Summary
Knockout of glutamine synthetase isogene Gln1;2 reduces nitrogen remobilization and the number and size of siliques and seeds in Arabidopsis. Gln1;1 affects the response of primary root development to exogenous nitrogen.
Key words: Arabidopsis, cytosolic glutamine synthetase, isoform, mutant, nitrogen, seed germination, seed productivity.
Abstract
Nitrogen (N) remobilization from reserves to sinks is essential for seedling establishment and seed production. Cytosolic glutamine synthetase (GS1) is up-regulated during both seed germination and seed filling in plants. However, the specific roles of the individual GS1 isogenes with respect to N remobilization, early seedling vigour, and final seed productivity are not known. In this study, impairment of seed germination and seedling establishment is demonstrated in the single knockout mutant gln1;2, and the double knockout mutant gln1;1:gln1;2. The negative effect of Gln1;2 deficiency was associated with reduced N remobilization from the cotyledons and could be fully alleviated by exogenous N supply. Following reproductive growth, both the single and double Gln1;2-knockout mutants showed decreased seed yield due to fewer siliques, less seeds per silique, and lower dry weight per seed. The gln1;1 single mutant had normal seed yield structure but primary root development during seed germination was reduced in the presence of external N. Gln1;2 promoter–green fluorescent protein constructs showed that Gln1;2 localizes to the vascular cells of roots, petals, and stamens. It is concluded that Gln1;2 plays an important role in N remobilization for both seedling establishment and seed production in Arabidopsis.
Introduction
Nitrogen (N) is an essential element for plant growth and development (Hawkesford et al., 2012). Plant roots absorb N as nitrate or ammonium (Yuan et al., 2013; Krapp et al., 2014) and these inorganic N forms must be processed before the N can be built into proteins, nucleic acids, and a range of secondary metabolites. A central component in the N processing chain is the enzyme glutamine synthetase (GS; EC 6.3.1.2) which catalyses the assimilation of ammonium into the amide glutamine (Gln) (Hirel et al., 2005). The generated Gln is the main N carrier in plants and can be transported via the xylem and phloem (Rentsch et al., 2007; Zhang et al., 2010).
Besides a key role in primary N assimilation, GS is crucial for reassimilation of NH4 + which is constantly generated in large quantities in plants via processes such as photorespiration, lignin biosynthesis, and protein turnover (Li et al., 2014). During the latter process, protein-bound N is converted to Gln, promoting recycling of N in storage proteins. This process is critical during seed germination in many plant species, including Arabidopsis, when N is remobilized from source organs (cotyledons and hypocotyls) to developing sinks, until the seedling establishes itself as a self-sufficient, autotrophic organism (Fait et al., 2006; Hong et al., 2012). In reproductive growth stages, senescence-induced degradation of leaf proteins provides the main source of N for incorporation into seed storage proteins, and GS activity has been shown to correlate with N remobilization efficiency (Kichey et al., 2005; Guiboileau et al., 2012).
Two GS isoforms exist in higher plants: the cytosolic glutamine synthetase isoform (GS1) encoded by a multigene family, and the chloroplastic glutamine synthetase isoform (GS2) encoded by one gene (Gln2) (Swarbreck et al., 2011). In Arabidopsis, GS1 is encoded by five individual isogenes with distinct tissue localization and expression patterns as well as distinct affinities for NH4 + and glutamate (Guo et al., 2004; Ishiyama et al., 2004a, b ; Li et al., 2006a ; Dragicevic et al., 2014). Phylogenetically, the nucleotide and amino acid sequences of these isoforms do not cluster with GS1 sequences from cereals (Thomsen et al., 2014). The function of Arabidopsis and cereal GS1 isogenes can thus not be compared directly, highlighting the importance of studying both model and crop species. However, two GS1 isogenes, Gln1;1 and Gln1;2, were up-regulated in Arabidopsis lines overexpressing a rice full-length cDNA library under the control of the Cauliflower mosaic virus (CaMV) 35S promoter (Albinsky et al., 2010), indicating a relationship between AtGS1 isogenes and the rice genes.
Individual GS1 isogenes have been demonstrated to play essential roles in plant development and yield structure in cereal species. This is the case, for example, in maize (Zea mays) where ZmGln1;3 and ZmGln1;4 are of critical importance for kernel germination (Limami et al., 2002) and for development of the cob with respect to kernel number and kernel size, respectively (Martin et al., 2006; Cañas et al., 2010). In rice (Oryza sativa), knockout of OsGS1;1 resulted in reduced growth and grain filling, while OsGS1;2 recently was identified as a critical player in primary root NH4 + assimilation as well as in tillering (Funayama et al., 2013). A crucial function of OsGS1;1 in coordinating the global metabolic network in rice plants exposed to ammonium as the N source was shown by Kusano et al. (2011). In the OsGS1;1 knockout mutant, the isogenes OsGS1;2 and OsGS1;3 were not able to compensate for the loss of OsGS1;1, which suggests that these GS1 isogenes are non-redundant (Tabuchi et al., 2005). A recent study in Brassica napus, a species closely related to Arabidopsis, identified 16 different GS1 isogenes and showed that they were differentially expressed in response to N regimes during leaf senescence (Orsel et al., 2014).
The exact physiological functions of individual GS1 isogenes in relation to plant development and seed productivity are still not known in Arabidopsis. The role of individual GS1 isogenes in plant development could partly be related to the quantity of N assimilated or remobilized, and partly to the maintenance of critical N flows and internal N sensing during essential growth stages. It is known that GS1 isogenes in Arabidopsis are involved in controlling carbon–nitrogen interactions (Bussell et al., 2013; de Jong et al., 2014) and responses to environmental stimuli such as N form (Engelsberger and Schulze, 2012) and salt stress (Debouba et al., 2013). However, very limited information is available on their specific roles with respect to seedling development and seed yield structure. Lothier et al. (2011) examined a gln1;2 knockout mutant and observed reduced rosette biomass, but only under ample nitrate supply or under provision of ammonium, while the mutant and the wild type (Wt) behaved similarly under nitrate-limiting conditions. In the study of Lothier et al. (2011), differences in N remobilization and changes in seed yield structure were only characterized in relative terms, namely as changes in harvest index (the ratio between seed yield and total above-ground biomass) and seed 15N partitioning (the ratio between seed 15N content and total plant 15N content). These parameters did not differ between the gln1;2 mutant and the Wt, and the same was the case for seed N concentration (Lothier et al., 2011).
The objectives of this work were to test the hypotheses that GS1 isogenes Gln1;1 and Gln1;2 play important roles with respect to: (i) N remobilization from reserves in germinating seeds of Arabidopsis; (ii) N loading into Arabidopsis seeds; and (iii) seed yield structure in Arabidopsis. In order to test these hypotheses, three different Gln1;1 and Gln1;2 knockout mutants were characterized, namely gln1;1, gln1;2, and gln1;1:gln1;2. It is shown that during germination, Gln1;2 plays an essential role in seed reserve N remobilization for sink establishment, while Gln1;1 promotes absorption of exogenous N. In the reproductive growth stage, Gln1;2 is significant for seed yield structure, affecting the number of siliques, their size, the number of seeds per silique, as well as the dry weight per seed.
