Summary
Highlight: A cell membrane-anchored protein governs ovary cell division and determines berry size mediated by a MADS-domain protein, suggesting a molecular link between ovary identity and growth in plants.
Key words: Berry size, cell cycle, fruit development, natural variation, Physalis, seed
Abstract
Physalis species show a significant variation in berry size; however, the underlying molecular basis is unknown. In this work, we showed that cell division difference in the ovaries might contribute to the ultimate berry size variation within Physalis species, and that mRNA abundance of Physalis floridana Cell Number Regulator1 (PfCNR1), the putative orthologue of the tomato fruit weight 2.2 (FW2.2), was negatively correlated with cell division in the ovaries. Moreover, heterochronic expression variation of the PfCNR1 genes in the ovaries concomitantly correlated with berry weight variation within Physalis species. In transgenic Physalis, multiple organ sizes could be negatively controlled by altering PfCNR1 levels, and cell division instead of cell expansion was primarily affected. PfCNR1 was shown to be anchored in the plasma membrane and to interact with PfAG2 (an AGAMOUS-like protein determining ovary identity). The expression of PfCYCD2;1, a putative orthologue of the mitosis-specific gene CyclinD2;1 in the cell cycle was negatively correlated with the PfCNR1 mRNA levels. PfAG2 was found to selectively bind to the CArG-box in the PfCYCD2;1 promoter and to repress PfCYCD2;1 expression, thus suggesting a PfAG2-mediated pathway for PfCNR1 to regulate cell division. The interaction of PfCNR1 with PfAG2 enhanced the repression of PfCYCD2;1 expression. The nuclear import of PfAG2 was essential in the proposed pathway. Our data provide new insights into the developmental pathways of a cell membrane-anchored protein that modulates cell division and governs organ size determination. This study also sheds light on the link between organ identity and organ growth in plants.
Introduction
The genetic mechanism underlying natural variations in plant organ size between species and within species has been elusive. Fruit size is an important agronomic trait and is the prime criteria for domestication. Developmental comparisons have suggested that, among equivalent (orthologous) organs from plant species of different size, larger organs result mainly from increased cell number rather than from larger cells (Mizukami, 2001). The Solanaceae family is a source of nutrition and culinary diversity for human populations. Five of the leading economic species in this family are potato (Solanum tuberosum), tomato (Solanum lycopersicum), eggplant (Solanum melongena), pepper (Capsicum spp.), and husk tomato (Physalis spp.). A number of key loci controlling fruit size and a subset of genes underlying these loci have been studied in this plant family, such as Fruit weight2.2 (FW2.2), fasciated (FAS), and locule number (LC) (Frary et al., 2000; Cong et al., 2008; Muños et al., 2011).
FW2.2 is the first quantitative trait locus (QTL) cloned in plants. The mutation in FW2.2 is supposed to be the first step in the domestication of larger tomato fruit, and FW2.2 alone controls up to 30% of fruit weight variation (Frary et al., 2000). FW2.2 encodes a repressor of cell division, and this function was fulfilled by negatively regulating expression of this gene, rather than via changes in protein structure (Frary et al., 2000; Cong et al., 2002). FW2.2-like genes have also been studied in several other species. In maize, the putative orthologue of tomato FW2.2 is cell number regulator1 (CNR1), which affects entire plant and multi-organ size by negatively regulating cell number (Guo et al., 2010). The avocado (Persea americana) fruit weight2.2-like (Pafw2.2-like) gene has been proposed to play a conserved role as a negative regulator of fruit cell division (Dahan et al., 2010). In soybean (Glycine max), fruit weight2.2-like1 (GmFWL1) is a homologue of the tomato FW2.2 gene and was found to be essential for soybean nodule organogenesis as a result of effects on plant cell division (Libault et al., 2010). In rice (Oryza sativa), fruit weight2.2 like3 (Osfwl3) controls the grain weight by negatively regulating cell division (Xu et al., 2013). Prunus avium cell number regulator (PavCNR), which is a homologue of the tomato FW2.2, is a cell number regulator gene in Prunus species and associates with fruit size in sweet and sour cherry (Franceschi et al., 2013). FW2.2-like genes play a conservative role in cell division in different species (Guo and Simmons, 2011; van der Knaap et al., 2014). However, they have been found to encode cell membrane-anchored proteins (Cong and Tanksley, 2006; Libault et al., 2010; Xu et al., 2013), and how they regulate cell division is an intriguing question. Little is known about the molecular and biochemical role of these proteins. FW2.2-like proteins were found to facilitate iron transport (Song et al., 2004; Nakagawa et al., 2007), but the role of iron change in regulating cell division is not established yet. Further yeast two-hybrid screens using FW2.2 as baits revealed that the encoded protein interacts with the regulatory subunit of casein kinase II (CKII) (Cong and Tanksley, 2006), a protein with broad activity that includes the control of cell division (Pepperkok et al., 1994; Espunya et al., 1999; Moreno-Romero et al., 2008), providing a step forward to understand the role of FW2.2 in cell division. However, details of the developmental pathway of FW2.2-like proteins that regulate cell division are largely unknown.
The genus Physalis has more than 70 species and has become a new model to study the evolution and developmental control of morphological novelty (He et al., 2004; He and Saedler, 2005; Wang et al., 2012; Zhao et al., 2013) because a Physalis fruit features a distinct fruiting clayx called ‘inflated calyx syndrome’ (ICS) or the ‘Chinese lantern’. However, study of the developmental and molecular control of Physalis berry size has long been neglected. Physalis species have a rich diversity in berry size (Fig. 1), and a few Physalis species have been cultivated for the production of the berries, for example Physalis philadelphica (Montes Hernández and Aguirre Rivera, 1994). Most of the species are diploid (2n=24), except for Physalis peruviana, which is tetraploid (Sinha, 1951; He and Saedler, 2005; Wang et al., 2012). The berry size seems to be uncoupled with the polyploidy level within Physalis species, but expression variation of several genes during flower and berry development might contribute to the berry size variation (Wang et al., 2012). In the present study, we showed that Physalis floridana Cell Number Regulator1 (PfCNR1), a putative orthologue of the tomato FW2.2, encodes a cell membrane-anchored protein and functions as a negative regulator of cell division. We further showed that PfCNR1 is involved in the cell division cycle through molecular interactions of PfCNR1 with P. floridana AG2 (PfAG2, an AGAMOUS-like MADS-domain regulatory protein) and of PfAG2 with P. floridana CyclinD2;1 (PfCYCD2;1, a putative CyclinD2;1 gene that encodes a key component at the G1/S phase in the cell cycle), thus directing cell division and contributing to natural variation of berry size within the Physalis species. Our work may also provide a crucial mechanistic link between organ patterning and growth.
