Abstract
Background:
The incidence of melanoma in older patients is on the rise. Prior studies have shown disparities in surgical management and poor survival of older patients with melanoma.
Methods:
This is a retrospective study of adult patients diagnosed with cutaneous invasive and in situ melanoma between 2000 and 2011 in the National Cancer Data Base. Characteristics and management of older patients (≥60 years) were compared with younger patients (20–59 years) using χ2 testing.
Results:
Of 476,623 total cases, 54% (n = 258,153) were diagnosed among older patients. The reported cases in the older patients increased by 1.74-fold between 2000 and 2011. The majority were white (96%), men (65%), with early-stage disease (76% stage 0-II), and superficial spreading melanoma histology (39%). Older patients, compared with younger patients, were more likely to be men (65% versus 49%, p < 0.0001), and have in situ melanoma (28% versus 21%, p < 0.0001); less likely to have nodal metastases (7% versus 9%, p < 0.0001), receive care in academic centers (30% versus 35%, p < 0.0001), undergo wide excision or major amputation for stage I–III disease (68% versus 72%, p < 0.0001) and systemic therapy for stage III (18% versus 45%, p < 0.0001) and IV disease (30% versus 50%, p < 0.0001).
Conclusion:
Older patients with melanoma are less likely to receive care in academic centers, undergo wide excision for stage I–III disease and receive systemic therapy for stage III–IV disease. Particularly, the utilization of systemic therapy is markedly low. This disparity is particularly important with the availability of less intense more effective therapies.
Keywords: academic medical center, age groups, cutaneous, melanoma, systemic therapy, wide excision
Background
Surveillance, Epidemiology, and End Results (SEER) data indicates a rise in the incidence of cutaneous melanoma over the last two decades. The annual percentage increase in cutaneous melanoma is 2.4 between 1992 and 2010 in men, and 1.7 between 1997 and 2010 in women [Siegel et al. 2014]. Six decades of data from the Connecticut tumor registry also highlight increased incidence rates of melanoma of 17-fold in men and 9-fold in women between 1950 and 2007 [Geller et al. 2013]. Age being a risk factor for melanoma [Geller et al. 2013; Siegel et al. 2014], the rise is, at least partly, the consequence of an aging population. Indeed the incidence of melanoma in older patients has dramatically increased in the last few decades [Geller et al. 2007]. In addition, age has been shown to be an independent prognostic factor for disease-free survival [Austin et al. 1994], overall survival [Balch et al. 2001] and disease-specific survival [Macdonald et al. 2011] in melanoma, albeit not consistently in all studies. For example, in one study, the 5-year disease-free survival of patients aged over 65 years with stage I and II melanoma was significantly worse than patients aged up to 65 years (55% versus 65%, p = 0.007). Age was a significant predictor of disease-free survival in a multivariate analysis [Austin et al. 1994]. The etiology for such disproportionately higher mortality rate in older patients is unclear. Such high incidence and mortality leads to substantial economic consequences of melanoma in older patients. A population-based analysis of the SEER Medicare data estimated an annual cost of $390 million for the management of melanoma in patients aged 65 years and over [Seidler et al. 2010].
As the baby boomers age, the incidence and mortality of older patients with melanoma, and the resulting economic burden are expected to further increase, thus highlighting the importance of understanding the biological behavior, and management trends of older patients with melanoma. We analyzed the clinicopathologic characteristics and management of patients with melanoma by age to identify the current trend.
Methods
This is a retrospective study of the National Cancer Data Base (NCDB) of patients with cutaneous melanoma diagnosed between 2000 and 2011 (the most recent available data). The NCDB, a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society initiated in 1989, contains oncology data from hospital cancer registries of more than 1500 accredited cancer programs in the United States and Puerto Rico. Approximately 70% of all newly diagnosed cases of cancer (including in situ carcinoma) in the United States, totaling about 29 million records, are reported to the NCDB. Certified tumor registrars at the Commission on Cancer-accredited cancer program registries collect and submit oncology data from patient charts using nationally standardized data item and coding definitions, and data transmission format specifications, in ways similar to SEER. All data are evaluated for data integrity, and undergo extensive quality monitoring [Bilimoria et al. 2008; American College of Surgeons 2013a].
