarely described. In FTC, the most common alterations includes RAS mutations and PAX8/peroxisome proliferator-activated receptor (PPARγ) rearrangements [Nikiforova and Nikiforov, 2008].
Figure 2.
Main oncogenic pathways and targeted therapies in thyroid carcinoma. HIF1, hypoxia-inducible factor 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; PI3K, phosphatidylinositide 3-kinase; PKC, protein kinase C; PPAR, peroxisome proliferator-activated receptor; PTEN, phosphatase and tensin homolog; VEGF, vascular endothelial growth factor; IKK, IB kinase; IB, inhibitor of kappa; B NF-KB, nuclear factor KB; TRK, Receptor thyrosine kinase; Grb2, Growth factor receptor-bound protein 2; SOS, son of sevenless; RAS, Rat sarcoma; BRAF, B-type rapidly accelerated fibrosarcoma; ERK, extracellular signal-regulated kinases; IGF-1R, insulin-like growth factor 1 receptor; IRS, Insulin receptor substrate; PI3K, phosphatidylinositide 3-kinasen; PKC, protein kinase C; mTOR, mammalian target of rapamycin;
HIF1, hypoxia-inducible factor; PTEN, phosphatase and tensin homolog; VEGF, vascular endothelial growth factor; MET, mesenchymal epithelial transition; PPAR, peroxisome proliferator-activated receptorn; PDK1, Phosphoinositide dependent kinase-1; FOXO3, forkhead box O3; BAD, Bcl-xL/Bcl-2-Associated Death Promoter Bcl2, B-cell lymphoma 2; GSK3, glycogen synthase kinase 3 beta 4EBP1: 4E-binding protein 1 EIF4E, eukaryotic translation initiation factor 4E; S6K1, S6 kinase 1; RASSF1A, Ras association domain family 1 isoform A; MST1, macrophage stimulating protein.
The RAS/RAF/MEK/MAPK/ERK pathway
RAS is activated through different membrane receptors and it recruits RAF for a subsequent phosphorylation of MAPK-ERK kinases. Once ERK is translocated to the nucleus, transcription factors, such as c-myc, c-jun, ets or c-fos are activated. Indeed, Erk may activate cytosolic apoptotic proteins, such as Bad, MCL-1, caspase 9 and cytoskeletal proteins, such as paxillin, calnexin and vinexin [Caronia et al. 2011]. Oncogenic modifications in RAS have been hypothesized to be one of the first steps in TC development because of the presence in well differentiated TCs (WDTCs) and PDTC or ATC and MTC. An initial analysis of hotspot mutations at codons 12, 13 and 61 of the three forms of RAS (HRAS, KRAS, NRAS) in 125 TC samples from 107 patients at different stages of disease demonstrated an overall incidence of a RAS mutation of 32.7% [Garcia-Rostan et al. 2003]. The most frequent mutation was KRAS (24.3%), followed by NRAS (8.4%) and HRAS (4.7%). PDTC and ATC were the histologic subtypes with the greater incidence of RAS mutations (55.2% and 51.7%, respectively). A significant association between the presence of an activating RAS mutation and poor survival was identified in patients with DTC (p < 0.001). A recent investigation in 58 resected FTC tumor samples also showed a significant association between the NRAS codon 61 mutation and the presence of distant metastasis (p = 0.020) and between the presence of any RAS mutation and worse prognosis (p = 0.042) [Fukahori et al. 2012]. In a different report with 65 PDTC tumor samples, the most common molecular alteration was RAS mutation identified in 25% of carcinomas. The most frequent RAS mutation was the point mutation at codon 61 of NRAS [Volante et al. 2009]. Once again, a strong relationship between the presence of a RAS mutation and poorer survival was detected (p = 0.004). However, a definitive conclusion about the prognostic value requires larger studies.
In MTC, a wide range of somatic RAS mutations have been reported from different investigations (7.9–68%), particularly in patients without a RET mutation [Agrawal et al. 2013; Moura et al. 2011]. Results from a meta-analysis including trials with complete screening showed an overall incidence of RAS mutations of 8.8% (HRAS 8.1%, KRAS 6.5% and NRAS 0.5%) [Ciampi et al. 2013].
From this particular pathway, BRAF mutation status has been the most common and established prognostic biomarker, particularly in PTC or in dedifferentiated tumors, probably developed from the first one [Xing et al. 2013]. Valine to glutamate amino acid substitution at residue 600 (V600E) is the most frequent point mutation in the BRAF gene (98–99%). Other alterations less frequently described have been the lysine to glutamine amino acid substitution (L601E), deletions or insertions around codon 600 or AKAP9/BRAF rearrangements. Mutations in BRAF have been associated with tumor recurrence and loss of response to radioiodine treatment, in part influenced by the secondary overexpression of vascular endothelial growth factor receptor (VEGFR) and MET [Elisei et al. 2008]. To overcome initial controversial results about its prognostic value, a meta-analysis conducted by Li and colleagues included 32 studies (only two were prospective) with 6372 patients with PTC (3244 patients with BRAF mutation) [Li et al. 2012]. A significant association between the presence of a BRAFV600E mutation and tumor size [odds ratio (OR) 1.57; 95% confidence interval (CI) 1.29–1.92], lymph node metastasis (OR 1.72; 95% CI 1.53–1.94), extrathyroid extension (OR 2.60; 95% CI 2.27–2.99), multifocality (OR 1.30; 95% CI 1.13–1.49), vascular invasion (OR 1.23; 95% CI 0.76–2.01), absence or infiltration of the tumoral capsule (OR 2.07; 95% CI 1.64–2.61) and advanced clinical stage (OR 1.82; 95% CI 1.58–2.10) were identified. Those results were also consistent with the results obtained from initial PTC stages (pT1/T2-N0) [Elisei et al. 2012]. Indeed, as a prognostic factor, only a BRAFV600E mutation was found to be significantly associated with disease-free survival.
In addition to upstream molecular alterations, secondary dysregulations in MAPK activation, such as hypometilation or genome-wide hypermetilation of many tumor suppressor genes (DAPK1, RARB, TIMP3, SLC5A8), and upregulation of oncogenic proteins may enhance the carcinogenic process [Hu et al. 2006; Xing, 2007]. Those alterations have an important role in cell metabolism and cell functions.
The PI3K/AKT/mammalian target of rapamycin pathway
The PI3K/AKT pathway is related to cell growth, proliferation, survival, motility and regulation of iodide uptake. In an oncological context, it also enhances angiogenesis, metastasis development and resistance to chemotherapy [de Souza et al. 2011]. This pathway is related to a progressive dedifferentiation (losing thyroid-stimulating hormone [TSH] signaling and increasing PI3K signaling) and acquisition of new oncogenic alterations. In vivo investigations have shown that persistent stimulation of TSH leads to an overactivation of mammalian target of rapamycin complex1/S6 kinase 1/S6 [Brewer et al. 2007]. A comprehensive analysis of a large panel of genes from FTC (n = 64) and ATC (n = 51) samples was carried out to establish the rationale for the development of targeted therapies in TC [Liu et al. 2008]. Frequent overexpression of VEGFR1, platelet-derived growth factor receptor 1 (PDGFR1), PDGFRβ or epidermal growth factor receptor (EGFR) was observed. The most frequent mutated genes were RAS (20.3% in FTC), PIK3Ca (12% in ATC) and phosphatase and tensin homolog (PTEN) (16.7% in ATC) and RET/PTC rearrangements were identified in 15% of ATC samples. A high percentage, 81% of ATC, had a genetic alteration involved in both the MAPK and PI3K/Akt pathways. In fact, genetic alterations in the second pathway are seen, predominantly in progressive dedifferentiated tumors, such as ATC and PDTC.