Fig. 1.
Organ size variation in Physalis species. (A) Mature berry in Physalis. Bar, 1cm. From left to right: P. philadelphica (P058), P. philadelphica (P064), and P. floridana (P106). The calyces were removed. (B) Size variation of flowers (blue), berries (red) and seeds (green) in 16 Physalis species. One accession for 15 species and two accessions of P. philadelphica (P058 and P064) were included and their information is shown in Supplementary Table S1 at JXB online. The dashed linkage of each accession is for clarity only. The mean and standard deviation are presented.
Materials and methods
Plant materials
The Physalis resources (Supplementary Table S1) were grown in a greenhouse at the Institute of Botany, Beijing, China. The stage of the mature flower was set as d 0. Flower buds at 9 (B1), 6 (B2), and 3 (B3) d before anthesis and mature flowers (F), as well as 5, 10, 15, 20, 25, 30, and 50 day post-anthesis (DPA) fruits and leaves, and seeds from 15 and 30 DPA fruits of P. floridana (P106), P. philadelphica (P064) and P. philadelphica (P058) were collected. The B2 flower buds of all species/accessions were harvested for quantitative transcript analysis.
Trait quantification of Physalis species
Three plants for each Physalis species/accession were transplanted into the experimental field in the summers of 2009, 2010, and 2012. The size of the mature flower was represented by the length between the receptacle and the tip of mature flowers. Mature berry size was defined by the weight, and the 100-seed weight was measured. For each plant/accession, the size of 15–60 mature flowers and 10–100 mature berries was quantified. Some accessions/species did not grow well in all 3 years, and the traits of these accessions were quantified based on 1- or 2-year observations. The seed weight was measured using three independent batches of 100 seeds for each species.
Cell number and size measurement
Ovaries from flower buds (B1, B2, and B3), mature flowers (F), and 5 DPA and 50 DPA berries (berries were cut longitudinally into eight equal parts and the seeds were stripped out) and the seeds of 50 DPA berries were fixed with 3.7% formaldehyde, 5% glacial acetic acid and 50% ethanol (FAA) for 48h at room temperature. The median transverse section area and cell size were determined by AxioVision Rel. 4.7 and cell number was counted manually as described previous (Frary et al., 2000; Cong et al., 2002). Five median transverse sections for each stage sample were analysed. The cell size of 150 cells for each tissue on each section was measured.
Quantitative transcript analyses
Total RNAs were isolated with a Plant Total RNA Extraction kit (Bioteke) and treated with RNase-free DNase I (Promega). First-strand cDNA was synthesized by reverse transcription with oligo(dT)17 using SuperScript® III Reverse Transcriptase (Invitrogen). The transcript abundance of genes was quantified using quantitative reverse transcription (qRT)-PCR via the SYBR® Premix Ex Taq™ kit (Takara). PfACTIN was used as an endogenous control. All PCR reactions were run on a Mx3000PTM Real Time PCR thermal cycler using the gene-specific primers (Supplementary Table S2 at JXB online).
RNA in situ hybridization
Floral buds B1, B2, and B3 as well as the ovaries from P106, P058, and P064 were fixed with FAA, dehydrated in a graded ethanol series, and embedded in Paraplast (Sigma). To generate probes for hybridization, a 269bp PfCNR1 cDNA fragment was designed as a template. Probes were synthesized using T7 RNA polymerase driven by a T7 promoter and were labelled with digoxigenin using a DIG-RNA labelling kit (Roche). Hybridization was performed as described previously (Carr and Irish, 1997), except that slides were washed in 2× SSC, 1× SSC, and then 0.5× SSC at 50 °C for 30min, respectively.
Yeast two-hybrid assays
Total RNA mixture from stems, leaves, flower buds (different developmental stages), mature flowers, and young fruits (with calyx) of Physalis species was used to construct a cDNA library (He et al., 2007). The baits PfCNR1, PfCNR11–42, and PfCNR1103–175 were transformed into Saccharomyces cerevisiae Y187 and used to screen the pGADT7-cDNA library. The yeast library system and procedure were similar to a previous description (Cong and Tanksley, 2006). To confirm the protein–protein interaction, the open reading frames (ORFs) of PfCNR1 and its putative interacting proteins were respectively inserted into pGBKT7 and pGADT7. The selective medium, detailed information of the Physalis library, screen procedures, yeast co-transformation, and the non-lethal galactosidase activity assays as described previously (He et al., 2007).
Yeast one-hybrid assays
The involved DNA fragments were respectively cloned into the pAbAi vector. pGADT7-PfAG2 and pGADT7-PfSEP1 were respectively transformed into the Y1HGold yeast strain that contained each derived pAbAi construct. Protein and DNA interaction was revealed by yeast cell survival on SD/–Leu medium supplemented with 200ng ml–1 of aureobasidin A (AbA). The yeast one-hybrid assay was performed following the manual of the Matchmaker Gold Yeast One-Hybrid System (Clontech).
Protein transient expression assays in plant cells
For subcellular localization, the ORFs of the involved genes were cloned into the expression vector Super1300 (Chen et al., 2009) and fused with the green fluorescence protein (GFP) gene. These derived constructs were transformed into Agrobacterium tumefaciens LBA4404, and the leaf epidermal cells of Nicotiana benthamiana were infected via Agrobacterium infiltration. For plasmolysis, 0.3g ml–1 of sucrose was injected into tobacco leaves expressing PfCNR1–GFP prior to observation. The detection of GFP indicated the subcellular localization of the protein we were interested in. For bimolecular fluorescence complementation (BiFC) assays, the ORFs of the involved genes were cloned into the pair of vectors pSPYNE-35S and pSPYCE-35S (Walter et al., 2004), which contained the N-terminal half (YFPn) and the C-terminal half (YFPc), respectively, of yellow florescence protein (YFP). These derived constructs were transformed into A. tumefaciens GV3101, and the construct recombination was injected into leaf epidermal cells of N. benthamiana via Agrobacterium infiltration. The detection of YFP signal indicated that the two proteins interacted in plant cells. The GFP or YFP signal was detected 48h after injection under a confocal laser-scanning microscope (Olympus FV1000MPE). Each portion of PfCNR1, combinations of these portions, and the deletion mutations of PfCNR1 and PfAG2 were introduced using a PCR approach.