Institutional review board waiver was obtained from the University of Nebraska Medical Center Institutional Review Board. All adults above the age of 20 years with melanoma were included in the study. Patients aged 60 years and above were categorized as older patients, which is the age cutoff frequently used by the United Nations to identify the older population [World Health Organization 2014]. In 2000, approximately 1330 hospitals reported cases of melanoma, which increased to approximately 1440 in 2011. The increase in the reported cancer cases (all types) from 2000 to 2011 was approximately 1.18-fold. Data abstracted in September 2013 included diagnosis year, age, gender, race, stage at diagnosis, histology, behavior, treatment received and hospital type. NCDB categorizes hospitals into community cancer centers (100–649 cancer cases annually, may need referral for a portion of therapy), comprehensive community cancer centers (≥650 cases annually, may need referral for a portion of therapy), academic comprehensive centers (associated with university medical schools or designated as National Cancer Institute Comprehensive Cancer Care Programs) and others based on services offered and case volume [American College of Surgeons 2013b].
Statistical analysis
Descriptive statistics were used to calculate the frequency of distribution of cases according to age groups. χ2 testing of independence was used to calculate any statistical difference in distribution between older and younger patients according to different patient-, disease- and treatment-related variables. Because data provided by the NCDB public website are pregrouped into age categories, we were unable to conduct any patient-level multivariate analyses.
Results
A total of 476,623 cases of invasive and in-situ cutaneous melanoma were diagnosed in NCDB hospitals between 2000 and 2011. Of these, 54.2% (n = 258,153) were diagnosed among patients over 60 years old (older population). Nearly three-quarters of these cases were invasive melanoma (71%). The majority of older patients were white (96%) and male (65%), and had early-stage disease [76% of all cases were stage 0–II; 81% of invasive melanoma (stages I–IV) were stage I–II]. The majority had Medicare insurance (64%) and received care in nonacademic centers (69%) (Table 1).
Table 1.
Characteristics of adult melanoma cases reported to National Cancer Data Base between 2000 and 2011.
| Variable | 20–59, N (%) | >60, N (%) | p value |
|---|---|---|---|
| N | 215,078 | 258,153 | |
| Man | 106,420 (49.5) | 169,019 (65.5) | <0.0001 |
| Woman | 108,658 (50.5) | 89,134 (34.5) | |
| White | 204,085 (94.9) | 248,151 (96.1) | <0.0001 |
| African American | 1214 (0.6) | 1599 (0.6) | |
| Hispanic | 3523 (1.6) | 2932 (1.2) | |
| Other/unknown | 6256 (2.9) | 5471 (2.1) | |
| Stage 0 | 42423 (19.7) | 67,074 (26) | <0.0001 |
| Stage I | 100,259 (46.6) | 92,047 (35.7) | |
| Stage II | 21,627 (10.1) | 37,797 (14.6) | |
| Stage III | 19,279 (9) | 18,528 (7.2) | |
| Stage IV | 7364 (3.4) | 10,960 (4.2) | |
| NA | 1955 (0.9) | 2423 (0.9) | |
| Unknown | 22,171 (10.3) | 29,324 (11.4) | |
| Malignant melanoma, NOS | 129,589 (60.3) | 148,663 (57.5) | <0.0001 |
| Nodular melanoma | 12,563 (5.8) | 18,278 (7.1) | |
| Malignant melanoma in lentigo maligna | 8632 (4) | 34,285 (13.3) | |
| Superficial spreading Melanoma | 56,082 (26.1) | 43,302 (16.8) | |
| Other types | 8212 (3.8) | 13,625 (5.3) | |
| In situ | 45,769 (21.3) | 73,305 (28.4) | <0.0001 |
| Invasive | 169,309 (78.7) | 184,848 (71.6) | |
| Not insured | 8368 (3.9) | 2510 (1) | <0.0001 |
| Private/managed | 178,856 (83.2) | 67,124 (26) | |
| Medicaid | 6732 (3.1) | 2099 (0.8) | |
| Medicare | 6231 (2.9) | 167,526 (64.9) | |
| Other government | 6858 (3.2) | 11,184 (4.3) | |
| Unknown | 8033 (3.7) | 7710 (3) | |
| Academic comprehensive program* (n = 254) | 34,801 (35.8) | 40,005 (30.5) | <0.0001 |
| Comprehensive community cancer program (n = 796) | 47,841 (49.2) | 64,171 (49) | |
| Community cancer program (n = 447) | 10,173 (10.4) | 13,328 (10.2) | |
| Other hospital (n = 112) | 4516 (4.6) | 13,443 (10.3) |
Only those patients, who received all or part of first course treatment in the hospital where they were diagnosed, were utilized to determine hospital-type.