Materials and methods
Plant material
Arabidopsis thaliana ecotype Columbia-0 (Col-0) lines with T-DNA insertions in the 5’-untranslated region, 62 nucleotides upstream of the start codon of Gln1;1 (gln1;1, SALK_000459), and the third intron, at 599 nucleotides downstream of the ATG of Gln1;2 (gln1;2, SALK_145235), were obtained from the European Arabidopsis Stock Centre, Nottingham, UK.
Homozygous gln1;1 and gln1;2 mutants were identified by PCR on genomic DNA (Ostergaard and Yanofsky, 2004) with the use of a primer annealing to the left border of the T-DNA (LBa1.3, 5ʹ-ATTTTGCCGATTTCGGAAC-3ʹ) and the following gene- specific primers: for Gln1;1 (fwd, 5ʹ-TTCACTGTCTTCACCA GGAGC-3ʹ; and rev, 5ʹ-TCCCAAATTTTATTTTAGCATTTAC AG-3ʹ) and for Gln1;2 (fwd, 5ʹ-CCACAACCACGAACTCTAA AG-3ʹ; and rev, 5ʹ-AACGGAGAATCGAAAAAGAGC-3ʹ). Two PCRs were needed for each plant. One reaction included two gene-specific primers (fwd and rev): the Wt and the heterozygous lines resulted in bands of 1106bp for Gln1;1 or 1208bp for Gln1;2, while the homozygous mutants gave no band. Another reaction was with one gene-specific primer (rev) and one T-DNA primer (LBa1.3): bands were obtained for the homozygous mutants and the heterozygous lines, while the Wt was blank.
gln1;1:gln1;2 double mutants were generated by crossing the single mutants. F1 progeny heterozygous for both genes (gln1;1, Gln1;1/gln1;2, Gln1;2) were identified by PCR using the primers above and were allowed to self-pollinate. The resulting F2 progeny (160 plants) were screened by PCR, and seven homozygous mutants, (gln1;1, gln1;1/gln1;2, gln1;2) were obtained. Seeds from self-pollination of these plants were used in the following experiments.
Plant growth conditions
In soil, single plants were grown in 5.5 litre pots with soil (PINDSTRUP Substrate 2, Pindstrup Mosebrug A/S, Denmark) under controlled growth chamber conditions with an 8 h:16h light:dark cycle, light intensity of 100 μmol m–2 s–1, 22 °C:20 °C day:night air temperatures, and 75% humidity of the air. N export experiments were performed at 2 weeks after germination (WAG), in order to study N remobilization in early growth stages. Two-week-old Arabidopsis seedlings had developed cotyledons and two rosette leaves. The cotyledons were fully expanded and were considered as N sources, and the two rosette leaves were considered to be sinks. Both source and sink organs were harvested in order to compare the N concentration in each part.
At 4 weeks after germination, the plants were transferred to a growth chamber with the same conditions except with a long-day cycle (16 h:8h light:dark) to induce flowering. In order to characterize the reproductive growth of the plants, the numbers of siliques formed on both the main inflorescence stem and the side branches were counted at 8 and 9 WAG. To compare silique development, four flowers per plant with long anthers extending above the stigma were labelled at the pedicel and the corresponding siliques were observed using a stereo fluorescence microscope (Leica MZ FLII) 10 d later. At 10 WAG, fully developed siliques from the main inflorescence stem (bottom 10 siliques) were collected to compare seed number per mature silique. At 12 WAG, desiccated seeds were collected, counted, and weighed.
On Petri plates, seeds were sterilized in 50% ethanol for 1min and in 50% NaClO (Klorin original, Colgate-Palmolive A/S, Denmark) with 0.05% (v/v) Triton X-100 for 10min, then rinsed five times with sterile water to remove the NaClO. Seeds were stratified for 48h at 4 °C in the dark and placed onto modified half-strength Murashige and Skoog (1/2 MS) medium in square Petri plates with three different N treatments. No-nitrogen medium contained 2.5mM KH2PO4, 2mM MgSO4, 2mM CaCl2, 0.1mM NaFe-EDTA, 70 μM H3BO3, 14 μM MnCl2, 0.5 μM CuSO4, 0.2 μM Na2MoO4, 10 μM NaCl, 1 μM ZnSO4, and 0.3% (w/v) phytagel, pH 5.8. Normal-nitrate medium and high-nitrate medium were similar to the no-nitrogen medium except for the addition of 5mM and 20mM KNO3, respectively. The Petri plates were placed quasi-vertically in a growth chamber with an 8 h:16h, light:dark cycle, light intensity of 100 μmol m–2 s–1, 22 °C:20 °C day:night air temperatures, and 75% humidity of the air. Observations were performed at day 1 and 2 after germination with a stereo fluorescence microscope (Leica MZ FLII). Primary root growth was measured 6 days after sowing (DAS).
RNA extraction and reverse transcription–PCR (RT–PCR)
Total RNA was extracted from 50–100mg of frozen roots using a solution of 35% (v/v) phenol, 1M guanidine thiocyanate, 1M ammonium thiocyanate, 0.1M sodium acetate, and 5% (v/v) glycerol. Proteins were removed by chloroform, and RNA was purified by isopropanol and washed with ethanol before resuspension in RNase-free water. Following DNA digestion with TURBO DNase (Applied Biosystems/Ambion) and confirmation of RNA quality by gel electrophoresis, reverse transcription was performed using M-MuLV Reverse Transcriptase (New England Biolabs). cDNA was synthesized from 2 μg of RNA estimated from the concentration measured by a nanodrop 2000 spectrophotometer (Thermo Scientific). The resulting cDNAs were used as templates for RT–PCR in order to characterize the mutants at the transcription level. cDNA-specific primers for Gln1;1 (fwd, 5ʹ-TTCACGTCACCCTCTTCCTC-3ʹ; and rev, 5ʹ-TCATGTCCATTCCAGAACCA-3ʹ) and Gln1;2 (fwd, 5ʹ-GGTTGGTGGTTCTGGTATGG-3ʹ; and rev, 5ʹ-CTCTCCCGCTGGAGTGTAAG-3ʹ) were synthesized.
Protein extraction and western blot analysis
Total proteins were extracted from 50mg of frozen shoots using 200 μl of solution [50mM TRIS, 2mM EDTA, 10% (v/v) glycerol, and 10mM 2-merceptoethonal, pH 8.0]. The homogenates were centrifuged at 16 099 g for 3min at 4 °C. The supernatant was analysed by western blot analysis. The total protein concentration was determined by a nanodrop 2000 spectrophotometer (Thermo Scientific). A 10 μg aliquot of crude proteins was separated on 12% Criterion XT Bis-Tris gels (Bio-Rad), electrophoretically transferred to 0.2 μm pore-size nitrocellulose membranes (Bio-Rad), and blocked with PBST [8mM K2HPO4, 3.9mM KH2PO4, 150mM NaCl, and 0.05% (v/v) Tween-20, pH 7.2] containing 5% skim milk. The blocked membrane was then incubated with 20 μg of anti-GS serum (rabbit IgG) in 20ml of PBST at 4 ºC overnight. After several washes with PBST, the membrane was incubated at room temperature for 2h with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10 000) (Thermo Scientific), and the immune complexes were detected using chemiluminescence reagents.