Transient luciferase (LUC) activity assays in P. floridana protoplasts
To produce the LUC reporter gene constructs, the indicated fragments were ligated into the YY96 vector (Yamamoto and Deng, 1998). The ORF of PfCNR1, PfAG2, PfAG2m (the encoded nuclear localization signal was deleted), PfSEP1, and PfCKIIβ1 was ligated into the pGFP221 vector (Han et al., 2005). Protoplasts of P. floridana (wild type, 35S:PfCNR1 OE9, and 35S:PfCNR1-RNAi R9) were prepared from young leaves, and transient expression assays were performed as described previously (Yamamoto and Deng, 1998). Each obtained reporter plasmid as well as the 35S:GUS- and/or pGFP221-derived constructs indicated were co-transformed into protoplasts, which were pelleted and resuspended in cell culture lysis reagent (Promega). The β-glucuronidase (GUS) fluorescence was measured using a Modulus luminometer/fluorometer with a UV fluorescence optical kit, and the LUC activity was detected with a luminescence kit (Promega). The relative LUC expression was expressed as the LUC/GUS ratio.
Expression vector construction and P. floridana transformation
A double-stranded RNA interference (RNAi) construct was assembled by introducing a 229bp fragment of PfFCNR1 cDNA into the pFGC1008 vector (He and Saedler, 2005). For overexpression, the ORF of PfCNR1 cDNA was cloned into the vector pRT100, and the 35S:PfCNR1 cut from the derived pRT100 was introduced into the pBAR-A vector (He and Saedler, 2005). All transgenic analyses were performed in P. floridana P106. Transformation was performed as described previously (He and Saedler, 2005). One of the plants derived from independent explants was kept. Nine independent transgenic lines for both RNAi and overexpression were created and analysed. The genotypes of the T2 transgenic Physalis lines were verified by qRT-PCR and semi-quantitative RT-PCR. In the virus-induced gene silencing (VIGS) analysis, the fragments of PfCNR1, PfCNR1L1, and PfCNR1L2 were introduced into the tobacco rattle virus system, which was then applied to the leaves of 2-week-old Physalis seedlings (Zhang et al., 2014). Half-flowers (halves of sepal, petal, and stamen) were collected for qRT-PCR, and the carpels were kept intact and labelled for berry weight determination. Half-flowers of the wild type were included as controls.
Phenotyping the transgenic P. floridana
Phenotypic variation of the T2 transgenic Physalis plants was analysed in comparison with the wild type. Five seeds of each T1 transgenic line were germinated, and at least three transgenic siblings of each transgenic line were included for phenotypic proofs. The segregating wild-type-like siblings of some transgenic lines were also analysed. The area of the sixth mature leaf (above the cotyledons) was measured. Flower length was recorded as the length between the receptacle and the petal tip. ICS area and weight of ovary, mature berry, and 100 seeds were measured. Epidermal cells of leaves, sepals, and ICS were imaged using scanning electron microscopy. Ovaries from mature flowers and mature berries of transgenic plants were made into paraffin sections for evaluating cell division and cell expansion in comparison with those of the wild type.
Statistical analyses
The correlation coefficient (r), a t-test, and the associations of three quantitative traits (berry weight, 100-seed weight, and flower length) with the variation in amino acid sites and with the variation in gene expression were analysed using SPSS 15.0.
Sequencing analyses
Using tomato FW2.2 to search the Tomato Genome Sequencing Project (http://mips.helmholtz-muenchen.de/plant/tomato/index.jsp) and the NCBI database (http://www.ncbi.nlm.nih.gov/), we identified another two sequences that were closely related to FW2.2 homologous genes (FW2.2L1 and FW2.2L2). Their orthologous cDNAs (PfCNR1, PfCNR1L1, and PfCNR1L2) in P106 were isolated using a 5’ and 3’ Rapid Amplification of cDNA Ends kit (Roche). Total DNA from the leaves of P106, P058, and P064 was extracted using a Plant Genomic DNA kit (Tiangen). Genomic sequences were isolated using rapid amplification of genomic DNA ends according to a Universal Genome Walker kit (Clontech). All primers used in the present work are shown in Supplementary Table S2. The phylogenetic trees were reconstructed under default parameters using the MEGA5 tool (Tamura et al., 2011). The transmembrane domain of PfCNR1 was predicted using the TopPred program online (http://www.sbc.su.se/~erikw/toppred2/). The nuclear localization signal (NLS) of PfAG2 was predicted by the cNLS Mapper (http://nls-mapper.iab.keio.ac.jp/). Sequence data from this study have been deposited in GenBank under accession numbers KJ155732–KJ155748.
Results
Berry size variation within the Physalis species
We collected 16 Physalis species (Supplementary Table S1). Considerable variations in the berry size among these Physalis species were observed, as seen in P058 (P. philadelphica), P064 (P. philadelphica), and P106 (P. floridana), which produced berries with different sizes (Fig. 1A). The berry weight (designated berry size) varied from 0.86±0.30g in P. floridana P106 to 11.0±0.60g in P. philadelphica P058 within the 16 Physalis species (Fig. 1B). The flower length and seed weight also varied significantly among these Physalis species (Fig. 1B). The flower length was recorded by the length between the receptacle and the tip of mature flowers. We observed that flower length varied from 7.5±0.2mm in P. philadelphica P064 to 20.4±0.5mm in P. Mexicana P038. The 100-seed weight was also measured for each species and ranged from 34±0.1mg in P. floridana P106 to 230±1.0mg in P. philadelphica P058. Unlike the observations in P. philadelphica (Wang et al., 2012), a correlation between flower length and berry weight was not observed among these Physalis species (r=0.05, P=0.84), nor between flower length and 100-seed weight (r=0.38, P=0.13). Interestingly, a positive correlation between berry weight and 100-seed weight was seen (r=0.88, P<0.01). Thus, among these traits, berry size is the prime breeding target for Physalis crops.