NA, not available; NOS, not otherwise specified.
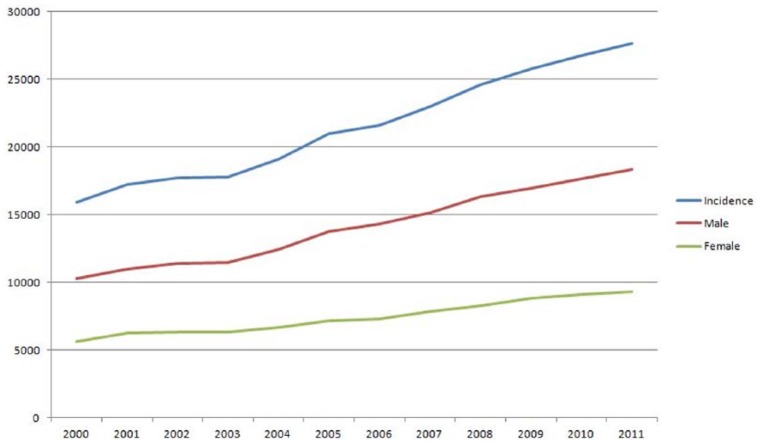
The new cases of melanoma in older patients reported to NCDB increased by 1.74-fold (Figure 1), from 15,910 cases in 2000 to 27,669 cases in 2011 (Table 2), compared with an increase of 1.18-fold in younger patients. A significant increase was observed in all subgroups by sex, race, stage and behavior (in situ versus invasive), but the greatest increase was noted in men (79%), white patients (74%), Hispanic patients (88%), stage 0 (96%), I (113%) and IV disease (123%).
Figure 1.
Incidence Reported cases of melanoma in older patients.
Table 2.
Trends of reported cutaneous invasive and in situ melanoma cases in older patients.
| Variables | 2000 | 2011 | Percentage increase between 2000 and 2011 |
|---|---|---|---|
| Incidence | 15,910 | 27,669 | 74% |
| Behavior | |||
| In situ | 4442 (28%) | 7976 (29%) | 80% |
| Invasive | 11,468 (72%) | 19,693 (71%) | 72% |
| Sex | |||
| Men | 10,269 (65%) | 18,362 (66%) | 79% |
| Women | 5641 (35%) | 9307 (34%) | 65% |
| Race | |||
| White | 15,290 (98%) | 26,592 (98%) | 74% |
| African American | 103 (1%) | 154 (1%) | 50% |
| Hispanic | 171 (1%) | 322 (1%) | 88% |
| Stage | |||
| Stage 0 | 4037 (30%) | 7902 (31%) | 96% |
| Stage I | 4886 (36%) | 10,407 (40%) | 113% |
| Stage II | 2421 (18%) | 4267 (16%) | 76% |
| Stage III | 1692 (12%) | 2009 (8%) | 19% |
| Stage IV | 598 (4%) | 1331 (5%) | 123% |
Patients with unknown race or stage were excluded from the analysis.