The PI3K/Akt deregulation may come from different genetic alterations as described below, such as the presence of thyroid hormone β receptor (TRβPV) mutant that binds with a greater affinity than the wild type TRβ to the p85 regulatory subunit of PI3K and may lead to Akt activation. Other deregulations involve RET/PTC rearrangements, RET mutations, overexpression of RTK (EGFR, VEGFR, FGFR, insulin-like growth factor 1 receptor [IGF-1R], KIT, MET), PIK3CA amplification or mutations (mainly in the catalytic domain region) [Ricarte-Filho et al. 2009], Akt activation in nuclear and cytoplasmic membrane, increased levels of pAkt or Akt mutations (AKT1E17K) [Ricarte-Filho et al. 2009] and loss of phosphatase and tensin homolog (PTEN) by mutations, gene methylation or reduced expression levels. In addition, phosphoinositide-dependent kinase-1 (PDK-1) gene amplification has been identified because it is recruited by activated PI3K and phosphorylates Akt at the cell membrane. However, its role in tumorigenesis of TC has not been clearly established [Liu et al. 2008]. Finally, RAS can also interact and activate PI3K downstream cascade. Initial results in cell lines have identified partial resistance to MEK inhibitors in cells with RAS mutations compared with cell lines harboring BRAFV600E mutation [Leboeuf et al. 2008].
RET point mutations and RET rearrangements
In thyroid tumors, RET can be activated by fusion to other genes in tumors derived from follicular cells or by point mutations in tumors arising from parafollicular cells.
RET/PTC rearrangements are suggested to be an initial step in TC related to childhood PTC and to radiation exposure [Hamatani et al. 2008]. There are more rearrangements identified, but RET/PTC1 (partner gene is a coiled-coil domain containing gene 6, CCDC6) and RET/PTC3 (partner gene is a nuclear receptor coactivator gene 4, NcoA4), which are intrachromosomal paracentric inversions, are the most common [Nikiforov, 2002]. RET/PTC is ligand independent, dimerized by autophosphorylation of thyrosine residues and binding to other adaptor proteins (GRB2, SOS, Shc, FRS2) for RAS/MAPK and PI3K downstream activation and interaction with different cytoplasmic substrates [Antonelli et al. 2012]. In most reported series, RET/PTC1 was the most frequent (60–70%), followed by RET/PTC3 (20–30%) and RET/PTC2 (<10%) [Nikiforov, 2002]. The presence of nonclonal RET/PTC has also been reported in benign lesions. The association to aggressiveness and tumor recurrence has not been well established.
Despite what was previously considered, dual mutation in BRAFV600E and RET/PTC can coexist in patients with well differentiated PTC. In an analysis of 72 tumor samples, 19.3% presented both alterations [Guerra et al. 2014]. Moreover, rearrangements of a different RTK, NTRK1 gene, have been reported from less than 2–15% of patients with PTC [Nikiforov and Nikiforova, 2011].
Germline point mutations have been identified in almost all patients with hereditary MTC and somatic point mutations in 40–50% of patients with sporadic MTC. The most frequent mutation in sporadic MTC is the substitution of a methionine to a threonine amino acid in the codon 918 that corresponds to the tyrosine kinase 2 domain. This mutation is present in 85% of patients [Frank-Raue et al. 2010]. In hereditary MTC, 95% of patients with MEN 2B present a germline mutation in codon 918, whereas 5% present in codon 883 (A883F). In contrast, 85% of patients with MEN 2A harbor a germline mutation in codon 634 (mostly C634R) corresponding to the extracellular cysteine-rich domain. In addition, patients with familiar MTC present more varied mutations involving codons belonging to the extracellular and intracellular domains [de Groot et al. 2006].
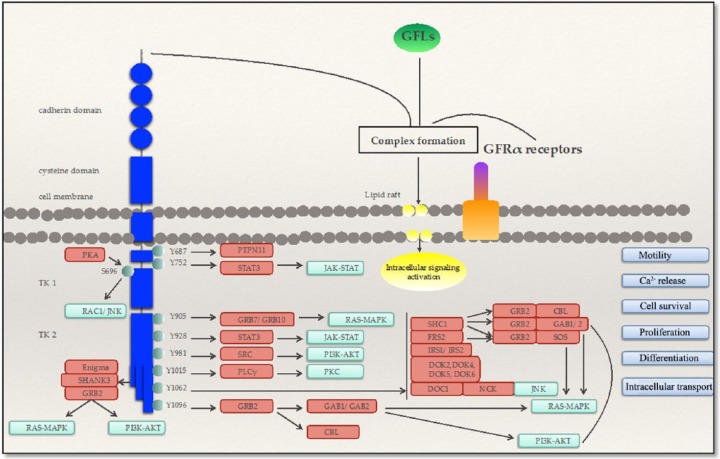
The oncogenic activation of RET depends on the location of the amino acid change leading to a ligand-independent activation through aberrant intermolecular disulfide bond formation, constitutive dimerization of the oncoprotein, activation of the tyrosine kinase domain or modifications of the substrate specificity and residue phosphorylation. Therefore, the binding of adaptor and effector proteins to docking sites enhances the activation of several pathways involved in embryogenesis, cell survival, proliferation, differentiation, motility, calcium release and intracellular transport. Consequently, it can induce oncogenic transformation through activation of the RAS/MAPK, PI3K/AKT and JAK/STAT pathways, protein kinase C (PKC) and direct signaling through SRC kinases, protein kinase A (PKA), focal adhesion kinase (FAK) and β-catenin domains [Mulligan, 2014] (Figure 3).
Figure 3.
RET signaling in thyroid cells. MAPK, mitogen-activated protein kinase; JNK, Janus kinase; PI3K, phosphatidylinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; PKA: protein kinase A; RAC1/JNK: Ras-related C3 botulinum toxin substrate 1/Janus kinase; SHANK3: SH3 and multiple ankyrin repeat domains 3; Grb2: Growth factor receptor-bound protein 2; MAPK: Mitogen-activated protein kinases; PI3K: phosphatidylinositide 3-kinaseAKT/PKB: protein kinase B; PTPN11: Tyrosine-protein phosphatase non-receptor type 11; STAT3: Signal transducer and activator of transcription 3; PLC: Phospholipase C; PKC: protein kinase C; GAB1/GAB2; CBL: Casitas B-lineage Lymphoma; SHC1: Src homology 2 domain containing transforming protein 1; FRS2: fibroblast growth factor receptor substrate 2; IRS1/IRS2: Insulin receptor substrate 1/ Insulin receptor substrate 2; DOK: downstream of tyrosine kinase; NCK: Non-Catalytic Region Of Tyrosine Kinase.
Novel pathways
Targeting the mesenchymal–epithelial transition (MET) has been recently investigated as a potential target in the treatment of TC. MET is involved in the disruption of cadherin-based cell to cell adhesion and cell motility that are decisive in embryogenesis and enhance molecular signaling for cell survival and proliferation, wound healing and organ homeostasis. The main downstream signaling activated by the ligand, HGF (hepatocyte growth factor), are the RAS/MAPK and PI3K/Akt pathways, FAK, Janus kinase 1 and PKC. The varied biological responses enhanced by MET will provide the ability to promote survival, angiogenesis, invasiveness and metastasis in tumor tissue. The overexpression of MET in TC may imply an aggressive phenotype favoring metastasis development [Peters and Adjei, 2012].
