Abstract
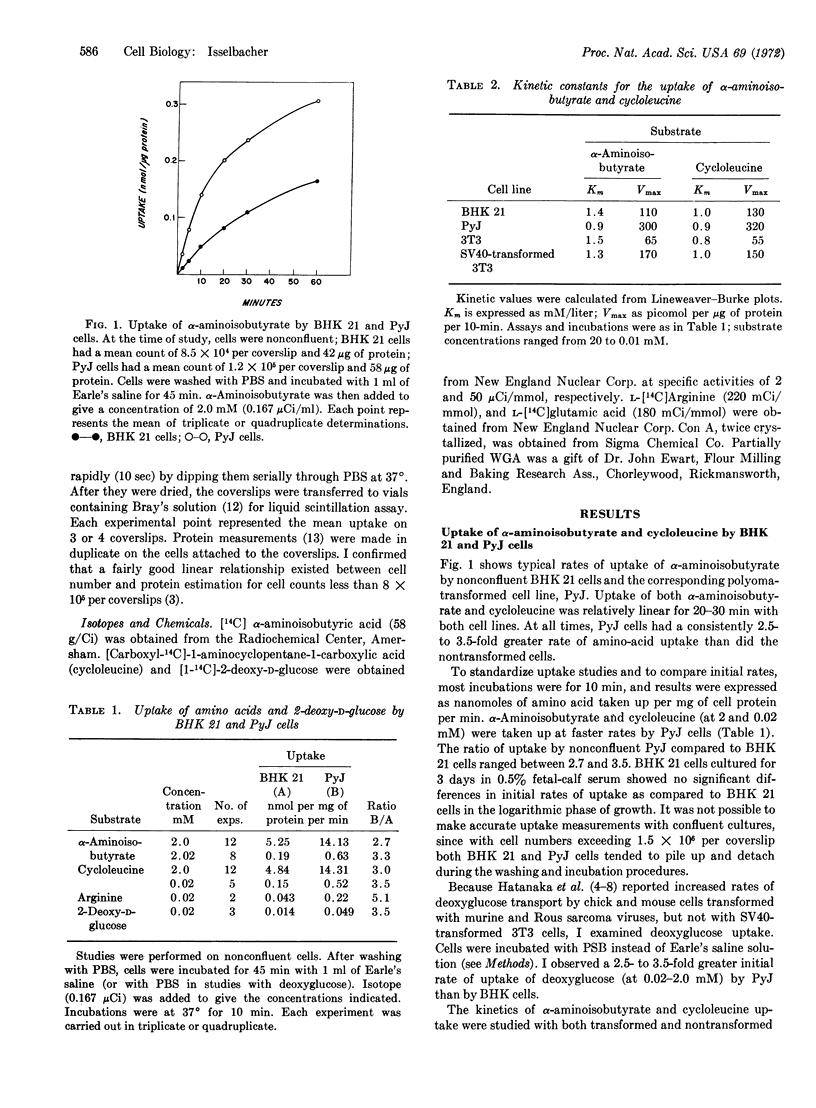
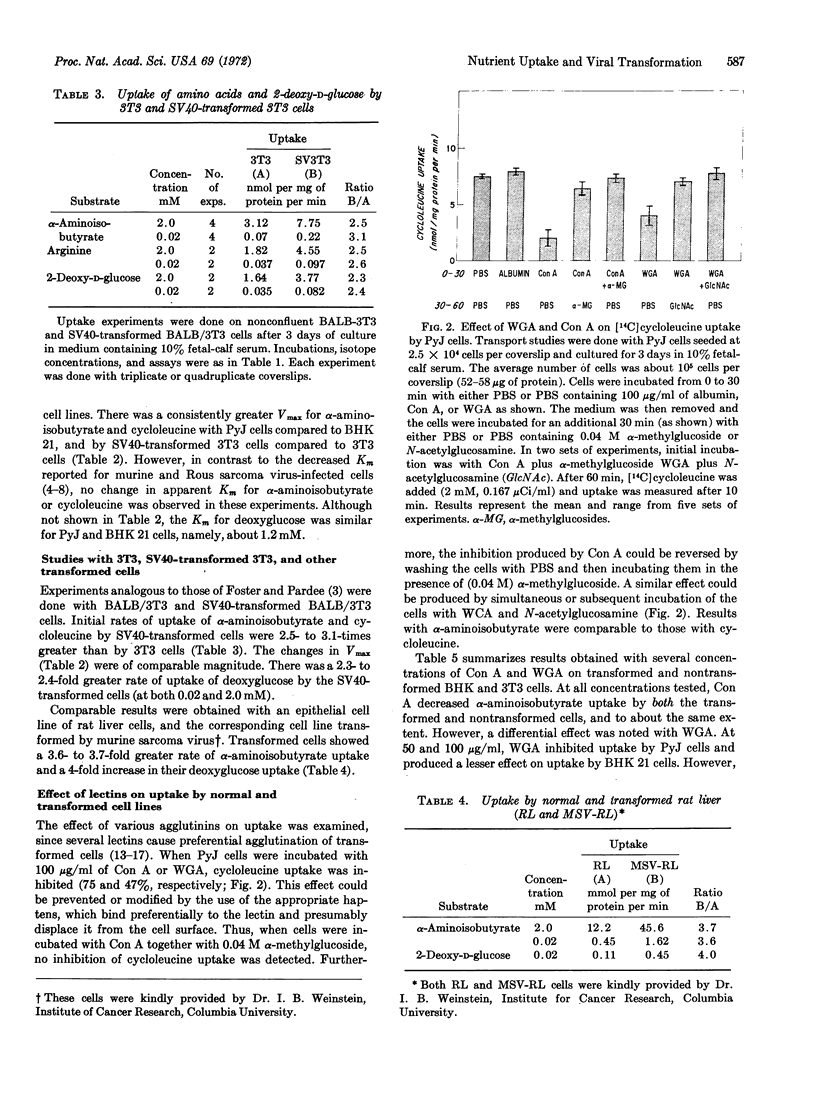
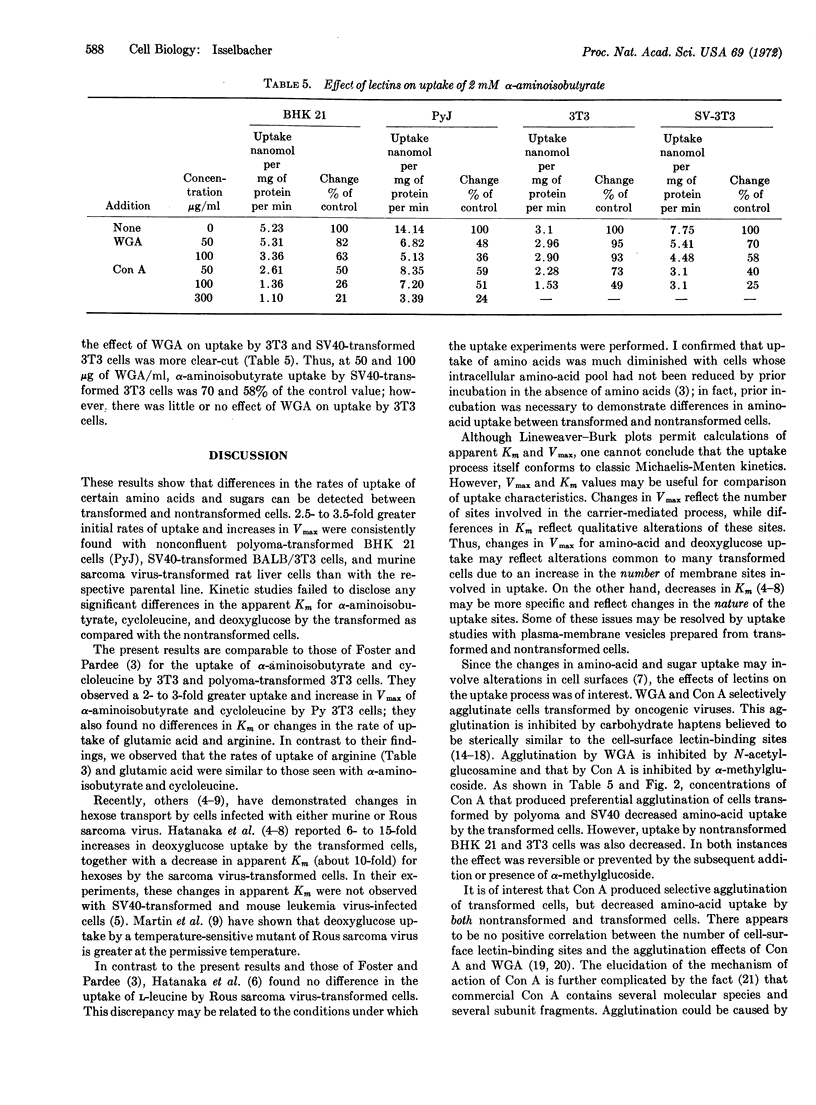
Transformed and nontransformed cells in tissue culture differ in their rate of uptake of certain nutrients, as determined by a polyester-coverslip technique. A 2.5- to 3.5-fold increased rate of uptake of α-aminoisobutyric acid, cycloleucine, and 2-deoxy-D-glucose was observed with polyoma virus-transformed baby hamster kidney (BHK) 21 cells and simian virus 40 (SV40)-transformed BALB/3T3 (mouse fibroblast) cells, compared to their nontransformed counterparts. Kinetic analysis suggested that the increased uptake by cells transformed with virus was associated with a 3-fold greater Vmax, with no detectable changes in apparent Km. Limited studies also revealed increased initial rates of uptake by murine sarcoma virus-transformed rat liver cells, as compared to the parental line. Exposure of cells to concanavalin A and wheat-germ agglutinin led to significant reductions in amino-acid uptake by both transformed and nontransformed cells; however, transformed cells showed a