Dynamics of cell activity during Physalis berry development
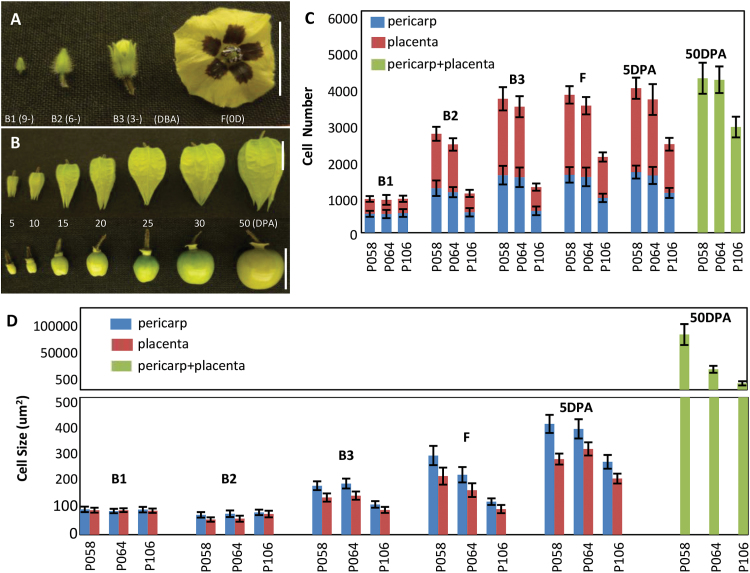
The berry expands from the ovary in response to fertilization. Cell division and cell expansion are believed to be responsible for the growth of the berry. To reveal any potential differences in these cellular levels among Physalis species, we decided to evaluate the cell division and cell expansion in ovary and developing berries. The mature flower (F) stage was set as d 0, and the developmental stages of a Physalis ovary and berry included 9 (B1), 6 (B2), and 3 (B3) d before anthesis, and 5, 10, 15, 20, 25, 30 and 50 DPA, which were exemplified by P. floridana (Fig. 2A, B). Median transverse sections of developing ovaries and berries were subjected to further evaluations. We found that the ovary size and the increase in the flesh (pericarp and placenta) size in developing berries might largely determine the mature berry size (Supplementary Fig. S1A, B, at JXB online). This was corroborated by the fact that cell division in the ovaries from floral buds (B1, B2, and B3) to mature flowers (F) was more active than that in the flesh of 5 and 50 DPA berries; however, cell division in the ovules and the developing seeds increased rapidly as the seeds developed (Fig. 2C; Supplementary Table S3 at JXB online). The different cell expansion from the B2 ovary stage to mature berries in the Physalis species might explain the variation in mature berry size, whereas different cell expansion in the fertilized ovules might explain the seed weight (Fig. 2D; Supplementary Table S4 at JXB online).
Fig. 2.
Berry development in Physalis species. (A) Flower development. The developmental stage of the mature flower (F) was set as d 0, and the floral buds at 9 (B1), 6 (B2), and 3 (B3) d before anthesis (DBA) are presented. (B) Fruit development. The developing berries of 5, 10, 15, 20, 25, 30, and 50 DPA are presented. The top images are intact fruits, and the bottom images represent the berries in which the inflated calyx was removed. The developmental stages in P. philadelphica were similarly defined for harvesting of tissues. Bars, 1cm. (C) Cell number variation. (D) Cell size variation. Quantification was performed according to median transverse section of the developing carpel and fruit from the floral buds (B1, B2, and B3), mature flower (F) and the developing berries of 5 and 50 DPA (see Materials and Methods for details). Five median transverse sections for each stage sample were analysed. The cell size of 150 cells for each tissue on each section was measured. The cell number for each section was counted. The mean and standard deviation are presented. The details are presented in Tables S3 and S4.
Comparison analyses among Physalis species (Fig. 2C, D, and Supplementary Tables S3 and S4) suggested that the cell division was undistinguished in different accessions (P058 and P064) of P. philadelphica, and thus berry size variation in the species was due to the difference in cell expansion originating in the B2 ovaries. Nonetheless, cell division activity in the ovaries was significantly different prior to anthesis in P. philadelphica (P058 and P064) and P. floridana (P106), partly contributing to berry size variation.
Characterization of the PfCNR1 gene family in Physalis species
FW2.2 controls tomato size mainly by regulating carpel cell division (Frary et al., 2000). We therefore focused on characterizing the homologous genes in Physalis species. Three cDNAs were isolated from P. floridana and designated as the PfCNR1 family. PfCNR1 encoded a protein that shared the highest identity (80%) with FW2.2, while the other two, which were designated as PfCNR1-like 1 (PfCNR1L1) and PfCNR1L2, had 52 and 50% identity, respectively, with PfCNR1. The two additional FW2.2 homologous genes, FW2.2L1 and FW2.2L2, were also found in the tomato genome. The orthology between PfCNR1 and FW2.2 was supported by phylogenetic analyses of the gene family (Supplementary Fig. S2 at JXB online) and local genomic microsynteny analyses (Fig. 3A). We assembled the genomic fragments harbouring the putative genes ORF38, PfCNR1, and mdtK through genomic walking. These were conserved among P106, P064, P058, and tomato. Considering the role of FW2.2 in tomato (Frary et al., 2000; Cong et al., 2002; Liu et al., 2003), we focused our investigation on the role of PfCNR1 in Physalis species. The two close parologues, PfCNR1L1 and PfCNR1L2, were used as controls in some cases.
Fig. 3.
Characterizations of the PfCNR1 gene family. (A) Local synteny of FW2.2 and PfCNR1 between tomato and Physalis species. PfCNR1 genes were isolated from P. philadelphica (P058), P. philadelphica (P064), and P. floridana (P106). The upstream ORF38 of FW2.2 (PfCNR1) encodes an unknown protein, while the downstream gene is mdtK. The full length of each assembled genomic fragment is given in parenthesis. (B) PfCNR1 expression in four floral whorls at anthesis. P. philadelphica (P058), P. philadelphica (P064), and P. floridana (P106) were tested. (C) PfCNR1 expression in Physalis species. Developmental stages were defined as in Fig. 2A and B. 15S, seeds from 15 DPA; 30S, seed from 30 DPA; L, leaf. PfACTIN was used as an internal control. The experiments were performed using three independent biological samples. The mean and standard deviation are presented. (D–i) In situ hybridization of PfCNR1 in P. floridana P106: B1 flower bud (D); B2 flower bud (E); carpel of the B2 flower bud (F); B3 flower bud (G); carpel of the mature flower (H); 5 DPA fruit (I). (J–M) In situ hybridization of PfCNR1 in P. philadelphica P058 and P064: B2 flower bud of P058 (J); B2 flower bud of P064 (K); carpel of the mature flower of P058 (L); carpel of the mature flower of P064 (M). A PfCNR1 anti-mRNA probe was used in (D)–(M). (N) In situ hybridization of PfCNR1 in a B2 flower bud of P. floridana P106 using a PfCNR1 sense probe as a negative control. Bars, 100 μm. Developmental stages were identical as defined in Fig. 2A and B.