Quantification of N concentration by isotope ratio-mass spectrometry (IR-MS)
The concentration of N was determined in samples of 4–5mg oven-dried finely ground plant material by IR-MS using a system consisting of an ANCA-SL Elemental Analyser coupled to a 20-20 Tracer Mass Spectrometer (SerCon Ltd, Crewe, UK) with acetanilide (Merck) as the standard.
Cloning of promoter–NLS-GFP-GUS/pCAMBIA 3300 DNA constructs
In order to analyse the expression patterns of Gln1;1 and Gln1;2, the DNA sequences upstream of the genes (promoter sequence) were cloned into a vector from which expression of green fluorescent protein (GFP) will be driven by the respective Gln1;1 and Gln1;2 promoters. A 1040bp DNA fragment containing the upstream region of Gln1;1 and a 1526bp DNA fragment containing the upstream region of Gln1;2 were commercially synthesized with restriction sites at the 5ʹ and 3ʹ ends of each fragment: GAGCTC and CCCGGG, for SacI and XmaI, respectively. The DNA fragments were cut with SacI and XmaI (New England Biolabs), and ligated with the plant expression vector pCAMBIA 3300 NLS-GFP-GUS (Nour-Eldin et al., 2006). PCR amplification of the two DNA fragments with the vector primers fwd, 5ʹ-GAAACAGCTATGACATGATTACGAA-3ʹ; and rev, 5ʹ-AGCAGTTCAACAGCATCATAGGT-3ʹ followed by DNA sequencing confirmed the vectors containing the insertions of Gln1;1 and Gln1;2 promoters.
Plant transformation
Wt Arabidopsis Col-0 plants were transformed with Gln1;1 and Gln1;2 promoter–NLS-GFP-GUS/pCAMBIA 3300 constructs using the floral dip method (Clough and Bent, 1998). Hundreds of seeds from the transformed plants were sown in soil and transformants were selected by spraying with BASTA. Spraying was performed 1 week after germination and repeated four times at 2 d intervals. At the end of the BASTA selection, transformants continued to grow and remained green, while non-transformed plants were small, turned white, and died. Seeds from self-pollination of the transformants were collected for a further selection of homozygotes.
Detection of the cell type specific expression of GFP
Observations of green fluorescent nuclei (Chytilova et al., 1999) from Gln1;1 and Gln1;2 expression were recorded by confocal microscopy Leica SP5. Roots or floral organs were cut and mounted in water for microscopic observation. A 488nm argon laser was used for the excitation, and the detector was set for emission in the 510–530nm range for GFP, and in the 650–700nm range for the autofluorescence from chloroplasts. Root tissues were from 3-week-old transgenic plants germinated on 1/2 MS medium (Sigma, M5519) containing 1% sucrose solidified with 0.3% (m/v) phytagel, pH 5.8. Floral and reproductive organs were from 6-week-old transgenic plants grown in soil. Similar images were captured from four independent transgenic plants.
Results
Characterization of Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2
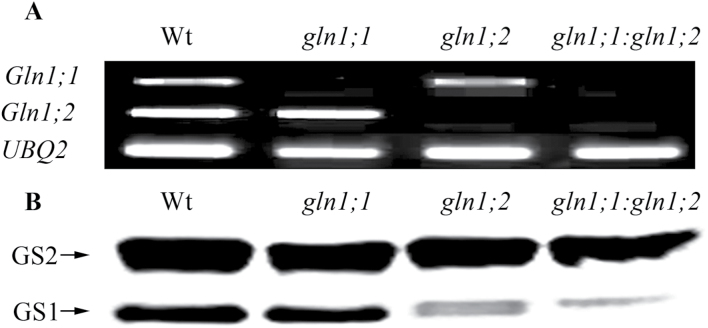
In order to study the effect of a loss of function of Gln1;1 and Gln1;2, homozygous single and double mutants were identified and analysed at the GS gene and protein level. RT–PCR analysis showed that Gln1;1 was expressed at a much lower level than Gln1;2 in Wt plants (Fig. 1A). Gln1;1 expression was absent in gln1;1 and gln1;1:gln1;2, and Gln1;2 expression was absent in gln1;2 and gln1;1:gln1;2 (Fig. 1A). At the protein level, the relative amounts of GS1 and GS2 in crude protein extracted from shoots of gln1;1, gln1;2, gln1;1:gln1;2, and the Wt were estimated by western blot analysis (Fig. 1B). In Wt shoots, two polypeptides were detected. The ~45kDa polypeptide corresponds to GS2, whereas the ~40kDa polypeptide corresponds to GS1. In gln1;2 and gln1;1:gln1;2, a prominent decrease in GS1 protein content was observed, showing that Gln1;2 is the main shoot isoform in the Arabidopsis GS1 multigene family. In gln1;1, a similar amount of GS1 protein to that in the Wt was observed (Fig. 1B). This reflects the contribution of protein from other GS1 isoforms, including an up-regulated Gln1;2 (Guan, 2014).
Fig. 1.
Identification of Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2. (A) RT–PCR analysis of Gln1;1 and Gln1;2 expression in the roots of the wild-type (Wt), gln1;1, gln1;2, and gln1;1:gln1;2 plants. UBQ2 was used as a reference gene for equal amounts of cDNA in different samples. Each sample contained a pool of roots from five individual plants. (B) Western blot analysis of GS1 and GS2 contents in shoots of Wt, gln1;1, gln1;2, and gln1;1:gln1;2 plants. The upper band corresponds to GS2, and the lower band corresponds to GS1. The same amount of crude protein was loaded in each lane, as described in the Materials and methods. Each sample contained a pool of shoots from five individual plants.
Early seedling establishment and N remobilization from cotyledons are impaired in Gln1;2 knockout mutants
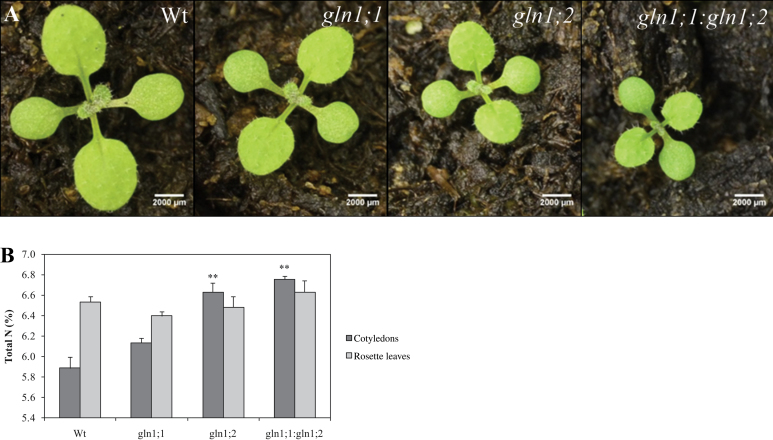
Seedling size after 2 weeks of growth in soil was distinctly smaller in the mutants compared with the Wt (Fig. 2A). The size of both the cotyledons and the first real leaf was reduced, most pronouncedly in the gln1;1:gln1;2 double mutant, followed by the gln1;2 mutant and the gln1;1 mutant. The slower seedling development in the mutants suggests a function for Gln1;2 and, to a lesser extent, Gln1;1 in N remobilization during seed germination and seedling establishment when N stored in the cotyledons is required to sustain the growth of new sink leaves.