Older patients, compared with younger patients, were more likely to be male (65% versus 49%, p < 0.0001), have in situ melanoma (28% versus 21%, p < 0.0001) and lentigo maligna (13% versus 4%, p < 0.0001). They were less likely to have superficial spreading melanoma (16% versus 26%, p < 0.0001), and nodal metastases (7% versus 9%, p < 0.0001), receive care in academic centers (30% versus 35%, p < 0.0001), undergo wide excision (>1 cm margin) or major amputation for stage I–III disease (68% versus 72%, p < 0.04) (Table 3) and receive systemic therapy, including chemotherapy or immunotherapy for stage III (18% versus 45%, p < 0.0001) and IV disease (30% versus 50%, p < 0.0001) (Table 4).
Table 3.
First course surgery in stage I to III melanoma reported to National Cancer Data Base between 2000 and 2011.
| Variable | 20–59, N (%) | >60, N (%) | p value |
|---|---|---|---|
| Wide excision >1 cm margin or major amputation | 102,530 (72.6) | 102,107 (68.8) | 0.0419 |
| Other surgery* | 36,957 (26.2) | 44,282 (29.9) | |
| No surgery | 1670 (1.2) | 1968 (1.3) | |
| Unknown if surgery performed | 8 (0) | 15 (0) |
This included gross excision, unspecified local excision, surgery and local tumor destruction.
Table 4.
Systemic therapy use in melanoma reported to National Cancer Data Base between 2000 and 2011.
| Variable | 20–59, N (%) |
>60, N (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Chemo | Immuno | C/I | None | Chemo | Immuno | C/I | ||
| Stage 0 | 41,594 (99.6) | 24 (0.1) | 106 (0.3) | 2 (0) | 65,741 (99.5) | 52 (0.1) | 303 (0.4) | 0 (0) | p<0.0001 |
| Stage I | 97,921 (99.5) | 140 (0.1) | 367 (0.4) | 13 (0) | 90,262 (99.6) | 133 (0.2) | 209 (0.2) | 5 (0) | |
| Stage II | 19,154 (90.8) | 364 (1.7) | 1530 (7.3) | 53 (0.2) | 35,854 (96.7) | 322 (0.9) | 851 (2.3) | 29 (0.1) | |
| Stage III | 10,183 (54.6) | 1515 (8.1) | 6480 (34.7) | 483 (2.6) | 14,714 (81.6) | 889 (4.9) | 2324 (12.9) | 105 (0.6) | |
| Stage IV | 3513 (49.4) | 2383 (33.5) | 752 (10.6) | 461 (6.5) | 7396 (69.6) | 2492 (23.4) | 548 (5.2) | 192 (1.8) | |
| NA | 1674 (87.9) | 70 (3.7) | 132 (6.9) | 28 (1.5) | 2253 (94.6) | 55 (2.3) | 65 (2.7) | 8 (0.4) | |
| Unknown | 19,859 (92.6) | 542 (2.5) | 919 (4.3) | 128 (0.6) | 27,295 (95.9) | 574 (2) | 535 (1.9) | 62 (0.2) | |
Patients with unknown age, with missing information and who received unspecified or other categories of systemic therapy (n = 9574) were excluded.
C/I, chemotherapy and immunotherapy; chemo, chemotherapy; immuno, immunotherapy; NA, not available.
Discussion
NCDB, which captures approximately 70% of all new cancer diagnoses, demonstrated a 1.74-fold increase in the reported cases of melanoma (invasive and in situ combined) in older patients in the last decade. This rapid increase is only partly related to the increase in the number of reporting hospitals, since the increase in younger patients as well as the increase in all types of reported cancer cases from 2000 to 2011 was only 1.18-fold. A significant increase in melanoma in older patients was observed in all subgroups. The highest absolute rise was observed in white men. Although the early-stage disease comprised the majority of upsurge, the percentage increase in metastatic disease was also dramatic.
Older patients accounted for more than half of all melanoma cases and disproportionately affected men and white patients. The increase in the reported cases is consistent with the results from the SEER database [Geller et al. 2007] and Connecticut tumor registry [Geller et al. 2013]. Between 1973 and 2002, the incidence of melanoma increased by threefold in non-Hispanic white patients aged 65 years and over, and fivefold in non-Hispanic white men aged 65 years and over [Geller et al. 2007].