Expression of PfCNR1 genes during flower/fruit development
We next investigated the mRNA accumulation pattern of PfCNR1 during flower and fruit development, and found that PfCNR1 was differentially expressed in the four whorls of Physalis flowers, and that it was relatively highly expressed in calyx and carpel before anthesis (Fig. 3B). In the three Physalis accessions, PfCNR1 had two peaks during flower and berry development. The first peak occurred before fertilization, while the second peak appeared in the 30 DPA berries (Fig. 3C). Unlike the second expression peak, which occurred simultaneously in different species, the first PfCNR1 expression peak occurred in the B2 stage in P. floridana (P106) with smaller berries. However, PfCNR1 heterochronically peaked in the mature flowers of P. philadelphica (P058 and P064) that produced bigger berries (Fig. 3C). The appearance of the PfCNR1 expression peak before anthesis occurred concomitantly with the repression of cell division activity (Fig. 3C; Supplementary Table S3). Moreover, PfCNR1 expression levels at the B2 stage showed a negative correlation to cell division in the ovaries, while the expression of PfCNR1L1 and PfCNR1L2 did not correlate with the cell division activity (Supplementary Fig. S3 and Supplementary Table S5 at JXB online). The second PfCNR1 expression peak was expressed mainly in the seeds (Fig. 3C). Thus, the PfCNR1 heterochronic expression and mRNA accumulation prior to anthesis were associated with berry development.
We then conducted in situ hybridization to detect the cellular distribution of PfCNR1 mRNA in the carpel. Consistent with the findings of qRT-PCR analyses (Fig. 3C), the reproducible PfCNR1 expression signals were only detected in B2 flower buds in P106 (Fig. 3D–I). The strongest PfCNR1 expression signals were visible in the ovules and the regions proximal to ovules in the placenta (Fig. 3E, F). The in situ PfCNR1 expression signals in P058 and P064 were not detectable in the floral organs of similar developmental stages as P. floridana P106 (Fig. 3J–M) due to the extremely weak expression of this gene in P. philadelphica (P058 and P064) as shown in qRT-PCR analyses (Fig. 3C). The PfCNR1 sense probe did not produce any signal in the B2 floral buds of P106 (Fig. 3N).
Therefore, differential expression of PfCNR1 seems to be important for organ size variation. To get further clues for this, we investigated the expression of the PfCNR1 alleles in our collected Physalis species using a qRT-PCR approach. We found that, among the 16 Physalis species, PfCNR1 transcript levels in B2 flower buds were negatively correlated with both mature berry weight (r=–0.67, P<0.01) and 100-seed weight (r=–0.78, P<0.01) (Supplementary Table S6 at JXB online). We also sequenced the PfCNR1 cDNA from the 16 Physalis species, and found that the encoded PfCNR1 proteins were highly conserved with nine polymorphic sites, but no significant association (P>0.05) was observed between sequence variations and the three quantitative traits (berry weight, 100-seed weight, and flower length) (Supplementary Table S6). All these findings suggested that altering expression of PfCNR1 is probably involved in the variation of the fruit size. To understand better the developmental role of this gene, molecular pathways associated with PfCNR1 were dissected in P. floridana, which is a well-established model of the genus Physalis (Zhang et al., 2014).
PfCNR1 is involved in the control of Physalis berry size
VIGS analyses were first performed in P. floridana to quickly obtain insight into the function of these genes. Half-flowers, which included halves of calyx, corolla, and stamens, were used for studying gene expression, while the carpels were kept intact and labelled for berry size determination. Altogether, 62, 56, and 60 labelled berries were finally harvested from the VIGS plants of PfCNR1, PfCNR1L1, and PfCNR1L2, respectively. The berry size of the PfCNR1-VIGS population ranged from 0.71 to 1.31g, and the PfCNR1 mRNA showed a significant negative correlation with the berry size (r=–0.52, P<0.001) (Supplementary Fig. S4A at JXB online). The berry size of the PfCNR1L1-VIGS and PfCNR1L2-VIGS populations varied from 0.74 to 1.20g and from 0.72 to 1.22g, respectively. Downregulation of either PfCNR1L1 (r=–0.07, P>0.05) or PfCNR1L2 (r=–0.09, P>0.05) had no effect on berry size (Supplementary Fig. S4B, C). Therefore, our observations in these transient analyses suggested that PfCNR1 downregulation specifically increased the berry size in Physalis species. We further analysed the consequence of the stable ‘altered PfCNR1 expressions’ in transgenic P. floridana plants.
PfCNR1 negatively controls cell proliferation and thus organ size in P. floridana
We created nine lines of transgenic P. floridana from independent explants where PfCNR1 expression was downregulated via RNAi (Fig. 4A). Multiple organ sizes, including leaves, floral organs, berries, and seeds, were significantly increased in comparison with the wild type (Fig. 4C–G; Supplementary Fig. S5 and Supplementary Table S7 at JXB online). A negative correlation was found between PfCNR1 transcript levels and organ sizes; nonetheless, seed number per berry, total fruit yield, and plant weight (fresh weight of the above-ground plant at harvest) were indistinguishable (P>0.05) between transgenic and wild-type plants (Supplementary Table S7). Thus, PfCNR1 is a negative regulator of organ size (weight) without altering the total biomass or yield.
Fig. 4.