Fig. 2.
N remobilization in Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2 during the first 2 weeks of seedling establishment. (A) Phenotype of the wild-type and mutant seedlings 2 weeks after germination. (B) Total N concentration (% dry weight) in cotyledons (source) and rosette leaves (sink) of the wild type and mutants. Plants were grown in soil for 2 weeks. Results are means ±SE (n=4). Asterisks indicate statistically significant differences between the lines, determined using Student’s t-test: *P<0.05; **P<0.01. Two independent experiments were carried out with similar results. Scale bars=2000 μm.
In order to investigate if there was a difference in N remobilization between mutants and the Wt, the N concentration in cotyledons and first rosette leaves was analysed. Plants were grown in soil for 2 weeks, during which period they developed two rosette leaves (Fig. 2A). The Wt plants had a markedly higher N concentration in the new sink leaves than in the source cotyledons (Fig. 2B). The same was the case for the gln1;1 single mutant, although the difference in N concentration between sink and source leaves was much smaller than in the Wt (Fig. 2B). Both the gln1;2 and the gln1;1:gln1;2 mutants behaved in an opposite manner to the Wt and the gln1;1 mutant by having a higher N concentration in the source cotyledons compared with the sink leaves (Fig. 2B). This difference was due to the fact that a significantly (P=0.01) higher N concentration was maintained in the cotyledons of the two Gln1;2 knockout mutants gln1;2 and gln1;1:gln1;2 relative to the Wt (Fig. 2B), showing that N remobilization from seed reserves in cotyledons was markedly reduced when Gln1;2 was lacking.
Seed germination is impaired in the gln1;2 knockout mutants but the defect can be alleviated by exogenous N
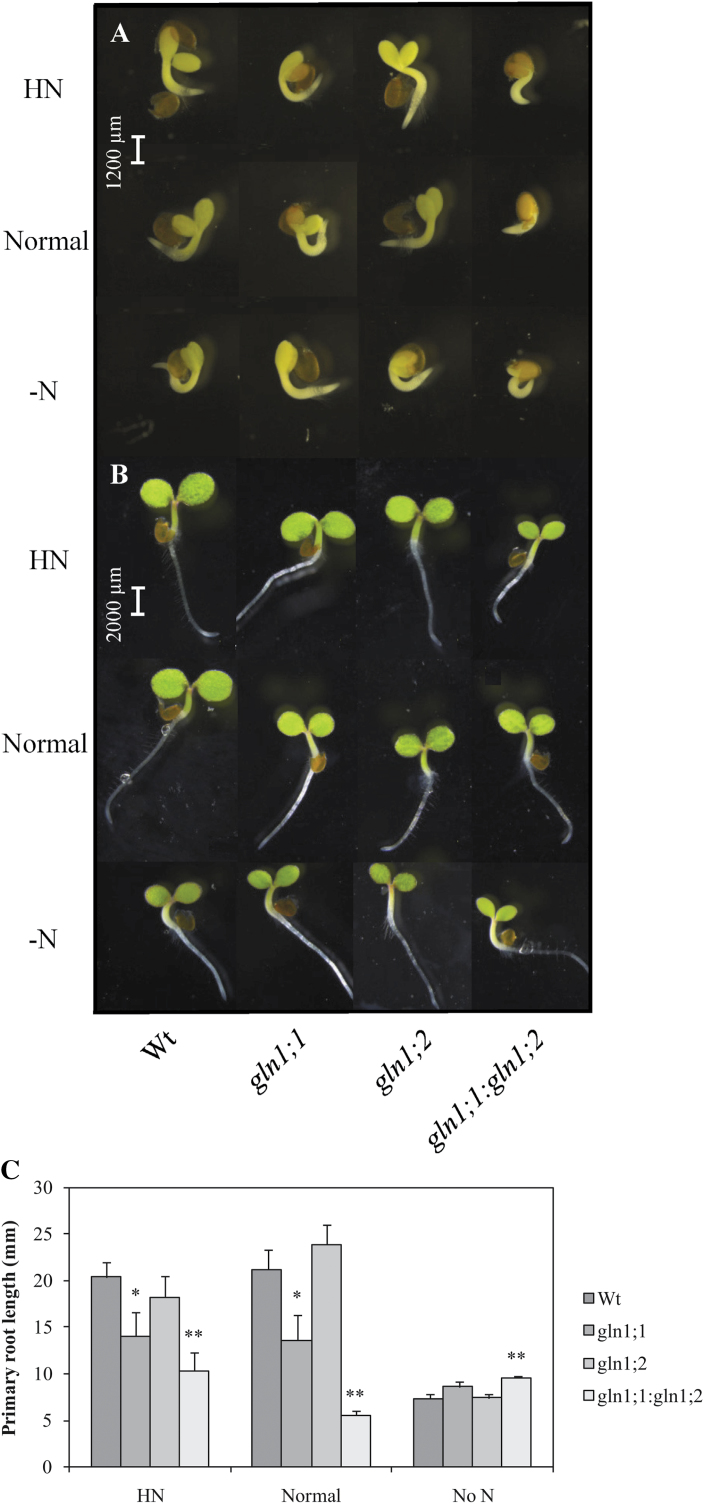
In the absence of exogenous N supply (–N), the cotyledons emerging during the first 2 DAS of gln1;2 and gln1;1:gln1;2 seeds were breaking through the seed coat more slowly (Fig. 3A) and were distinctly smaller than those from Wt and gln1;1 seeds (Fig. 3B). In order to study if this phenotype was associated with N deficiency due to reduced N remobilization from seed reserves, an exogenous N supply was applied in the form of nitrate at a normal (5mM) or high (20mM) level to modified 1/2 MS medium.
Fig. 3.
Characterization of Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2 in early stages of seed germination. (A) Stereo microscopy images of the wild type and mutants on day 1. (B) Stereo microscopy images of the wild type and mutants on day 2. (C) Primary root length of the wild type and mutants at day 6 (n=16–18). The wild-type and mutant seeds were placed onto three different N treatment plates modified from 1/2 MS medium: high-nitrate medium (HN), normal-nitrate medium (Normal), and no-nitrogen medium (–N), as indicated on the left hand side. Plates were placed quasi-vertically. Similar images for (A) and (B) were acquired from four individual seedlings.
Addition of N enhanced seed germination (Fig. 3A) and cotyledon size (Fig. 3B) of the gln1;2 single mutant compared with the –N treatment. At the high level of N supply, the seedling establishment of gln1;2 was almost fully recovered. In the gln1;1 mutant, the length of the primary root was negatively affected (P=0.05) by exogenous N supply (Fig. 3C), suggesting a function for Gln1;1 with respect to primary root development in response to external N. The gln1;1:gln1;2 double mutant had a noticeably retarded germination at all N levels (Fig. 3). Only the radicle was visible at day 1 (Fig. 3A), and the cotyledons were much smaller and had started to turn yellow at day 2 (Fig. 3B). The primary roots developed by gln1;1:gln1;2 were significantly (P=0.01) shorter than in the Wt under high and normal N conditions, but longer in the –N treatment (Fig. 3C).