Rearrangement of paired box 8/peroxisome proliferator-activated receptor (PAX8/PPARγ) results in overexpression of a quimeric protein that downregulates the tumor suppressor activity of PPARγ and is detected in benign follicular adenomas (2–13%) and malignant FTC (30–35%) or PTC with follicular variants (1–5%) [Omur and Baran, 2014].
Loss of function of p53 is extremely rare in WDTC and correlates with tumor dedifferentiation. This alteration is seen in PDTC and ATC with a reported frequency of 15–30% and 60–80%, respectively [Volante et al. 2009]. Similarly, mutations in ALK are associated with PDTC.
The Wnt signaling pathway is involved in embryonic development, cell differentiation and proliferation and, in addition, in metastatic disease development due to its involvement in the migration process; the epithelial–mesenchymal transition. The canonical Wnt signaling is related to the cytoplasmic protein β catenin and, in the absence of Wnt, this protein is phosphorylated through a destruction complex [axin, adenomatous polyposis coli (APC) and glycogen synthase kinase 3 beta (GSK3β). The oncogenic mechanism in TC is related to the accumulation of β catenin in cytoplasmic cells because of the inability to be degraded through the ubiquitin-dependent pathway and the disassembly of the destruction complex. Consequently, transduction signaling is activated by frequent nuclear translocation of β catenin and binding to the lymphoid enhancer-binding factor 1/transcription factor (LEF-1/TCF) complex for gene transduction, such as c-myc and bcl-1 [Garcia-Rostan et al. 1999; Rezk et al. 2004]. In PDTC and in ATC, a cadherin-associaed protein beta 1 (CTNNB1) point mutation in exon 3 has been detected in 0–20% and 60% of patients respectively and are suggested to be associated with poor outcome [Garcia-Rostan et al. 2001]. Other mutations have been found in different proteins, such as APC and axin. Also, upregulation of this pathway is secondary to GSK3β inactivation due to PI3K/Akt downstream activation, which can be stimulated by RET/PTC [Xing, 2013].
Nuclear factor κB (NF-κB) is a transcription factor activated by the upstream MAPK signaling pathway and has an important role in inflammatory reactions during tumorigenesis. Particularly, BRAFV600E seems to stimulate IκB (inhibitor of NF-κB) degradation.
Hypoxia-inducible factor 1 α (HIF-1α) pathway regulates genes involved in angiogenesis by binding to HIF-1β for HIF-1 transcription factor formation, particularly influenced by VEGF-A. It is involved in tumor development in ATC, PTC and MTC by the downstream signaling enhanced by MAPK and the PI3K/mTOR pathway.
Alterations in micro-RNAs, involved in gene expression regulation, have been found in TC. A deregulation has been observed in miR-222, miR-221 and miR-146b in PTC [Pallante, 2006], possibly associated with a worse outcome and to p27kip1 and KIT. Furthermore, alterations in miR-197, miR-346, miR-155 and miR-224 in FTC and miR-30d, miR125b, miR26a and miR-30a-5p in ATC have also been described [Nikiforov and Nikiforova, 2011].
Mutational status of BRAF and RET: are they ready for primetime?
Classical cytotoxic drugs have demonstrated limited activity in TC, urging the need for new treatment options. The extensive improvement in the recognition of the primordial pathways and subsequent alterations in most TCs have led to the development of new treatment agents that have changed the landscape in such an orphan disease.
Sorafenib
Sorafenib is a multikinase inhibitor of RET, VEGFR1–3, Flt-3, KIT and CRAF/BRAF (wild type and V600E mutated). Based on the overexpression of VEGFR/PDGFR in TC and the key value of constitutive activation of RAS/BRAF in TC oncogenesis [Gupta-Abramson et al. 2008], several retrospective and phase II clinical trials have investigated the role of sorafenib in all types of thyroid tumors and have showed promising results that are presented in Table 1. The data observed support the efficacy of sorafenib in DTC and MTC. With regard to ATC, it is suggested that those tumors coming from a dedifferentiation of WDTC or those tumors harboring areas of differentiated PTC should obtain a better response with sorafenib [Savvides et al. 2013]. However, the low number of patients included in the trials does not allow any definitive conclusion.
Table 1.
Initial trials of sorafenib in thyroid carcinoma: phase II and retrospective studies.
| Study design | Inclusion criteria | Number of patients | BRAF mutation | Response rate | PFS (months) | OS (months) | |
|---|---|---|---|---|---|---|---|
| Gupta-Abramson et al. [2008] | Phase II prospective | PTC (18)
FTC (9) MTC (1) PD/ATC (2) |
30 | – | PR = 7 (23%)
SD = 16 (53%) |
21 | – |
| Kloos et al. [2009] | Phase II prospective | Arm A:
PTC (19) Arm B: PTC (22) FTC (11) ATC (4) |
46 | PTC = 17/22
nPTC = 0/6 |
PRPTC = 6 (14%)
PRnPTC = 0 (0%) SDPTC = 25 (61%) SDnPTC = 10 (67%) |
10–16 (nPTC = 4.5) | 23–37 (nPTC = 24.2) |
| Cabanillas et al. [2010] | Retrospective | PTC (8)
FTC (7) |
15 | PTC = 4/7 | 12 (80%)
PR = 3 (20%) SD = 9 (60%) |
19 | 67% at 2 years |
| Lam et al. [2010] | Phase II
prospective |
sMTC (16)
hMTC (5) |
21 | RET mutation:
sMTC = 10/12 hMTC = 5/5 |
PR = 1 + 1 (9.5%)
SD = 8 + 1 (43%) |
17.9 | – |
| Ahmed et al. [2011] | Phase II prospective | PTC (8)
FTC (9) PDTC (2) MTC (15) |
34 | DTC = 1/3 | PR = 5 (15%)
SD = 25 (73%) |
71% at 2 years | 79% at 2 years |
| Schneider et al. [2012] | Phase II prospective | DTC | 31 | BRAF: 10/32
K/N-RAS: 3/9 PIK3CA: 2/6 |
PR=8 (31%)
SD=11 (42%) |
18 | 34.5 |
| Capdevila et al. [2012] | Retrospective | PTC (7)
FTC (9) MTC (15) ATC (3) |
34 | – | PRDTC = 3 (19%)
PRMTC = 7 (47%) PRATC = 1 (33%) SDDTC = 8 (50%) SDMTC = 6 (40%) SDATC = 0 (0%) |
10.5
DTC = 13.3 MTC = 10.5 ATC = 4.4 |
23.6
DTC = 23.6 MTC = NR ATC = 5 |
| Savvides et al. [2013] | Phase II prospective | ATC | 20 | – | PR = 2 (10%)
SD = 5 (25%) |
1.9 | 3.9 |
ATC, anaplastic thyroid carcinoma; DTC, differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; hMTC, hereditary medullary thyroid carcinoma; MTC, medullary thyroid carcinoma; nPTC, nonpapillary thyroid carcinoma; OS, overall survival; PDTC, poorly differentiated thyroid carcinoma; PFS, progression-free survival; PR, partial response; PTC, papillary thyroid carcinoma; SD, stable disease; sMTC, sporadic medullary thyroid carcinoma.