Altering PfCNR1 expression in P. floridana using transgenic approaches. (A) Genotyping PfCNR1 RNAi transgenic plants. The expression of the three genes (PfCNR1, PfCNR1L1, and PfCNR1L2) in the PfCNR1 family was investigated among the wild type (WT) and nine lines of 35S:PfCNR1-RNAi designated R1–R9. PfACTIN was used as an internal control. Three independent biological samples of each line were subjected to qRT-PCR. The mean and standard deviation are presented. (B) Genotyping of PfCNR1 overexpression transgenic plants. The expression of three genes (PfCNR1, PfCNR1L1, and PfCNR1L2) in the PfCNR1 family was investigated among the wild type (WT) and nine lines of 35S:PfCNR1, designated OE1–OE9. PfACTIN was used as a loading control. Three independent biological samples of each line were subjected to RT-PCR, and a typical gel image is presented. (C–F) Comparison of organs among wild type (WT), 35S:PfCNR1 (OE9), and 35S:PfCNR1-RNAi (R9). (C) flowers of the wild-type (WT), PfCNR1 overexpressor (OE9) and RNAi plant (R9). Bar 1mm. (D) Carpels of WT, OE9, and R9. Bar, 1mm. (E) Mature fruit of WT, OE9, and R9. Bar, 1cm. (F) Mature seed of WT, OE9, and R9. Bar, 1mm. (G) Quantification of organ size variation among the WT, OE9 and R9. Three plants for each line were cultivated. Nine mature flowers from each line and the ovaries and sepals from these flowers were measured. Berry weight, ICS size and 100-seed weight were recorded from 10 fruits per plant. The total berry yield for each plant was measured. In each measurement, the mean and the standard deviation are presented. (H) Cell number variation among the WT, OE9 and R9. The details were presented in Table S8. The significance compared with WT was evaluated using a two-tailed t-test. A star indicates a significant difference (P<0.05).
Organ size alteration may result from alterations in cell size and/or cell number. We found that the size of the epidermal cells of leaves, calyces, and ICSs in the 35S:PfCNR1-RNAi transgenic lines was not altered in comparison with the wild type (Supplementary Table S8 at JXB online), which was corroborated by an unchanged cell number per unit area (Supplementary Fig. S5B, C, G). In contrast, cell number was significantly increased but no significant difference in cell size was observed in the ovaries, developing berries, and seeds between the RNAi and wild-type plants (Fig. 4H; Supplementary Fig. S5D, E, and Supplementary Table S8). In mature berries of 35S:PfCNR1-RNAi transgenic plants (i.e. R9), the cell size decreased as the cell number increased compared with the wild type (Supplementary Table S8). This can be attributed to the compensation effect between cell number and cell size that has been reported previously (Doonan, 2000).
The findings were further substantiated by overexpressing PfCNR1 in P. floridana. Nine independent transgenic lines were generated from independent explants (Fig. 4B). Most of the nine transgenic plants showed a significant reduction in multiple organ sizes; i.e. in leaves, flowers, ICSs, berries, and seeds (Fig. 4C–G; Supplementary Fig. S5 and Supplementary Table S7). Nonetheless, seed number per berry, berry yield per plant, and plant weight were not significantly altered (P>0.05) in these transgenic plants overexpressing PfCNR1 (Supplementary Table S7). Cell number was reduced significantly but no significant changes (P>0.05) in the cell size of organs were seen in the transgenic plants (Fig. 4H; Supplementary Fig. S5B–E, G and Supplementary Table S8). In comparison with the wild-type P. floridana plants, the siblings that segregated from the T2 populations of some transgenic lines did not show any phenotypic variation, and the PfCNR1 expression was also not altered in these plants (Supplementary Fig. S5H–J). Thus, PfCNR1 controls organ size by inhibiting cell division.
PfCNR1 is a plasma membrane-anchored protein
To understand how PfCNR1 exerts its function, we next determined the subcellular localization of its encoded protein. In subcellular localization analyses, GFP alone was distributed in both the nucleus and cytoplasm (Supplementary Fig. S6A at JXB online). In marked contrast, the fused protein PfCNR1–GFP was localized exclusively at the periphery of the cell (Supplementary Fig. S6B, D), and the GFP signal did not surround the nuclei (Supplementary Fig. S6B, C), which suggests that no protein was anchored on the vacuolar or the nuclear membrane. When the plant cells were plasmolysed, PfCNR1–GFP was clearly demonstrated to be located in the plasma membrane regardless of the residual GFP signals adhering proximate to the cell wall (Supplementary Fig. S6D, E). This suggested the unique localization of PfCNR1 in the plasma membrane.
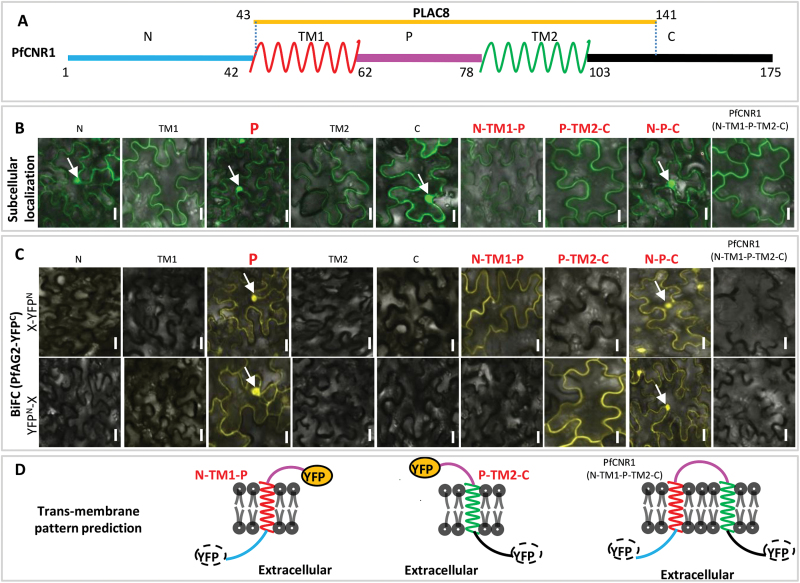
Based on the computational prediction (Supplementary Fig. S6F), PfCNR1 was expected to consist of the N1–42, TM143–61, P62–78, TM279–103, and C104–175 portions (Fig. 5A). To elucidate further the cell membrane localization of PfCNR1, we made different constructs that synthesized the GFP fusion with each portion or portion combinations (e.g. N–P–C), respectively. In plant cells, all expressed constructs that did not contain any transmembrane portion (TM1 or TM2) generated a GFP signal in the nuclei; otherwise, no nuclear GFP signal was seen (Fig. 5B). Thus, PfCNR1 is very probably localized in the cell membrane. PfCNR1 also had the PLAC8 domain aa 43–141, which mainly covered TM1, P, and TM2 (Fig. 5A). PLAC8 is an FW2.2-like feature and this domain is suggested to be involved in transmembrane localization and protein–protein interaction (Libault and Stacey, 2010).
Fig. 5.