Seed yield is significantly reduced in Gln1;2 knockout mutants
Seed formation requires N remobilization from senescing vegetative plant parts as the N uptake and assimilation during seed filling is insufficient to fulfil the high N demand of the seeds. In order to resolve how knockout of Gln1;1 or/and Gln1;2 affects seed production, the seed yield structure in the reproductive growth stage of single and double knockout mutants was characterized.
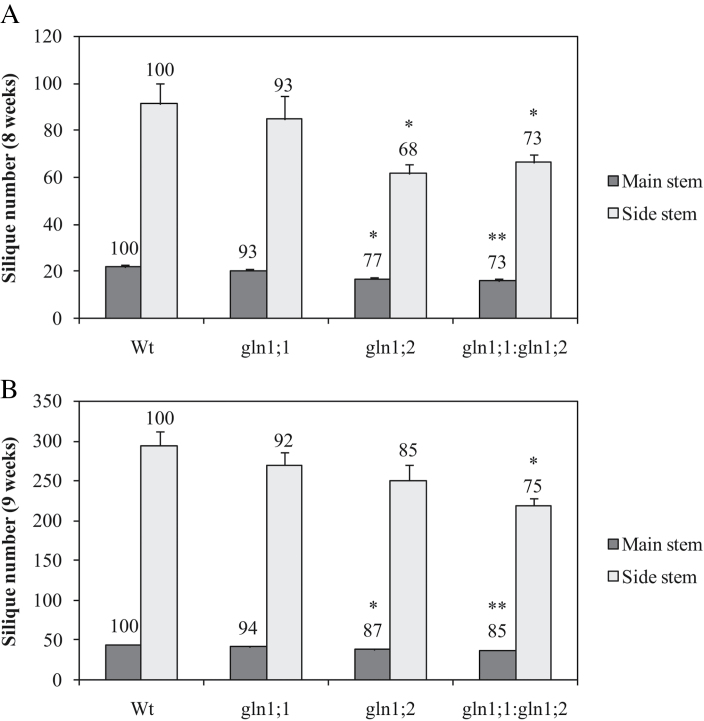
Both mutants and the Wt bolted after 7 weeks of growth and developed a main inflorescence stem, with side branches developing after 8 weeks (Fig. 4A). A significantly lower number of siliques was formed in gln1;2 and gln1;1:gln1;2 compared with the Wt (Figs 4A, 5A). The decrease in silique number was due to both a lower silique set on the main inflorescence stem (23% reduction for gln1;2 and 27% for gln1;1:gln1;2 relative to Wt) and a lower silique set on the side branches (32% and 27% reduction for gln1;2 and gln1;1:gln1;2, respectively; Fig. 5A). One week later, at 9 weeks, a significantly lower number of siliques on both the main inflorescence stem and the side branches was still observed in the gln1;1:gln1;2 double mutant (Fig. 5B).
Fig. 4.
Phenotype of Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2 in reproductive growth stages. (A) Images of gln1;1, gln1;2, gln1;1:gln1;2 and the wild-type (Wt) plants 8 weeks after germination. (B) Comparison of silique development in the Wt and mutant plants 10 d after flowering. From top to bottom: Wt, gln1;1, gln1;2, and gln1;1:gln1;2. (C) Comparison of length and width of mature seeds harvested from the 10-week-old wild-type and mutant plants. Values (μm) on the vertical and horizontal curly brackets are means ±SE (n=10) of seed length and width, respectively. Plants were grown in soil for 4 weeks under short-day conditions, then transferred to long-day conditions in order to induce flowering. Similar images were acquired from four individual plants. Scale bars=1200 μm.
Fig. 5.
Characterization of siliques from Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2. (A) Number of siliques per plant on the main stem and side branches after 8 weeks of growth. (B) Number of siliques per plant on the main stem and side branches after 9 weeks of growth. Plants were grown in soil for 4 weeks under short-day conditions, then transferred to long-day conditions in order to induce flowering. Results represent means ±SE (n=8). Asterisks indicate statistically significant differences between the lines, determined using Student’s t-test: *P<0.05; **P<0.01. Values above columns are expressed relative to the wild type set to 100.
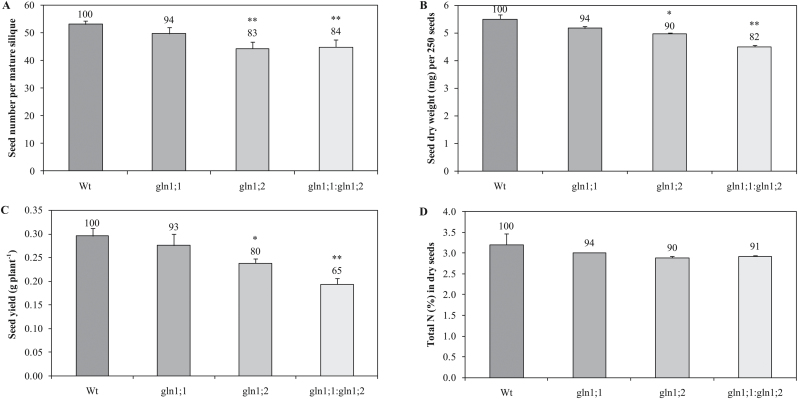
Shorter siliques were observed in gln1;2 and gln1;1:gln1;2 (Fig. 4B), and the size of the mature viable seeds of gln1;2 and gln1;1:gln1;2 was smaller relative to that of Wt seeds (Fig. 4C). The seed number per fully developed silique was determined in 10 mature siliques collected from the bottom part of the main inflorescence stem at 10 weeks. Both gln1;2 and gln1;1:gln1;2 had an ~17% (P=0.01) lower number of seeds per mature silique compared with the Wt (Fig. 6A). In addition, the dry weight of the individual seeds was reduced by 10% in gln1;2 (P=0.05) and by 18% in gln1;1:gln1;2 (P=0.01) (Fig. 6B). The lower seed number and seed dry weight resulted in 20% lower (P=0.05) seed yield in the single mutant gln1;2 and a 35% lower (P=0.01) seed yield in the double mutant gln1;1:gln1;2 (Fig. 6A–C). This clearly shows a crucial role for Gln1;2 with respect to the seed yield structure in Arabidopsis.
Fig. 6.
Characterization of mature seeds in Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2. (A) Seed number per mature silique 10 weeks after germination (n=10). (B) Dry weight (mg) of 250 seeds harvested 12 weeks after germination (n=4). (C) Total seed yield (g) per plant 12 weeks after germination (n=8). (D) Total N concentration (% dry weight) in dry seeds 12 weeks after germination (n=8). Plants were grown in soil for 4 weeks in short-day conditions, then transferred to long-day conditions in order to induce flowering. Results are means ±SE (n≥4). Asterisks indicate statistically significant differences between the lines, determined using Student’s t-test: *P<0.05; **P<0.01. Values above columns are expressed relative to the wild type set to 100. Two independent experiments with similar results were carried out.