The predictive value of several biomarkers could not be well established. Controversial results concerning the decrease of tumor markers [carcinoembryonic antigen (CEA), calcitonin and thyroglobulin (Tg)] and radiological response were observed [Lam et al. 2010; Ahmed et al. 2011; Capdevila et al. 2012]. In addition, the role of BRAF overactivation on tumor response could not be established. However, a significant decrease in pVEGFR, pERK and increase in pVEGF were observed in a subgroup analysis harboring a BRAFV600E mutation [Kloos et al. 2009].
Adverse events associated with sorafenib treatment were hand–foot syndrome (80%; 95% CI 68–91), diarrhea (68%; 95% CI 59–77), fatigue (67%; 95% CI 57–78), rash (66%; 95% CI 50–82), weight loss (52%; 95% CI 33–72) and hypertension (31%; 95% CI 21–42) [Shen et al. 2014].
To address definitive conclusions about the activity of sorafenib in DTC, a phase III randomized, double-blind, placebo-controlled trial was conducted [Brose et al. 2014]. The DECISION trial included 417 patients (57% PTC, 25% FTC, 10% PDTC) who were randomized to sorafenib 400 mg/12 h (N = 207) or placebo (N = 210) until disease progression. At that time, patients were offered to crossover to sorafenib according to the investigator’s decision. The primary endpoint of progression-free survival (PFS) was met, showing a significant benefit in the experimental group [10.8 months in the sorafenib group versus 5.8 months in the placebo group; hazard ratio (HR) 0.59; 95% CI 0.45–0.76, p < 0.0001]. This benefit was observed in all subgroups analyzed (age, sex, histologic subtypes, metastasis location, fludeoxyglucose uptake, tumor size, total I131 dose received and mutational status). Median PFS in patients with a BRAF mutation was 20.5 months versus 9.4 months in the sorafenib and placebo group, respectively (HR 0.46; 95% CI 0.24–0.90; p = 0.02). In patients without a BRAF mutation, median PFS was 8.9 months versus 3.8 months in the sorafenib and placebo group, respectively (HR 0.55; 95% CI 0.38–0.79; p < 0.001). Patients harboring a RAS mutation showed a median PFS of 5.5 months versus 3.5 months in the sorafenib and placebo group, respectively (HR 0.49; 95% CI 0.24–1.0; p = 0.045). In addition, in patients without a RAS mutation, median PFS was 10.8 months versus 5.8 months in the sorafenib and placebo group, respectively (HR 0.60; 95% CI 0.42–0.85; p = 0.004). The BRAF mutation was more frequently identified in patients with PTC (46.2%) and the RAS mutation in PDTC (32.3%), suggesting that differences in PFS were associated with the tumor subtype because the magnitude of effect of sorafenib was similar in all groups demonstrated by a similar HR. However, neither BRAF nor RAS mutational status were associated with prognosis. Radiological objective response rate (ORR) was 12.2% versus 0.5% and stable disease (SD) at 6 months was 42% versus 33% in the sorafenib versus placebo group, respectively. Changes in serum Tg were observed according to radiological tumor response and treatment designation. However, they were not enough for an individual recommendation as a definitive predictive value. At the time of analysis, median overall survival (OS) was not reached in both groups (HR 0.802; 95% CI 0.54–1.2, p = 0.14). Despite a longer follow up, a difference in OS will be difficult to achieve due to crossover: 71% of patients in the placebo group and 27% of patients in the sorafenib group received off-label sorafenib. Based on these results, sorafenib was approved in November 2013 by the US Food and Drug Administration for the treatment of late-stage (metastatic) DTC.
Vemurafenib
Searching for more potent and directed inhibitors of BRAF for effective tumor control growth in patients with DTC and a BRAF mutation, vemurafenib has been investigated in this context.
Vemurafenib is a potent kinase inhibitor of BRAFV600E and CRAF and less potent for BRAF wild type. Initial results came from preclinical investigations in human TC cell lines with and without the BRAFV600E mutation [Nucera et al. 2011]. Vemurafenib was able to inhibit downstream phosphorylation of ERK1/ERK2 involved in cell proliferation, as well as migration and invasion in 8505c cells harboring the BRAFV600E mutation and in PTC1 cells with wild type BRAF. Those results were confirmed in in vivo models of ATC with a BRAFV600E mutation. Considering the clinical relevance of inhibiting BRAF in PTC, vemurafenib was given to three patients with metastatic PTC harboring a BRAFV600E mutation in a phase I trial [Kim et al. 2013]. One patient achieved a partial response (PR) and two patients had disease stabilization. Clinical outcomes showed a median PFS of 11.4–13.2 months and median OS of 15–31.7 months. Investigation is ongoing, further studying the role of vemurafenib in selected patients, as well as the combination of an irreversible inhibitor of BRAFV600E/K/D and CRAF, dabrafenib, with a selective inhibitor of MEK1/MEK2, trametinib (Table 4).
Table 4.
Ongoing clinical trials in thyroid carcinoma.
| Treatment | Study design | Inclusion criteria | Primary endpoint | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Vemurafenib | Phase II
Nonrandomized |
PTC + BRAFV600E | Best overall response rate | NCT01286753 |
| Dabrafenib ± trametinib | Phase II
randomized |
TC + BRAFV600E | ORR | NCT01723202 |
| Vandetanib | Phase III | DTC | PFS | NCT01876784 |
| randomized | ||||
| Vandetanib 300 versus 150 mg/day | Phase IV
randomized |
MTC | ORR | NCT01496313 |
| Vandetanib + bortezomib | Phase I/II
nonrandomized |
Solid tumors (MTC) | ORR + biomarker response | NCT00923247 |
| Cabozantinib | Phase II | DTC | Efficacy + safety | NCT02041260 |
| nonrandomized | ||||
| Decitabine | Phase II
nonrandomized |
PTC + FTC | Restoration radioiodine uptake | NCT00085293 |
| Sunitinib | Phase II | PTC + FTC + MTC | ORR | NCT00381641 |
| nonrandomized | ||||
| Sorafenib + everolimus | Phase II
nonrandomized |
TC | ORR | NCT01141309 |
DTC, differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; ORR, objective response rate; PFS, progression-free survival; TC, thyroid carcinoma.
Vandetanib
Vandetanib is a potent tyrosine kinase inhibitor (TKI) that competes with the adenosine triphosphate (ATP) binding site in the catalytic domain of RET, VEGFR2–3 and EGFR, which are important targets in TC [Deshpande et al. 2011]. It was the first targeted drug approved for the treatment of unresectable or metastatic MTC.
Its activity was initially demonstrated in NIH3T3 RET/PTC3, RET/MEN2A (C634R) mutant, RET/MEN2B (M918T) mutant, EGFR/RET and v-Ha-Ras transfected cells [Carlomagno et al. 2002]. NIH3T3 cells are mouse embryonic fibroblast cells whose characteristics make them suitable for the transfection host. In those cell lines, vandetanib demonstrated a potent inhibition of the downstream phosphorylation and colony formation activated by RET and EGFR, as well as in vivo tumor formation in nude mice.
Two dose-escalation phase I trials assessed the security of vandetanib in solid tumors. The first one included 77 patients and assessed the percentage of dose-limiting toxicities (DLTs) of diarrhea and rash at a dose of at least 500 mg per day. Steady-state concentrations were achieved after 28 days [Holden et al. 2005]. The other phase I trial conducted in 18 Japanese patients established the maximum tolerated dose at 400 mg per day. The authors recommended a dose of 300 mg per day in further clinical trials [Tamura et al. 2006].