Older patients with invasive melanoma frequently presented with early-stage disease (81% stage I–II), which is consistent with results from the SEER database. According to the SEER database, between 2003 and 2009, 84% of melanoma cases presented at a localized stage [Siegel et al. 2014]. Superficial spreading melanoma was the most common histology irrespective of age [Linos et al. 2009; Tsai et al. 2010; Ciocan et al. 2013]. Although there were differences in histology of older patients with melanoma, compared with the younger patients, in our study, the majority of histology was categorized as melanoma not otherwise specified in NCDB, thus limiting the comparison. Older patients were more likely to have in situ disease and less likely to have nodal metastases at presentation. Prior studies have shown that older patients with melanoma are more likely to have adverse prognostic features such as elevated mitotic rate and tumor thickness, histologic ulceration, nodular subtype, head and neck location, but less likely to have lymph node metastases [Tsai et al. 2010; Ciocan et al. 2013]. Altered lymphatic flow with age may account for decreased lymph node metastases [Conway et al. 2009].
Older patients were less likely to have private or managed insurance and more likely to have Medicare, as expected. They were also less likely to receive care in academic centers. A previous NCDB analysis using 2003–2007 data reported only as an abstract had shown similar results [Tsai et al. 2011]. This is important since the insurance and hospital types, geographic area of treatment and oncology background of surgeons have been shown to influence compliance with National Comprehensive Cancer Network (NCCN) treatment guidelines for melanoma [Erickson et al. 2008; Bilimoria et al. 2009]. Medicaid or Medicare insurance, hospitals other than NCCN/National Cancer Institute designated hospitals, Northeast, South or West geographic area of treatment [Bilimoria et al. 2009] as well as nononcology background of surgeons [Erickson et al. 2008] were factors associated with poor compliance with NCCN guidelines in a study.
Although the majority of older patients were diagnosed with potentially curable localized disease, the use of wide excision in stage I–III disease was somewhat less common. Prior analysis has also shown that older patients are less likely to undergo wide local excision, sentinel lymph node biopsy for clinically node-negative melanoma over 1 mm thick, and regional lymphadenectomy for stage III disease [Tsai et al. 2011]. Delay in definitive excision of over 6 weeks [Ciocan et al. 2013] and poor surgical management have also been reported in other studies [Bilimoria et al. 2009; Tsai et al. 2010; Ciocan et al. 2013]. Although a less aggressive approach may be appropriate in select older patients with poor life expectancy from other comorbidities, fit patients should have an adequate resection to improve outcomes.
Our study also revealed that the use of systemic chemotherapy or immunotherapy in stage III and IV disease was less common in older patients. In a population-based French study, adjuvant interferon therapy was less frequently proposed (18% versus 58%, p < 0.001), started (9% versus 36%, p < 0.001) and completed (36% versus 65%, p = 0.004) in patients aged 70 years and over compared with younger patients. The development of adverse effects (50%), disease progression (30%) and patient’s choice or unspecified reasons (20%) were the reasons for discontinuation of adjuvant therapy in older patients [Ciocan et al. 2013]. Another prospective population-based study in Germany showed age up to 60 years (odds ratio 3.7) and insurance type (odds ratio 2.4) were the only independent factors associated with the initiation of adjuvant therapy [Livingstone et al. 2011]. The modest benefit of adjuvant therapy and anticipated poor tolerance in older patients are considered the reasons for low utilization of adjuvant therapy. A recent analysis of a prospectively collected database, however, revealed comparable safety and efficacy profile of high-dose interleukin 2 in patients aged 65 years and over (n = 22) compared with younger patients (n = 82) [Clark et al. 2013]. Although selection bias may be at play, this study illustrates that the use of systemic therapy may be appropriate for older patients with good performance status. Older patients with comorbidities may not be able to tolerate conventional chemotherapy and immunotherapy; however, with the availability of less intense more effective therapies, systemic therapy should not be underutilized. Importantly, these patients should be offered enrollment in clinical trials of novel therapeutic options.