Seed N concentration is unaffected in Arabidopsis mutants gln1;1, gln1;2, and gln1;1:gln1;2
The N concentration in the seed dry matter was determined for the mutants and the Wt (Fig. 6D). No significant differences were found between mutants and the Wt with respect to seed N concentration.
Gln1;1 and Gln1;2 are expressed in different cell types in Arabidopsis
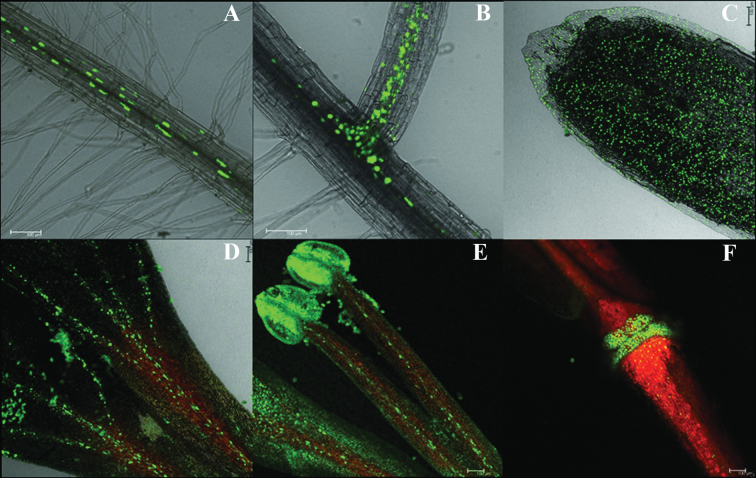
In order to investigate the localization of Gln1;2 expression, the ProGln1;2–NLS-GFP-GUS promoter–reporter construct was transformed into Arabidopsis. Confocal microscopy of the transgenic roots showed a strong GFP fluorescence in the endodermis of the mature root and in the vasculature of the basal region of lateral roots (Fig. 7A, B). In vegetative shoots, the expression of Gln1;2 occurred in mesophyll and vascular bundle cells (Guan, 2014). During reproductive growth, bright GFP signals were detected in the epidermal cells of sepals (Fig. 7C). Besides that, fluorescence from Gln1;2 expression was restricted to cells in the vascular bundle along the veins of petals (Fig. 7D), in the vascular bundle of stamens (Fig. 7E), and in nodes between the pedicel and the developing silique (Fig. 7F). This localization of Gln1;2 in vascular bundles of floral and reproductive organs agrees with a role for Gln1;2 in ammonium assimilation for seed production.
Fig. 7.
Localization of Gln1;2 in Arabidopsis expressing a ProGln1;2–NLS-GFP-GUS promoter–reporter construct. (A) Localization of Gln1;2 in the endodermis of the mature root. (B) Localization of Gln1;2 in the vasculature of the basal region of lateral roots. (C–F) Localization of Gln1;2 in floral and reproductive organs. (C) Sepal. (D) Petal. (E) Stamen. (F) The node between the pedicel and the developing silique. Root tissues for (A) and (B) were from 3-week-old plants germinated on 1/2 MS medium containing 1% sucrose. Floral and reproductive organs for (C–F) were from 6-week-old plants grown in soil. Similar images were acquired from four independent transgenic plants. Scale bars=100 μm.
The GFP signal driven by the Gln1;1 promoter was recorded in the epidermal cells of the root elongation zone (Supplementary Fig. S1 available at JXB online). This localization supports the observed role of Gln1;1 in controlling primary root development in interaction with external N availability during seedling early growth (Fig. 3C).
Discussion
Gln1;1 and Gln1;2 play specific roles in seed germination and seedling establishment in Arabidopsis
In Arabidopsis, >90% of seed N is incorporated into storage proteins (Baud et al., 2002; Li et al., 2006b ). When these storage proteins are degraded during germination (Hong et al., 2012), ammonium is produced and needs to be reassimilated into Gln for subsequent remobilization to support seedling growth (Rentsch et al., 2007). However, the specific roles of the individual GS1 isogenes with respect to N remobilization from seed storage proteins during germination have not been clarified. In the present study, it is reported that lack of Gln1;2 implies negative consequences for both seed germination (Fig. 3) and early seedling establishment (Fig. 2). Although the defect in seed germination of the gln1;2 mutant could be almost fully recovered by exogenous N supply (Fig. 3), early seedling establishment after 2 weeks of germination in soil with access to external N was nevertheless markedly reduced (Fig. 2). This illustrates that germination and remobilization of seed reserves are regulated independently (Pritchard et al., 2002; Fait et al., 2006) and, although primary roots are capable of absorbing external N during germination (Kircher and Schopfer, 2012), the transition between the use of seed and external resources may not be able to fully restore early growth. As shown here, Gln1;2 plays an essential role with respect to early seedling establishment by promoting N remobilization from seed reserves, and loss of this function cannot be fully compensated for by absorption of N from the surroundings.
In order to support seedling establishment, N absorbed from the soil must be assimilated into Gln in the roots, then transported to the shoots (Rentsch et al., 2007; Zhang et al., 2010). The GFP signal driven by the Gln1;1 promoter was recorded in the epidermal cells of the root elongation zone (Supplementary Fig. S1 at JXB online; see also Ishiyama et al., 2004b ). This localization suggests that Gln1;1 plays a role with respect to assimilation and sensing of external N. Root architecture is modulated in response to external N availability (Giehl et al., 2014; Ma et al., 2014) and also the provision of ammonium or nitrate may trigger lateral root branching and reduce the length of the primary root axis (Lima et al., 2010; Yan et al., 2014). The shorter primary roots developed by the gln1;1 mutant in response to application of external N (Fig. 3) suggest that Gln1;1 constitutes part of the signalling pathway mediating modifications of the root system in response to external N supply.
In the gln1;1:gln1;2 double mutant, a cumulative effect by knockout of both Gln1;1 and Gln1;2 was observed, which severely retarded both seed germination and seedling establishment (Figs 2, 3). The stunted phenotype reflects both impaired utilization of external N due to lack of Gln1;1 and decreased N remobilization from cotyledons due to lack of Gln1;2. When exposed to external N, the double mutant had 40–80% shorter primary roots than the Wt and the gln1;2 mutant (Fig. 3C). In contrast, in the absence of external N, the double mutant had the longest roots among the lines (Fig. 3C), suggesting a more pronounced N deficiency causing a relative stimulation of root extension (Fischer et al., 2013).
Gln1;2 plays an important role in seed yield structure
Uptake and assimilation of N during vegetative growth stages are critical processes for generation of the biomass infrastructure and phytohormonal signals which are required for initiation and maintenance of inflorescences and seed meristems (de Jong et al., 2014). Later on during reproductive growth stages, N in senescing leaves must be remobilized to ensure N for proper growth of the developing seeds (Diaz et al., 2008; Guiboileau et al., 2013). For efficient N remobilization to seeds, Gln is loaded into the phloem of the source organs and transported to the reproductive sinks (Taylor et al., 2010; Zhang et al., 2010). Direct evidence for specific roles for individual GS1 isogenes in seed yield structure and N remobilization in Arabidopsis has so far not been obtained.