The activity of vandetanib over cell lines harboring RET/PTC rearrangements or RET point mutations motivated its development in DTC and MTC. The efficacy and safety in 30 patients with hereditary MTC were assessed in a phase II open-label, single-arm study [Wells et al. 2010]. A confirmed PR was achieved in six (20%) patients and SD at 6 months in 16 (53%) patients. The important decrease in CEA (53%) and calcitonin (80%) was not correlated with radiological response. Recent data suggest independent changes in calcitonin levels and changes in tumor growth during RET inhibition [Akeno-Stuart et al. 2007].
Finally, a pivotal phase III trial was conducted including 331 patients with unresectable locally advanced or metastatic MTC receiving vandetanib 300 mg per day until disease progression [Wells et al. 2012] (Table 2). At that time, patients were offered inclusion in an open-label phase with vandetanib. Excluding data from the open-label phase, the median PFS was 19.3 months in the placebo arm and not reached in the vandetanib arm (Weibull model predicted median of 30.5 months). HR for PFS was 0.27 (95% CI 0.18–0.41; p < 0.001). ORR was 13% in the placebo arm versus 45% in the vandetanib arm (p < 0.001; 12 patients in placebo arm responded during the open-label phase). Grade 3 and over QTc prolongation was observed in 19 patients. Consequently, regulatory agencies considered it mandatory for stringent electrocardiogram and electrolyte monitoring by expert physicians treating patients with vandetanib. Patients with sporadic MTC harboring a RET mutation, particularly the M918T mutation, significantly benefited from vandetanib. Responses to vandetanib were also observed in patients with RET unknown tumors and in patients with M918T negative tumors, suggesting that other RET mutations may also be susceptible to vandetanib inhibition.
Table 2.
Phase III clinical trials of vandetanib and cabozantinib in MTC.
| Clinical trial | ZETA | EXAM |
|---|---|---|
| Study design | Randomized (2:1), double-blind, placebo- controlled, phase III trial | Randomized (2:1), double-blind, placebo-controlled, phase III trial |
| Treatment | Vandetanib 300 mg/24 h versus placebo | Cabozantinib 140 mg/24 h versus placebo |
| Number of patients | 331 (231 vandetanib + 100 placebo) | 330 (219 cabozantinib + 111 placebo) |
| Tumor stage | Locally unresectable/metastatic | Locally unresectable/metastatic |
| Documented RECIST progression | ||
| Previous treatment lines | 132 (40%) patients previously treated | 128 (40%) patients previously treated: Previous TKI: 44 (cabozantinib), 24 (placebo) |
| ≥ 2 previous systemic therapies: 52 (cabozantinib), 31 (placebo) | ||
| RET mutational status: RET+/RET–/RET unknown | 56% (187)/2.4% (8)/41% (136) | 48% (159) / 12% (41) / 39% (130) |
| Primary endpoint | PFS | PFS |
| Progressive disease | Not mandatory | Yes |
| Overall response rate | 45% versus 13% | 28% versus 0% |
| Disease control rate | 87% versus 71% | 55.3% versus 13.5% |
| Calcitonin response | 69% versus 3% (p < 0.001) | −45.2% versus +57.2% (p < 0.001) |
| CEA response | 52% versus 2% (p<0.001) | −23.7% versus +88.7% (p < 0.001) |
| Progression-free survival (PFS) | NR (30.5 months) versus 19.3 months (HR 0.27) | 11.2 versus 4.0 months (HR 0.28) |
| Overall survival | HR 0.83 (95% CI 0.60–1.14)* | HR 0.83 (95% CI 0.60–1.14) |
| Adverse events ≥ G3 | Diarrhea, hypertension, QTc prolongation, fatigue | Diarrhea, palmo-plantar erithrodermia, hypertension, fatigue |
| Treatment dose reductions | 35% versus 3% | 79% versus 9% |
| Treatment discontinuations due to adverse event | 12% versus 3% | 16% versus 8% |
Less than 50% of events had occurred.
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; NR, not reached; TKI, tyrosine kinase inhibitor.
The role of vandetanib in DTC was evaluated in a randomized phase II trial including 145 patients [Leboulleux et al. 2012]. Median PFS was 11.1 months in the vandetanib arm and 5.9 months in the placebo arm (HR 0.63, 95% CI 0.43–0.92, p = 0.017). The benefit was greater in the PTC subgroup than in the FTC/PDTC subgroup (16.2 and 7.7 months with vandetanib versus 5.9 and 5.6 months with placebo, respectively). Only one patient in the vandetanib group achieved a PR. However, SD was achieved in 56% of patients in the vandetanib group and 36% of patients in the placebo group (p = 0.017). A correlation between Tg decrease and radiologic response was not reported. These data supported the development of a phase III trial, currently ongoing (Table 4).
Cabozantinib
Cabozantinib is a potent ATP competitive inhibitor of VEGFR2, MET, KIT and RET followed by AXL and Flt3 [Viola et al. 2013] and is approved by the regulatory agencies for the treatment of progressive advanced MTC. Preclinical studies (in vitro and in vivo) demonstrated the activity of cabozantinib on key receptors in angiogenesis, invasiveness and cell growth [Yakes et al. 2011]. In cultured cells including human umbilical vein endothelial cells that are used for the investigation of endothelial cell pathophysiology, with human diploid fibroblasts and VEGF (60 ng/ml), cabozantinib (4.6 nmol/liter) was able to inhibit tubule formation. In addition, in thyroid tumor tissue, cabozantinib (100 mg/kg) was able to inhibit MET phosphorylation by its ligand, hepatocyte growth factor (HGF).
Thirty-seven patients with MTC were included in the expanded cohort of a phase I trial with a capsule dose of 175 mg daily [Kurzrock et al. 2011]. Sixteen (43.2%) patients had received previous TKI therapy, 17 (46%) overexpressed MET and 25 (67.5%) had RET mutation. DLT was observed at three level doses (intermittent suspension 11.52 mg/kg, daily suspension 265 mg and daily capsule 250 mg): hand–foot syndrome, mucositis and alanine transaminase and lipase elevations. Steady-state concentrations were achieved after 15 days. ORR was identified in 10 patients (29%), three of them were previously treated. SD at 6 months was seen in 15 patients (41%) and median duration of response was not reached after 17 months of follow up.
Based on those promising results, a phase III clinical study was conducted in 330 patients with locally advanced or metastatic MTC with documented RECIST progression. Patients were randomly assigned to cabozantinib 140 mg per day or placebo until disease progression or unacceptable toxicity, but crossover was not allowed [Elisei et al. 2013] (Table 2). The study met its primary endpoint showing a longer PFS in the cabozantinib group compared with placebo (11.2 months versus 4.0 months; HR 0.28, 95% CI 0.19–0.40, p < 0.001). In contrast to previous analysis, significant correlation was detected between individual changes in calcitonin at week 12 and radiological response of target lesions at week 12, only in patients treated with cabozantinib (p < 0.0001). At the American Society of Clinical Oncology (ASCO) 2013 meeting, the results from the subgroup mutational analysis were presented. Patients harboring a RET mutation had a significant benefit in PFS (N = 169; 60 weeks versus 20 weeks; HR 0.23; 95% CI 0.14–0.38, p < 0.0001). Moreover, PFS results in patients with an M918T mutation also correlated with an improvement in OS (HR 0.53; p = 0.0179) in an interim analysis with 75% of total events achieved [EMA, 2014]. In addition, in patients with unknown RET mutation status, a benefit in PFS was shown with cabozantinib (N = 115; 48 weeks versus 13 weeks; HR 0.30; 95% CI 0.16–0.57, p = 0.0001) [Sherman et al. 2013]. Definitive conclusions about the RET mutation negative group were difficult to draw due to the small and heterogeneous sample size (N = 46; 25 weeks versus 23 weeks; HR 0.53, p = 0.21), but an ORR of 22% was reported. Interestingly, but limited by the small sample size, patients who were RET mutation negative and RAS mutation positive seemed to benefit from cabozantinib in PFS (N = 16; 47 weeks versus 8 weeks; HR 0.15, 95% CI 0.02–1.10, p = 0.0317) with an ORR of 31%. The role of cabozantinib as first-line treatment in radioiodine-refractory DTC is currently being evaluated in a phase II trial (Table 4).