greater decrease in uptake after exposure to wheat-germ agglutinin. Increased initial rates of uptake of certain amino acids and sugars may be a feature common to transformed cells, compared to their parental control.
Keywords: lectins, mouse, hamster, SV40, polyoma
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUB J. C., TIESLAU C., LANKESTER A. REACTIONS OF NORMAL AND TUMOR CELL SURFACES TO ENZYMES. I. WHEAT-GERM LIPASE AND ASSOCIATED MUCOPOLYSACCHARIDES. Proc Natl Acad Sci U S A. 1963 Oct;50:613–619. doi: 10.1073/pnas.50.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Bürk R. R. One-step growth cycle for BHK21-13 hamster fibroblasts. Exp Cell Res. 1970 Dec;63(2):309–316. doi: 10.1016/0014-4827(70)90218-1. [DOI] [PubMed] [Google Scholar]
- Clarke G. D., Stoker M. G., Ludlow A., Thornton M. Requirement of serum for DNA synthesis in BHK 21 cells: effects of density, suspension and virus transformation. Nature. 1970 Aug 22;227(5260):798–801. doi: 10.1038/227798a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H., PIEZ K. A., LEVY M. The intracellular amino acid concentrations required for protein synthesis in cultured human cells. J Biol Chem. 1961 Jul;236:2039–2042. [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Hare J. D. Location and characteristics of the phenylalanine transport mechanism in normal and polyoma-transformed hamster cells. Cancer Res. 1967 Dec;27(12):2357–2363. [PubMed] [Google Scholar]
- Hatanaka M., Augl C., Gilden R. V. Evidence for a functional change in the plasma membrane of murine sarcoma virus-infected mouse embryo cells. Transport and transport-associated phosphorylation of 14C-2-deoxy-D-glucose. J Biol Chem. 1970 Feb 25;245(4):714–717. [PubMed] [Google Scholar]
- Hatanaka M., Gilden R. V. Virus-specified changes in the sugar-transport kinetics of rat embryo cells infected with murine sarcoma virus. J Natl Cancer Inst. 1970 Jul;45(1):87–89. [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Hatanaka M., Todaro G. J., Gilden R. V. Altered glucose transport kinetics in murine sarcoma virus-transformed BALB-3T3 clones. Int J Cancer. 1970 Mar 15;5(2):224–228. doi: 10.1002/ijc.2910050209. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]