Total seed yield in the gln1;2 single mutant and in the gln1;1:gln1;2 double mutant was significantly reduced compared with the Wt. The reduction in seed yield was due to a lower number of siliques per plant (Figs 4A, 5), a lower number of seeds per silique (Figs 4B, 6A), and lower weight per seed (Figs 4C, 6B). Thus, all three components of the seed yield structure were negatively affected by the knockout of Gln1;2, which is the main GS1 isoform in the shoot (Fig. 1B). Lothier et al. (2011) studied a different Atgln1;2 mutant and concluded that Gln1;2 was not essential for seed production or internal N distribution between vegetative and reproductive shoot components. However, the study by Lothier et al. (2011) was limited to a measurement of relative parameters only, such as the harvest index (seed weight as a proportion of total shoot weight) and the seed 15N partitioning (the ratio between seed 15N content and total plant 15N content). Ratios between yield parameters can be similar even though the absolute values are markedly different (Zheng et al., 2013). This was also the case in the present work where the proportion of 15N in different organs of gln1:2 and Wt plants at maturity was similar despite a significantly lower absolute quantity of 15N in the different plant parts of the mutant compared with the Wt (Supplementary Fig. S2 at JXB online). Much more N was thus handled by the Wt plants, reflecting the larger size of these plants (Fig. 4), but in relative terms gln1;2 did not affect the pattern of N distribution. This suggests that Gln1;2, as well as having an effect on the absolute quantity of N remobilized, also plays a role in establishment of the actual yield capacity by maintaining sufficient N flows during critical growth stages. In maize, ZmGln1;3 and ZmGln1;4 were shown to play specific roles in the development of the cob with respect to kernel number and kernel size, respectively (Martin et al., 2006; Cañas et al., 2010) suggesting a similar role for GS1 isogenes with respect to internal N signalling.
While seed size and weight were reduced in the Gln1;2-knockout mutants (Fig. 6B), seed N concentration was not affected (Fig. 6D). During seed filling, the rate of increase in seed weight following import of photoassimilates is normally higher than the rate of increase in seed N accumulation (Corby et al., 2011). However, in the Gln1;2 knockout mutants, the smaller seed size reduced the volume to be filled with N during seed growth, thus allowing the N concentration in the individual seeds to be sustained.
Using promoter–GFP constructs, a clear tissue- and cell type-specific localization of Gln1;2 expression was observed not only in the root vasculature and in the leaf veins (Fig. 7; Ishiyama et al., 2004b ; Lothier et al., 2011), but also in the epidermal cells of sepals, in the veins of petals and stamens, and in nodes between the pedicel and the developing silique (Fig. 7). This localization ties in with a role for Gln1;2 in N translocation and redistribution via the xylem and phloem, respectively (Martin et al., 2006; Bernard et al., 2008). In conjunction with, for example, NH4 + transporters in the stamen (Yuan et al., 2009), Gln1;2 may further specifically catalyse N transport to reproductive sinks such as pollen and siliques, thereby constituting a causal link with seed yield structure.
Conclusions
The glutamine synthetase isogene Gln1;2 plays an important role in N remobilization from reserves in germinating seeds and in development of seed yield components in Arabidopsis. The isogene Gln1;1 affects the response of primary root development to exogenous N provision during seed germination in Arabidopsis.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Localization of Gln1;1 in epidermis cells in the elongation zone of 3-week-old Arabidopsis roots.
Figure S2. 15N content in different tissues of gln1;2 and Arabidopsis wild type.
Acknowledgements
This work was supported by the China Scholarship Council [2009618037] and the Danish Council for Independent Research | Technology and Production Sciences (09-065893).
References
- Albinsky D, Kusano M, Higuchi M, et al. 2010. Metabolomic screening applied to rice FOX Arabidopsis lines leads to the identification of a gene-changing nitrogen metabolism. Molecular Plant 3, 125–142. [DOI] [PubMed] [Google Scholar]
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C. 2002. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiology and Biochemistry 40, 151–160. [Google Scholar]
- Bernard SM, Moller ALB, Dionisio G, et al. 2008. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Molecular Biology 67, 89–105. [DOI] [PubMed] [Google Scholar]
- Bussell JD, Keech O, Fenske R, Smith SM. 2013. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis. The Plant Journal 75, 578–591. [DOI] [PubMed] [Google Scholar]
- Cañas RA, Quillere I, Lea PJ, Hirel B. 2010. Analysis of amino acid metabolism in the ear of maize mutants deficient in two cytosolic glutamine synthetase isoenzymes highlights the importance of asparagine for nitrogen translocation within sink organs. Plant Biotechnology Journal 8, 966–978. [DOI] [PubMed] [Google Scholar]
- Chytilova E, Macas J, Galbraith DW. 1999. Green fluorescent protein targeted to the nucleus, a transgenic phenotype useful for studies in plant biology. Annals of Botany 83, 645–654. [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Corby HDL, Smith DL, Sprent JI. 2011. Size, structure and nitrogen content of seeds of Fabaceae in relation to nodulation. Botanical Journal of the Linnean Society 167, 251–280. [Google Scholar]
- Debouba M, Dguimi HM, Ghorbel M, Gouia H, Suzuki A. 2013. Expression pattern of genes encoding nitrate and ammonium assimilating enzymes in Arabidopsis thaliana exposed to short term NaCl stress. Journal of Plant Physiology 170, 155–160. [DOI] [PubMed] [Google Scholar]
- de Jong F, Thodey K, Lejay LV, Bevan MW. 2014. Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPORTER2.1 expression. Plant Physiology 164, 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz C, Lemaitre T, Christ A, Azzopardi M, Kato Y, Sato F, Morot-Gaudry JF, Le Dily F, Masclaux-Daubresse C. 2008. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiology 147, 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic M, Todorovic S, Bogdanovic M, Filipovic B, Misic D, Simonovic A. 2014. Knockout mutants as a tool to identify the subunit composition of Arabidopsis glutamine synthetase isoforms. Plant Physiology and Biochemistry 79, 1–9. [DOI] [PubMed] [Google Scholar]
- Engelsberger WR, Schulze WX. 2012. Nitrate and ammonium lead to distinct global dynamic phosphorylation patterns when resupplied to nitrogen-starved Arabidopsis seedlings. The Plant Journal 69, 978–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait A, Angelovici R, Less H, Ohad I, Urbanczyk-Wochniak E, Fernie AR, Galili G. 2006. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiology 142, 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JJ, Beatty PH, Good AG, Muench DG. 2013. Manipulation of microRNA expression to improve nitrogen use efficiency. Plant Science 210, 70–81. [DOI] [PubMed] [Google Scholar]
- Funayama K, Kojima S, Tabuchi-Kobayashi M, Sawa Y, Nakayama Y, Hayakawa T, Yamaya T. 2013. Cytosolic glutamine synthetase1;2 is responsible for the primary assimilation of ammonium in rice roots. Plant and Cell Physiology 54, 934–943. [DOI] [PubMed] [Google Scholar]