Even though older patients are more likely to have adverse prognostic features, such as elevated mitotic rate, thick tumors and ulceration, nodal metastases are less frequent [Tsai et al. 2010; Ciocan et al. 2013]. Despite these differences, age has been shown to be an independent prognostic factor for disease-free survival [Austin et al. 1994] overall survival [Balch et al. 2001] and disease-specific survival [Macdonald et al. 2011], albeit not in all studies. The reasons for poor survival in older patients is unclear but speculated to be related to the differences in tumor biology, host biology, healthcare discrepancies [Tsai et al. 2010] or competing causes of death. The poor utilization of potentially curative wide excision, as well as systemic therapy, as shown in our study, may possibly contribute to poor survival in older patients with melanoma; however, other factors including the presence of comorbidities can certainly play an important role.
Limitations and strengths
There are several limitations of a study utilizing a database, which include retrospective design, possibility of administrative errors in entering data and lack of availability of information on possible confounders. The data collection of NCDB is similar to SEER; all data are evaluated for data integrity and undergo extensive quality monitoring [Bilimoria et al. 2008], hence the accuracy of the NCDB is reliable. The rise in cases of older patients with melanoma may be related to increased reporting; however, the rise was substantially higher than younger patients, thus suggesting an actual increase. Both invasive and in situ melanoma were lumped into one category for calculation of the trend; however, as illustrated in table 2, the trend of invasive melanoma has also increased. Although some of the differences between the two groups such as wide excision are statistically significant, the actual clinical difference may be small. We acknowledge such findings are noticeable in studies with very large sample size. Patient-level data allow a more detailed analysis of different factors associated with the disparities identified. Since the publicly accessible NCDB through its website provides data pregrouped by different categories, we were unable to perform a patient-level multivariate analysis. However, the current study is the largest study, to our knowledge, to provide a focused analysis of clinicopathologic and management trends in older patients with melanoma using a large database. The majority of the previously published studies included a population size of a few thousand or less. Additionally, this is the first population-based US study, to our knowledge, to compare systemic therapy use between older and younger patients. The healthcare disparities are very relevant as we move from intensive but less effective systemic therapy to better tolerated and more effective therapy options.
In conclusion, older patients with melanoma comprise more than half of all cases of adult melanoma, and are less likely to receive care in an academic center, undergo wide excision for stage I–III disease and receive systemic therapy for stage III–IV disease. In particular, the utilization of systemic therapy is markedly lower. These healthcare discrepancies should be monitored and changed as we move to an era of highly effective and better tolerated therapy options.
Acknowledgments
The authors would like to thank Mrs Sujana Panta, University of Nebraska Omaha, for her help in data collection and synthesis.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: AKG reports serving as a consultant for Boehringer Ingelheim and Otsuka Pharmaceuticals. PTS reports receiving payment for lectures from Bristol Myers and Celgene in the past. There are no conflict of interest for any other authors.
Contributor Information
Vijaya Raj Bhatt, Division of Hematology-Oncology, Department of Internal Medicine, University of Nebraska Medical Center, 987680 Nebraska Medical Center, Omaha, NE 68198-7680, USA.
Rajesh Shrestha, Department of Internal Medicine, Memorial Hospital of Rhode Island, Pawtucket, RI, USA.
Jairam Krishnamurthy, Department of Internal Medicine, Division of Hematology-Oncology, University of Nebraska Medical Center, Omaha, NE, USA.
Kailash Mosalpuria, Department of Internal Medicine, Division of Hematology-Oncology, University of Nebraska Medical Center, Omaha, NE, USA.
Fausto R. Loberiza, Jr, Department of Internal Medicine, Division of Hematology-Oncology, University of Nebraska Medical Center, Omaha, NE, USA.
Apar Kishor Ganti, Division of Hematology-Oncology, Department of Internal Medicine, Veteran’s Affairs Nebraska-Western Iowa Health Care System and University of Nebraska Medical Center, Omaha, NE, USA.