Interaction between PfAG2 and the membrane-anchored PfCNR1. (A) Subsections of PfCNR1 based on computational prediction. Different sections of the protein are highlighted in colour. N, N-terminal region; TM (TM1 and TM2), transmembrane domain; P, the portion between two transmembrane domains; C, C-terminal region. The number indicates the position of the amino acids of the two ends of each section. (B) Subcellular localization of each section of PfCNR1. The fusion protein of each section with GFP was transiently expressed in plant cells. (C) BiFC assays. PfAG2–YFPc and each section of PfCNR1 were co-expressed in plant cells. X indicates different sections of PfCNR1. The arrow indicates the nuclei. Bars, 10 μm. (D) Subcellular localization predication of each section of PfCNR1 and the mutated PfCNR1. The predication was performed according to the observations in (B) and (C). The dash-circled YFP indicates that the YFP signal was not seen when YFPn was fused at the extracellular end, while the solid-circled yellow YFP indicates that the YFP signal was detected once the YFPn was fused at the intracellular end. The colours of each PfCNR1 section are as used in (A).
Characterization of interacting proteins of PfCNR1
To exert its role in the cell cycle, we assumed that the cell membrane-anchored PfCNR1 should interact with other proteins, and then transduce the signal into the nucleus to modulate cell division. Therefore, we decided to search for PfCNR1 interacting proteins. PfCNR1 and its different portions (defined by clues from Fig. 5F) did not self-activate in yeast, so they were used as respective baits to screen a Physalis expressing library (He et al., 2007). Sequencing analyses revealed that PfGIP (a homologue of gibberellin-induced protein in P. floridana), PfSEP1 [a SEPALLATA (SEP)-like MADS-domain protein in P. floridana], and PfAG2 [an AGAMOUS (AG)-like MADS-domain regulatory protein in P. floridana] probably interacted with PfCNR1 (Supplementary Fig. S7A at JXB online). They were either partial or full sequences in length; for example, five recombinant yeast colonies inserting the coding sequence of PfAG2 were obtained, and two full-length sequences were seen (Supplementary Fig. S7A and B). To further confirm their interactions, we isolated the full-length sequences of all these putative partners, and the interactions were then tested in yeast via co-transformation (see Materials and methods). We found that interaction of PfCNR1 and PfAG2 was robust in transformed yeast cells; however, the interaction was not revealed in BiFC assays in plant cells (Supplementary Fig. S7C, F). PfAG2 was a homologue of the homeotic MADS-domain protein AG in Arabidopsis (Supplementary Fig. S7G). We further examined the dimerization of PfCNR1, and found that PfCNR1 and its different portions did not form dimers in yeast and plant cells (Supplementary Fig. S7F). The putative orthologue of the casein kinase II β-subunit 1 (CKIIβ1) is a known interacting partner of tomato FW2.2 (Cong and Tanksley, 2006); therefore, we also isolated the putative orthologue P. floridana PfCKIIβ1 and included it in our protein–protein interaction studies (Supplementary Fig. S7H). PfCNR1 was found to interact with PfCKIIβ1 in yeast as well; however, no YFP signal was detected in the plant cells under any of the circumstances tested (Supplementary Fig. S7F). In BiFC assays, YFPn was fused to both the N and C termini of PfCNR1-derived constructs and each of them was co-expressed with the fusion protein PfAG2 or PfCKIIβ1 with YFPc in plant cells. In principle, the YFP signal should be reconstituted once the two proteins interact. The failure to detect a YFP signal may have been because of the non-overlapping localization of PfCNR1 and its putative interacting proteins. To clarify this, subcellular localization of these putative partners was checked, and they were localized in either the nuclei or the cytoplasm (Supplementary Fig. S7F). Thus, it is likely that the two ends (N- and C-terminal ends) of PfCNR1 might be extracellular, as suggested by bioinformatics prediction (Fig. 5F). To confirm this experimentally, we used different portions of PfCNR1 to check the interactions with the above-mentioned PfCNR1 partners using BiFC assays. The YFP signal was only observed when the intracellular PfCNR1 62–78 encountered either PfAG2 or PfCKIIβ1 in plant cells (Supplementary Fig. S7C–F), thus corroborating our assumption regarding PfCNR1 transmembrane pattern. Moreover, the PfCNR1 62–78 portion was required for interacting with its partners PfAG2 and PfCKIIβ1.
To the best of our knowledge, this is the first reported finding of an FW2.2-like protein interacting with an AG-like MADS-domain protein, and thus the interaction of PfCNR1 and PfAG2 was analysed further. YFPn was fused to both the N and C termini of PfCNR1-derived constructs (Fig. 5A) and each was co-expressed with PfAG2–YFPc in plant cells. The YFP signal was only detected once the P portion of PfCNR1 was included; moreover, the presence of YFP was dependent on the position of the YFPn fusion if any one of the transmembrane domains TM1 and/or TM2 was included (Fig. 5C). These results further suggested that the 16 aa peptide of PfCNR1 is required for its interaction with PfAG2 and also suggested the cell membrane localization pattern of PfCNR1and its derived proteins (Fig. 5D).
Transcriptional correlation of PfCNR1 and PfCYCD2;1
Transcriptional regulation by the PfCNR1 signalling pathway were also investigated. The expression of the putative PfCNR1 interacting partner genes was first checked, and they were all expressed but no differential expression in the B2 flower buds was observed among P058, P064, P106, 35S: PfCNR1 (OE9), and 35S: PfCNR1-RNAi (R9) plants (Fig. 6A). Since cell division in eukaryotic organisms is controlled by highly conserved basic cell-cycle machinery (Dewitte and Murray, 2003), we identified five putative Cyclin genes from P. floridana that are key genes in the plant cell division cycle (Mizukami, 2001). We designated these genes PfCYCA2;1, PfCYCB1;1, PfCYCB2;1, PfCYCD2;1, and PfCYCD3;1. In the B2 flower buds of P058, P064, R9, and OE9 plants, only PfCYCD2;1 was significantly elevated in P058, P064, and R9 plants, and it was slightly repressed in OE9 plants relative to the wild-type P106 (Fig. 6A). The expression of CyclinD2;1 positively correlated with cell division (Oh et al., 2008; Talengera et al., 2012). Therefore, PfCNR1 may function as a negative modulator of the cell division cycle by directly or indirectly influencing the expression of PfCYCD2;1.
Fig. 6.