Other targeted agents
VEGFR inhibitors
Based on the relevance of angiogenesis in TC progression, additional TKIs to the ones already discussed have been investigated in different TC subtypes, demonstrating activity in phase II clinical trials (Table 3). Further clinical and investigational experience is improving with those agents. However, until now, lenvatinib is the only one that has achieved a randomized, double-blind, placebo-controlled phase III trial at the moment. Lenvatinib is a FGFR1 inhibitor that is upregulated in follicular thyroid cells and is involved in tumor progression through MAPK signaling pathway activation [Kondo et al. 2007]. The SELECT trial included 392 patients with progressive radioiodine-refractory DTC randomized to lenvatinib 24 mg daily (N = 261) or placebo (N = 131). Patients were allowed to receive one prior VEGF or VEGFR targeted agent (N = 93). The results were presented at the ASCO 2014 Meeting, showing a significant benefit in PFS for patients treated with lenvatinib compared with placebo (18.3 months versus 3.6 months; HR 0.21, p < 0.0001). ORR was 65% in the lenvatinib group and 2% in the placebo arm (p < 0.0001). The most frequent grade 3 and over adverse events related to lenvatinib treatment were hypertension, proteinuria, loss of weight, fatigue and diarrhea [Schlumberger et al. 2014].
Table 3.
Phase II clinical trials of other targeted agents with activity over RAS/RAF/MAPK and PI3K/AKT pathways.
| Molecular target | Study | Tumor type | N | Treatment doses | Mutational profile | Response (PR/SD) | PFS (months) | Adverse events | |
|---|---|---|---|---|---|---|---|---|---|
| Axitinib | VEGFR1–3, PDGFRβ, KIT | Phase II [Cohen et al. 2008] | DTC (60)
MTC (11) |
71 | 5 mg/12 h | – | 2/3 (45%) | 18 | Hypertension, fatigue, GI, anorexia, proteinuria |
| Lenvatinib | VEGFR1–3, FGFR1, PDGFR | Phase II [Schlumberger et al. 2012] | MTC | 59 | 24 mg/24 h | – | 21/– (36%) | 9 | |
| Motesanib | VEGFR1–3, PDGFR, KIT, RET | Phase II [Schlumberger et al. 2009] | MTC | 91 | 125 mg/24 h × 48 weeks/DP/toxicity | RET 33/47 | 2/44 (50.5%) | 12 | Diarrhea, hypertension, fatigue, weight loss, hypothyroidism |
| Phase II [Sherman et al. 2008] | DTC | 93 | 125 mg/24 h × 48 weeks/DP/toxicity | BRAFV600E 10/33
RAS 6/33 RET 0/33 |
13/33 (49%) | 10 | |||
| Pazopanib | VEGFR1–3, PDGFRα-β, KIT | Phase II [Bible et al. 2010] | DTC | 39 | 800 mg/24 h | 18/– (49%) | 11.7 | Hypertension, fatigue, diarrhea, anorexia, nausea, mielotoxicity, hepatotoxicity | |
| Sunitinib | VEGFR1–3, PDGFR, KIT, FLT3, CSF-1R, RET | Phase II [De Souza et al. 2010] | MTC | 25 | 50 mg/24 h 4/2 | RET 11/13 (85%) | 8/13 (92%) | – | Asthenia, diarrhea, hand foot syndrome, neutropenia, leukopenia |
| Phase II [Carr et al. 2010] | DTC (28)
MTC (7) |
35 | 37.5 mg/24 h continuously | – | 11/13 (80%) | 12.8 | |||
| Phase II [Ravaud et al. 2008] | PTC (8)
ATC (1) MTC (4) Miscellaneous |
17 | 50 mg/24 h 4/2 | – | 1/12 (76.4%) | – | |||
| Everolimus | mTOR | Phase II | DTC (25)
ATC (6) MTC (9) |
40 | 10 mg/24 h | – | 2/17 (50%) | 11.7 | Mucositis, anorexia, ALT/AST elevations |
| Selumetinib | MEK1/2 | Phase II [Ho et al. 2013] | DTC | 20 | 75 mg/12 h | BRAFV600E 9
RAS 5 RET/PTC 3 |
5/3 (40%) | – | Fatigue, maculopapular rash, AST elevation |
| Phase II [Hayes et al. 2012] | PTC | 39 | 100 mg/12 h | BRAFV600E 12/26 | 1/12 (40.6%) | 8 | |||
| NRAS Q61R 1/26 |
ALT, alanine transaminase; AST, aspartate transaminase; ATC, anaplastic thyroid carcinoma; CSF-1R, colony-stimulating factor 1 receptor; DTC, differentiated thyroid carcinoma; FGFR, fibroblast growth factor receptor; GI, gastrointestinal; MAPK, mitogen-activated protein kinase; MTC, medullary thyroid carcinoma; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositide 3-kinase; PFS, progression-free survival; PR, partial response; PTC, papillary thyroid carcinoma; SD, stable disease; VEGFR, vascular endothelial growth factor receptor.
PI3K/AKT/mTOR inhibitors
Investigations in cultured cells and animal models with FTC have shown modest activity of everolimus on tumor growth control, but not over metastasis development. However, a phase II clinical trial including all TC histologic subtypes showed a low response rate, but moderate disease stabilization and significant clinical benefit in half of patients [Lim et al. 2013]. These results suggest a better role of mTOR inhibitors in a combination strategy or in more advanced or aggressive tumors, considering the role of the PI3K/Akt pathway in TC dedifferentiation. In MTC, the activity of this drug over hyperactivation of PI3K was more effective by inhibiting downstream phosphorylation (mTOR and S6K1). However, inhibition of the negative feedback of S6K1 on Insulin receptor substrate 1 and the resistance to mTORC2 inhibition may limit the activity of the rapamycin analogs. Other targeted agents to overcome those limitations are currently being investigated: dual PI3K/mTOR inhibitors (BEZ 235, BGT 226, XL-765, GDC0980) alone or in combination with Raf inhibitors (RAF265) [Jin et al. 2011], PI3K inhibitors, Akt inhibitors, mTOR complex catalytic site inhibitors (AZD8055 that demonstrated greater activity by inhibiting the phosphorylation of p70S6K and 4E-BP1, substrate of mTORC1 and Akt, substrate of mTORC2) and molecules that reduce protein stability by interfering in protein interactions (heat shock protein 90) or by proteosomal degradation [Garcia-Echeverria and Sellers, 2008].
MEK inhibitors
A number of molecular alterations in the RAS/RAF/MAPK pathway harbor a common downstream effector, MEK1/2. Therefore, the inhibition of these molecules represents a relevant target in inhibiting tumor progression. Selumetinib is a non-ATP competitive MAPK kinase inhibitor (MEK1/2) whose activity has been demonstrated in preclinical trials [Leboeuf et al. 2008]. In a phase I trial, the pharmacokinetic results showed a median half life of 8 h. DLT was rash and the recommended dose for clinical safety was 100 mg/12 h [Adjei et al. 2008].