Peter T. Silberstein, Division of Hematology-Oncology, Department of Internal Medicine, Veteran’s Affairs Nebraska-Western Iowa Health Care System and Creighton University Medical Center, Omaha, NE, USA
References
- American College of Surgeons (2013a) National Cancer Data Base. 12 February Available at: http://www.facs.org/cancer/ncdb/ (accessed 1 January 2014).
- American College of Surgeons. (2013b) NCDB Public Benchmark Reports. Hospital Type or Health Care System. Available at: http://cromwell.facs.org/bmarks/bmpub/ver10/help/hcr_09_hosp_typesys.cfm (accessed 1 January 2014).
- Austin P., Cruse C., Lyman G., Schroer K., Glass F., Reintgen D. (1994) Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol 1: 487–494. [DOI] [PubMed] [Google Scholar]
- Balch C., Soong S., Gershenwald J., Thompson J., Reintgen D., Cascinelli N., et al. (2001) Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer Melanoma Staging System. J Clin Oncol 19: 3622-3634. [DOI] [PubMed] [Google Scholar]
- Bilimoria K., Balch C., Wayne J., Chang D., Palis B., Dy S., et al. (2009) Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. Journal of Clinical Oncology 27: 1857-1863. [DOI] [PubMed] [Google Scholar]
- Bilimoria K., Stewart A., Winchester D., Ko C. (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15: 683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocan D., Barbe C., Aubin F., Granel-Brocard F., Lipsker D., Velten M., et al. (2013) Distinctive features of melanoma and its management in elderly patients: a population-based study in France. JAMA Dermatol 149: 1150-1157. [DOI] [PubMed] [Google Scholar]
- Clark J., Kelley B., Titze J., Fung H., Maciejewski J., Nathan S., et al. (2013) Clinical and safety profile of high-dose interleukin-2 treatment in elderly patients with metastatic melanoma and renal cell carcinoma. Oncology 84: 123-126. [DOI] [PubMed] [Google Scholar]
- Conway W., Faries M., Nicholl M., Terando A., Glass E., Sim M., et al. (2009) Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol 16: 1548-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J., Velasco J., Hieken T. (2008) Compliance with melanoma treatment guidelines in a community teaching hospital: time trends and other variables. Ann Surg Oncol 15: 1211-1217. [DOI] [PubMed] [Google Scholar]
- Geller A., Clapp R., Sober A., Gonsalves L., Mueller L., Christiansen C., et al. (2013) Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol 31: 4172-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A., Swetter S., Brooks K., Demierre M., Yaroch A. (2007) Screening, early detection, and trends for melanoma: current status (2000–2006) and future directions. J Am Acad Dermatol 57: 555-572; quiz 573-576. [DOI] [PubMed] [Google Scholar]
- Linos E., Swetter S., Cockburn M., Colditz G., Clarke C. (2009) Increasing burden of melanoma in the United States. J Invest Dermatol 129: 1666-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone E., Windemuth-Kieselbach C., Eigentler T., Rompel R., Trefzer U., Nashan D., et al. (2011) A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. European Journal of Cancer 47: 1977-1989. [DOI] [PubMed] [Google Scholar]
- Macdonald J., Dueck A., Gray R., Wasif N., Swanson D., Sekulic A., et al. (2011) Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer 2: 538-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler A., Pennie M., Veledar E., Culler S., Chen S. (2010) Economic burden of melanoma in the elderly population: population-based analysis of the Surveillance, Epidemiology, and End Results (SEER) – Medicare data. Arch Dermatol 146: 249-256. [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9-29. [DOI] [PubMed] [Google Scholar]
- Tsai S., Balch C., Lange J. (2010) Epidemiology and treatment of melanoma in elderly patients. Nat Rev Clin Oncol 7: 148-152. [DOI] [PubMed] [Google Scholar]
- Tsai S., Soong S., Balch C., Lange J. (2011) Disparities in the management of melanoma in elderly patients: an analysis of patients from the National Cancer Database. ASCO Meeting Abstracts 29: 8522. [Google Scholar]
- World Health Organization (2014) Definition of an older or elderly person. Available at: http://wwwwhoint/healthinfo/survey/ageingdefnolder/en/ (accessed 1 February 2014).