- Giehl RFH, Gruber BD, von Wiren N. 2014. Its time to make changes: modulation of root system architecture by nutrient signals. Journal of Experimental Botany 65, 769–778. [DOI] [PubMed] [Google Scholar]
- Guan M. 2014. Functions of glutamine synthetase isoforms in the nitrogen metabolism of plants . PhD thesis, University of Copenhagen. [Google Scholar]
- Guiboileau A, Avila-Ospina L, Yoshimoto K, Soulay F, Azzopardi M, Marmagne A, Lothier J, Masclaux-Daubresse C. 2013. Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. New Phytologist 199, 683–694. [DOI] [PubMed] [Google Scholar]
- Guiboileau A, Yoshimoto K, Soulay F, Bataille MP, Avice JC, Masclaux-Daubresse C. 2012. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytologist 194, 732–740. [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S. 2004. Transcriptome of Arabidopsis leaf senescence. Plant, Cell and Environment 27, 521–549. [Google Scholar]
- Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Moller I, White P. 2012. Functions of macronutrients. In: Marschner P, ed. Marschner’s mineral nutrition of higher plants, 3rd edn. Amsterdam: Elsevier, 135–189. [Google Scholar]
- Hirel B, Martin A, Terce-Laforgue T, Gonzalez-Moro MB, Estavillo JM. 2005. Physiology of maize I: a comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiologia Plantarum 124, 167–177. [Google Scholar]
- Hong YF, Ho THD, Wu CF, Ho SL, Yeh RH, Lu CA, Chen PW, Yu LC, Chao AL, Yu SM. 2012. Convergent starvation signals and hormone crosstalk in regulating nutrient mobilization upon germination in cereals. The Plant Cell 24, 2857–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama K, Inoue E, Tabuchi M, Yamaya T, Takahashi H. 2004. a Biochemical background and compartmentalized functions of cytosolic glutamine synthetase for active ammonium assimilation in rice roots. Plant and Cell Physiology 45, 1640–1647. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H. 2004b. Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. Journal of Biological Chemistry 279, 16598–16605. [DOI] [PubMed] [Google Scholar]
- Kichey T, Le Gouis J, Sangwan B, Hirel B, Dubois F. 2005. Changes in the cellular and subcellular localization of glutamine synthetase and glutamate dehydrogenase during flag leaf senescence in wheat (Triticum aestivum L.). Plant and Cell Physiology 46, 964–974. [DOI] [PubMed] [Google Scholar]
- Kircher S, Schopfer P. 2012. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109, 11217–11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Mery S, Meyer C, Daniel-Vedele F. 2014. Nitrate transport and signalling in Arabidopsis. Journal of Experimental Botany 65, 789–798. [DOI] [PubMed] [Google Scholar]
- Kusano M, Tabuchi M, Fukushima A, et al. 2011. Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. The Plant Journal 66, 456–466. [DOI] [PubMed] [Google Scholar]
- Li BH, Li GJ, Kronzucker HJ, Baluska F, Shi WM. 2014. Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends in Plant Science 19, 107–114. [DOI] [PubMed] [Google Scholar]
- Li RJ, Hua W, Lu YT. 2006a. Arabidopsis cytosolic glutamine synthetase AtGLN1;1 is a potential substrate of AtCRK3 involved in leaf senescence. Biochemical and Biophysical Research Communications 342, 119–126. [DOI] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J. 2006b. Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67, 904–915. [DOI] [PubMed] [Google Scholar]
- Lima JE, Kojima S, Takahashi H, von Wiren N. 2010. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. The Plant Cell 22, 3621–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limami AM, Rouillon C, Glevarec G, Gallais A, Hirel B. 2002. Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase. Plant Physiology 130: 1860–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothier J, Gaufichon L, Sormani R, et al. 2011. The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. Journal of Experimental Botany 62, 1375–1390. [DOI] [PubMed] [Google Scholar]
- Ma WY, Li JJ, Qu BY, He X, Zhao XQ, Li B, Fu XD, Tong YP. 2014. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. The Plant Journal 78, 70–79. [DOI] [PubMed] [Google Scholar]
- Martin A, Lee J, Kichey T, et al. 2006. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. The Plant Cell 18, 3252–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour-Eldin HH, Hansen BG, Norholm MHH, Jensen JK, Halkier BA. 2006. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Research 34, e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M, Moison M, Clouet V, Thomas J, Leprince F, Canoy AS, Just J, Chalhoub B, Masclaux-Daubresse C. 2014. Sixteen cytosolic glutamine synthetase genes identified in the Brassica napus L. genome are differentially regulated depending on nitrogen regimes and leaf senescence. Journal of Experimental Botany 65, 3927–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Yanofsky MF. 2004. Establishing gene function by mutagenesis in Arabidopsis thaliana. The Plant Journal 39, 682–696. [DOI] [PubMed] [Google Scholar]
- Pritchard SL, Charlton WL, Baker A, Graham IA. 2002. Germination and storage reserve mobilization are regulated independently in Arabidopsis. The Plant Journal 31, 639–647. [DOI] [PubMed] [Google Scholar]
- Rentsch D, Schmidt S, Tegeder M. 2007. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Letters 581, 2281–2289. [DOI] [PubMed] [Google Scholar]
- Swarbreck SM, Defoin-Platel M, Hindle M, Saqi M, Habash DZ. 2011. New perspectives on glutamine synthetase in grasses. Journal of Experimental Botany 62, 1511–1522. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Sugiyama K, Ishiyama K, Inoue E, Sato T, Takahashi H, Yamaya T. 2005. Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. The Plant Journal 42, 641–651. [DOI] [PubMed] [Google Scholar]
- Taylor L, Nunes-Nesi A, Parsley K, Leiss A, Leach G, Coates S, Wingler A, Fernie AR, Hibberd JM. 2010. Cytosolic pyruvate,orthophosphate dikinase functions in nitrogen remobilization during leaf senescence and limits individual seed growth and nitrogen content. The Plant Journal 62, 641–652. [DOI] [PubMed] [Google Scholar]
- Thomsen HC, Eriksson D, Møller IS, Schjoerring JK. 2014. Cytosolic glutamine synthetase: a target for improvement of crop nitrogen use efficiency? Trends in Plant Science (in press). [DOI] [PubMed] [Google Scholar]
- Yan YS, Wang HC, Hamera S, Chen XY, Fang RX. 2014. miR444a has multiple functions in the rice nitrate-signaling pathway. The Plant Journal 78, 44–55. [DOI] [PubMed] [Google Scholar]
- Yuan LX, Graff L, Loque D, Kojima S, Tsuchiya YN, Takahashi H, von Wiren N. 2009. AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant and Cell Physiology 50, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LX, Gu RL, Xuan YH, Smith-Valle E, Loque D, Frommer WB, von Wiren N. 2013. Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo . The Plant Cell 25, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LZ, Tan QM, Lee R, Trethewy A, Lee YH, Tegeder M. 2010. Altered xylem–phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. The Plant Cell 22, 3603–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FX, Wang XK, Zhang WW, Hou PQ, Lu F, Du KM, Sun ZF. 2013. Effects of elevated O-3 exposure on nutrient elements and quality of winter wheat and rice grain in Yangtze River Delta, China. Environmental Pollution 179, 19–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.