Two recent phase II trials have been conducted. Hayes and colleagues demonstrated a modest activity of selumetinib in unselected patients, but with greater results in the BRAFV600E subpopulation [Hayes et al. 2012]. Ho and colleagues investigated the role of selumetinib in the inhibition of the constitutive activation of MAPK signaling involved in thyroid hormone expression genes for a recovery in the ability of radioiodine uptake [Ho et al. 2013]. Interestingly, all patients harboring a NRAS mutation showed an increase in iodine uptake and some grade of tumor reduction. These results were not observed in patients with a BRAFV600E mutation. Recent findings suggest an additional upregulation of NF-κB independently of MEK-ERK activation [Xing, 2013]. Dual inhibition of MEK-ERK and NF-κB may be effective in the patients who were BRAFV600E mutation positive. Further investigation with optimal patient selection may determine the best therapeutic role for selumetinib in TC.
PPARγ inhibition
The possible influence of the quimeric oncoproteins PPARγ/PAX8 in tumorigenesis has been the basis for the investigation of oral PPARγ regulators currently administered in patients with diabetes mellitus. Rosiglitazone has been studied in a phase II trial with patients with radioiodine-refractory DTC showing a RR of 25% [Kebebew et al. 2009].
Epigenetic modulating agents
Targeted agents targeting epigenetic changes such as histone deacetylase inhibitors or hypomethylating agents have been investigated in TC [Harris and Bible 2011]. The histone deacetylase inhibitors vorinostat, depsipeptide and romidepsin were studied in phase I and II trials. Limited activity with hardly any tumor responses and moderate rates of disease stabilization (46–71%) with considerable adverse events (fatigue, ataxia, cardiac toxicity, thrombosis) have restricted its investigation in TC. Hypomethylating agents have been studied for recovery of radioiodine uptake. Decitabine, a better tolerated agent compared with 5-azacytidine, is currently being investigated in a phase II trial (Table 4).
Conclusion
The identification of the components of downstream signaling from the RAS/RAF/MAPK and PI3K/Akt activated pathways involved in tumorigenesis have helped to identify novel effective targeted agents in TC that was previously without active treatments. The exploration of different therapies according to their mechanism of action has demonstrated not only efficacy in phase II trials, but also a significant benefit in survival in phase III clinical trials. In addition, this knowledge allows the investigation of potential prognostic or predictive biomarkers, such as BRAFV600E, that will help for therapy optimization and patient selection.
However, a relatively high percentage of patients, approximately 30–45% including all histologic subtypes of TC, suffer the development of a TC with unknown genetic alterations, so further investigation for underlying aberrations is warranted.
From now on, several unresolved questions require further data from consistent trials: the sequential order of the demonstrated effective treatments, the benefit of combination therapies, the development of defined subgroups of patients according to histology or mutational profile that may benefit from directed agents, and the investigation of consistent predictive or prognostic biomarkers.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
T. Alonso-Gordoa, Medical Oncology Department, Ramon y Cajal University Hospital, Madrid, Spain
J.J. Díez, Endocrinology Department, Ramon y Cajal University Hospital, Madrid, Spain
M. Durán, Surgery Department, Rey Juan Carlos University Hospital, Mostoles, Spain
Enrique Grande, Servicio de Oncología Médica, Hospital Universitario Ramón y Cajal, Carretera de Colmenar Km 9100, 28034 Madrid, Spain.
References
- Adjei A., Cohen R., Franklin W, Morris C, Wilson D, Molina J, et al. (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26: 2139–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N., Jiao Y., Sausen M., Leary R., Bettegowda C., Roberts NJ, et al. (2013) Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab 98: E364–E369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold K, Viros A, et al. (2011) Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol 165: 315–322. [DOI] [PubMed] [Google Scholar]
- Akeno-Stuart N, Croyle M, Knauf JA, Malaguarnera R, Vitagliano D, Santoro M, et al. (2007) The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res 67: 6956–6964. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Fallahi P, Ferrari S, Mancusi C, Colaci M, Santarpia L., et al. (2012) RET TKI: potential role in thyroid cancers. Curr Oncol Rep 14: 97–104. [DOI] [PubMed] [Google Scholar]
- Bible K., Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. (2010) Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11: 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C., Yeager N., Di Cristofano A. (2007) Thyroid-stimulating hormone initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Res 67: 8002–8006. [DOI] [PubMed] [Google Scholar]
- Brose M, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L, et al. (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. The Lancet 384: 319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanillas M, Waguespack S, Bronstein Y, Williams M, Feng L, Hernandez M, et al. (2010) Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M.D. Anderson experience. J Clin Epidemiol Metab 95: 2588–2595. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Iglesias L, Halperin I, Segura A, Martinez-Trufero J, Vaz M, et al. (2012) Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer 19: 209–216. [DOI] [PubMed] [Google Scholar]
- Carlomagno F., Vitagliano D., Guida T. (2002) ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62: 7284–7290. [PubMed] [Google Scholar]
- Caronia L., Phay J., Shah M. (2011) Role of BRAF in thyroid oncogenesis. Clin Cancer Res 17: 7511–7517. [DOI] [PubMed] [Google Scholar]
- Carr L., Mankoff D., Goulart B. (2010) Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16: 5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi R, Mian C, Fugazzola L, Cosci B, Romei C, Barollo S, et al. (2013) Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid 23: 50–57. [DOI] [PubMed] [Google Scholar]
- Cohen E, Rosen LS, Vokes E, Kies M, Forastiere A, Worden FP, et al. (2008) Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26: 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot J, Links T, Plukker JTM, Lips CJM, Hofstra RMW, et al. (2006) RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev 27: 535–560. [DOI] [PubMed] [Google Scholar]
- De Souza E., Ferreira A., de Carvalho D. (2011) The mTOR protein as a target in thyroid cancer. Exp Opin Ther Targets 15: 1099–1112. [DOI] [PubMed] [Google Scholar]
- De Souza JA, Busaidy NL, Zimrin A, Seiwert TY, Villaflor VM, Poluru KB, et al. (2010) Phase II trial of sunitinib in medullary thyroid cancer (MTC). J Clin Oncol 28(15 Suppl.): abstract 5504. [Google Scholar]
- Deshpande H, Roman S, Jaykumar T, Ann Sosa J, et al. (2011) Vandetanib (ZD6474) in the treatment of medullary thyroid cancer. Clin Med Insights Oncol 5: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. (2008) BRAF V600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93: 3943–3949. [DOI] [PubMed] [Google Scholar]
- Elisei R., et al. (2012) The BRAFV600E mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 97: 4390–4398. [DOI] [PubMed] [Google Scholar]
- Elisei R, Schlumberger M, Muller SP, Schoffski P, Brose MS, Shah M, et al. (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (2014) Cabozantinib. Summary of Product Characteristics. London: European Medicines Agency. [Google Scholar]
- Frank-Raue K., Rondot S., Raue F. (2010) Molecular and cellular endocrinology. Mol Cell Endocrinol 322: 2–7. [DOI] [PubMed] [Google Scholar]
- Fukahori M, Yoshida A, Hayashi H, Yoshihara M, Matsukuma S, Sakuma Y, et al. (2012) The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid 22: 683–689. [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C., Sellers W. (2008) Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 27: 5511–5526. [DOI] [PubMed] [Google Scholar]
- Garcia-Rostan G, Tallini G, Herrero A D, Aquila T, Carcangiu M, Rimm D., et al. (1999) Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 59: 1811–1815. [PubMed] [Google Scholar]
- Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G., et al. (2001) Beta-catenin dysregulation in thyroid neoplasms. Down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 158: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, et al. (2003) ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21: 3226–3235. [DOI] [PubMed] [Google Scholar]
- Guerra A, Zeppa P, Bifulco M, Vitale M. (2014) Concomitant BRAF V600E mutation and RET/PTCRe arrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid 24: 254–259. [DOI] [PubMed] [Google Scholar]
- Gupta-Abramson V, Troxel A, Nellore A, Puttaswamy K, Redlinger M, Ransone K V., et al. (2008) Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26: 4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, et al. (2008) RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 68: 7176–7182. [DOI] [PubMed] [Google Scholar]
- Harris P., Bible K. (2011) Emerging therapeutics for advanced thyroid malignancies: rationale and targeted approaches. Exp Opin Investig Drugs 20: 1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D, Lucas A, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, et al. (2012) Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res 18: 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AL, Grewal R, Leboeuf R, Sherman E, Pfister DG, Deandreis D, et al. (2013) Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, et al. (2005) Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16: 1391–1397. [DOI] [PubMed] [Google Scholar]
- Hu S, Liu D, Tufano R, Carson K, Rosenbaum E, Cohen Y, et al. (2006) Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer 119: 2322–2329. [DOI] [PubMed] [Google Scholar]
- Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW, et al. (2011) Synergistic action of a RAF inhibitor and a dual PI3K/mTOR inhibitor in thyroid cancer. Clin Cancer Res 17: 6482–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebebew E, Lindsay S H CO, Woeber K, Hawkins RE, Greenspan F, et al. (2009) Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid 19: 953–956. [DOI] [PubMed] [Google Scholar]
- Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, et al. (2013) Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF V600E mutation. Thyroid 23: 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. (2009) Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27: 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Zheng L, Liu W, Kurebayashi J, Asa SL, Ezzat S., et al. (2007) Epigenetically controlled fibroblast growth factor receptor 2 signaling imposes on the RAS/BRAF/mitogen-activated protein kinase pathway to modulate thyroid cancer progression. Cancer Res 67: 5461–5470. [DOI] [PubMed] [Google Scholar]
- Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. (2011) Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29: 2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, et al. (2010) Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28: 2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboeuf R, Baumgartner JE, Benezra M, Malaguarnera R, Solit D, Pratilas CA, et al. (2008) BRAF V600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab 93: 2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboulleux S, Bastholt L, Krause T, De la, Fouchardiere C, Tennvall J, Awada A, et al. (2012) Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 13: 897–905. [DOI] [PubMed] [Google Scholar]
- Li C, Lee KC, Schneider EB, Zeiger MA, et al. (2012) BRAFV600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 97: 4559–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, et al. (2013) A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol 24: 3089–3094. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. (2008) Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 93: 3106–3116. [DOI] [PubMed] [Google Scholar]
- Moura MM, Cavaco BM, Pinto AE, Leite V, et al. (2011) High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 96: E863–E868. [DOI] [PubMed] [Google Scholar]
- Mulligan L. (2014) RET revisited: expanding theoncogenic portfolio. Nat Rev Cancer 14: 173–186. [DOI] [PubMed] [Google Scholar]
- Nikiforov Y. (2002) RET/PTC rearrangement in thyroid tumors. Endocr Pathol 13: 1–14. [DOI] [PubMed] [Google Scholar]
- Nikiforova M., Nikiforov Y. (2008) Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Exp Rev Mol Diagn 8: 83–95. [DOI] [PubMed] [Google Scholar]
- Nikiforov Y., Nikiforova M. (2011) Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 7: 569–580. [DOI] [PubMed] [Google Scholar]
- Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, et al. (2011) Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. The Oncologist 16: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omur O., Baran Y. (2014) An update on molecular biology of thyroid cancers. Crit Rev Oncol Hematol 90: 233-252. [DOI] [PubMed] [Google Scholar]
- Pallante P. (2006) MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer 13: 497–508. [DOI] [PubMed] [Google Scholar]
- Peters S., Adjei A. (2012) MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol 9: 314–326. [DOI] [PubMed] [Google Scholar]
- Pusztaszeri M., Bongiovanni M., Faquin W. (2014) Update on the cytologic and molecular features of medullary thyroid carcinoma. Adv Anat Pathol 21: 26–35. [DOI] [PubMed] [Google Scholar]
- Ravaud A, La Fouchardiere De C, Courbon F, Asselineau J, Klein M, Nicoli-Sire P, et al. (2008) Sunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial. J Clin Oncol 26(15 Suppl.): abstract 6058. [Google Scholar]
- Rezk S, Brynes RK, Nelson V, Thein M, Patwardhan N, Fischer A, et al. (2004) Beta-catenin expression in thyroid follicular lesions: potential role in nuclear envelope changes in papillary carcinomas. Endocr Pathol 15: 329–338. [DOI] [PubMed] [Google Scholar]
- Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. (2009) Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69: 4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, et al. (2013) Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 23: 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, et al. (2009) Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 27: 3794–3801. [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Jarzab B, Cabanillas ME, Robinson BG, Pacini F, Ball DW, et al. (2012) A phase II trial of the multitargeted kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer (MTC). J Clin Oncol 30(Suppl.): abstract 5591. [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. (2014) A phase 3, multicenter, randomized, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT). J Clin Oncol 32(5 Suppl.): abstract 6008. [Google Scholar]
- Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JWA, Kapiteijn E, et al. (2012) Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 167: 643–650. [DOI] [PubMed] [Google Scholar]
- Shen C., Qiu Z., Luo Q. (2014) Sorafenib in the treatment of radioiodine-refractory differentiated thyroid cancer: a meta-analysis. Endocr Relat Cancer 21: 253–261. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. (2008) Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359: 31–42. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Cohen EEW, Schoffski P, Elisei R, Schlumberger M, Wirth LJ, et al. (2013) Efficacy of cabozantinib (Cabo) in medullary thyroid cancer (MTC) patients with RAS or RET mutations: results from a phase 3 study; J Clin Oncol 31(15 Suppl.): abstract 6000. [Google Scholar]
- Tamura T, Minami H, Yamada Y, Yamamoto N, Shimoyama T, Murakami H, et al. (2006) A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol 1: 1002-1009. [PubMed] [Google Scholar]
- Tuttle R., Ball D., Byrd D. (2013) Thyroid carcinoma. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Version 2.2013. Fort Washington, PA: National Comprehensive Cancer Network. [Google Scholar]
- Viola D., Cappagli V., Elisei R. (2013) Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Fut Oncol 9: 1083–1092. [DOI] [PubMed] [Google Scholar]
- Volante M, Rapa I, Gandhi M, Bussolati G, Giachino D, Papotti M, et al. (2009) RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab 94: 4735–4741. [DOI] [PubMed] [Google Scholar]
- Wells SA, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. (2010) Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. (2007) Gene methylation in thyroid tumorigenesis. Endocrinology 148: 948–953. [DOI] [PubMed] [Google Scholar]
- Xing M. (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13: 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M., Haugen B., Schlumberger M. (2013) Progress in molecular-based management of differentiated thyroid cancer. The Lancet 381: 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10: 2298–2308. [DOI] [PubMed] [Google Scholar]