Regulation of the PfCYCD2;1 expression. (A) Gene expression analysis of the putative PfCNR1 interacting partners and the putative Cyclin. Total RNA from the B2 flower buds was subjected to qRT-PCR. PfACTIN was used as an internal control. Expression of each gene was investigated in three independent biological samples and its expression in the wild-type P106 was set as 1. The mean and standard deviation are presented. (B) Characterization of the putative PfCYCD2;1 promoter. Three CArG-boxes (C1, C2, and C3) are highlighted in red circles beyond the ATG. The mutated promoters resulting from either a point mutation (C1pm) or deletion mutation (C1dm) are shown. The putative promoter sequences are presented in Supplementary Fig. S8. (C) Yeast one-hybrid assays between PfAG2 and the related DNA fragments. Pro, promoter; 3×, three tandem repeats of the CArG-box; C1, CArG-box1; C2, CArG-box2; C3, CArG-box3; dm, deleted mutation; pm, point mutation. The survival of yeast cells on SD/–Leumedium supplemented with aureobasidin A (AbA) suggested that the protein could bind to the corresponding DNA fragment. (D) LUC relative activity assay in P. floridana protoplasts. LUC activities were normalized using 35S:GUS as an internal control. The wild-type PfAG2 associated interactions are highlighted in pink and the interactions of PfAG2m that excluded the NLS are indicated in red. The mean and standard deviation from at least six independently repeated assays are presented. The star and the square, respectively, indicate a significant difference (P<0.05) compared with ProPfCYCD2;1:LUC or ProPfCYCD2;1:LUC with PfAG2. (E) The NLS in PfAG2 and its deleted version (PfAG2m). The 25 aa sequences of the NLS are given between the PfAG2 and PfAG2m structures. (F) Subcellular localization of PfAG2. The arrow indicates the nuclei. (G) Subcellular localization of PfAG2m. Bar, 10 μm (F, G).
Molecular interactions of the PfCNR1 interacting proteins and PfCYCD2;1
In order to elucidate the molecular interactions of PfCYCD2;1 with the PfCNR1 protein and its interacting partners, we first checked the protein-protein interactions among these proteins. We found no evidence to support any possible reciprocal direct contact among PfAG2, PfCKIIβ1 and PfCYCD2;1 (Supplementary Fig. S7I). Moreover, PfCNR1 did not interact with PfCYCD2;1 either (Supplementary Fig. S7I).
Since PfAG2 is a putative MADS-domain transcription factor (Supplementary Fig. S7G), its molecular interaction with the PfCYCD2;1 gene is of potential interest. To reveal the molecular interaction, we isolated the 1.7kb PfCYCD2;1 promoter. We found three CArG-boxes (C1, C2, and C3) in this region (Fig. 6B; Supplementary Fig. S8 at JXB online). Interestingly, only one CArG-box was found in the orthologous region in tomato (Supplementary Fig. S8). The CArG-box is a well-known binding site for the MADS-domain transcription factors (Kaufmann et al., 2005). In yeast one-hybrid assays, the survival of yeast cells on SD/–Leu medium supplemented with AbA suggested that the protein could bind to the DNA fragment. We therefore found that PfAG2 could bind to the PfCYCD2;1 promoter but not to the SlCYCD2;1 and PfCNR1 promoters (Fig. 6C). In particular, PfAG2 could specifically interact with the C1 CArG-box. Once the CArG-box was deleted or mutated, PfAG2 did not bind to the mutated PfCYCD2;1 promoters. PfSEP1, another MADS-box protein, did not bind to the promoter of PfCYCD2;1, SlCYCD2;1, or PfCNR1, nor did it bind to any CArG-box in the PfCYCD2;1 promoter (Supplementary Fig. S9 at JXB online).
We next substantiated the consequence of the specific binding. We exploited this in P. floridana protoplasts using the LUC gene as a reporter (Fig. 6D). We found that PfAG2 could significantly (P<0.05) repress LUC expression driven by the PfCYCD2;1 promoter and co-expression of PfCNR1 and PfAG2 could further inhibit LUC expression (P<0.05). Furthermore, when the C1 CArG-box in the PfCYCD2;1 promoter was mutated or deleted, the repression of LUC expression by PfAG2 and PfCNR1 disappeared. Nonetheless, altering PfCNR1 expression in the leaf protoplasts alone did not affect the expression of the reporter gene (Fig. 6D; Supplementary Fig. S10A at JXB online). Furthermore, altering expression of PfCKIIβ1 or PfSEP1, and co-expression of PfCNR1 with either PfCKIIβ1 or PfSEP1, also did not affect LUC expression driven by the PfCYCD2;1 promoter either (Fig. 6D). Nevertheless, PfAG2 mRNA was not detected in the protoplasts from young leaves (Supplementary Fig. S10B). These data therefore suggested that PfAG2 could directly and specifically repress PfCYCD2;1 expression.
The interaction between PfCNR1 and PfAG2 was able to suppress LUC expression driven by the PfCYCD2;1 promoter, and the nuclear import of PfAG2 seems to be essential. The NLS of PfAG2 was deleted to produce PfAG2m (Fig. 6E). Unlike PfAG2, PfAG2m could not be imported into the nucleus (Fig. 6F, G). In transient LUC assays, LUC expression driven by the PfCYCD2;1 promoter was no longer suppressed once the PfAG2m was expressed instead of PfAG2 (Fig. 6D). Therefore, the nuclear import of PfAG2 is essential to suppress PfCYCD2;1 expression, thereby regulating cell division and organ size. Moreover, we found that among Physalis species, PfCNR1 transcript levels at B2 flower buds were negatively correlated with PFCYCD2;1 mRNA levels (r=–0.50, P=0.04), which were positively correlated with organ size (Supplementary Table S6).
Discussion
Fruit size is a prime target in the breeding of Solanaceous crops. As the first cloned QTL, FW2.2 accounts for approximately 30% of the fruit size variation between cultivated tomato and its wild-type relatives (Frary et al., 2000). Its homologues are all associated with organ size or cell division in maize, rice, avocado, cherry, soybean, and melon (Dahan et al., 2010; Guo et al., 2010; Libault et al., 2010; Guo and Simmons, 2011; Franceschi et al., 2013; Xu et al., 2013; Monforte et al., 2014); thus, they were also named Cell Number Regulator (CNR) genes. Nonetheless, how they regulate cell division is not understood. In the present study, we characterized FW2.2-like genes in P. floridana, also termed PfCNR1-like, and suggested a novel regulatory pathway of PfCNR1 (characterized as the putative FW2.2 orthologue) in cell division, and found that the heterochronic expression of the PfCNR1 genes in the ovaries might explain interspecific variation in berry size and seed weight within Physalis species.
A novel working model for membrane-anchored PfCNR1 in the cell cycle
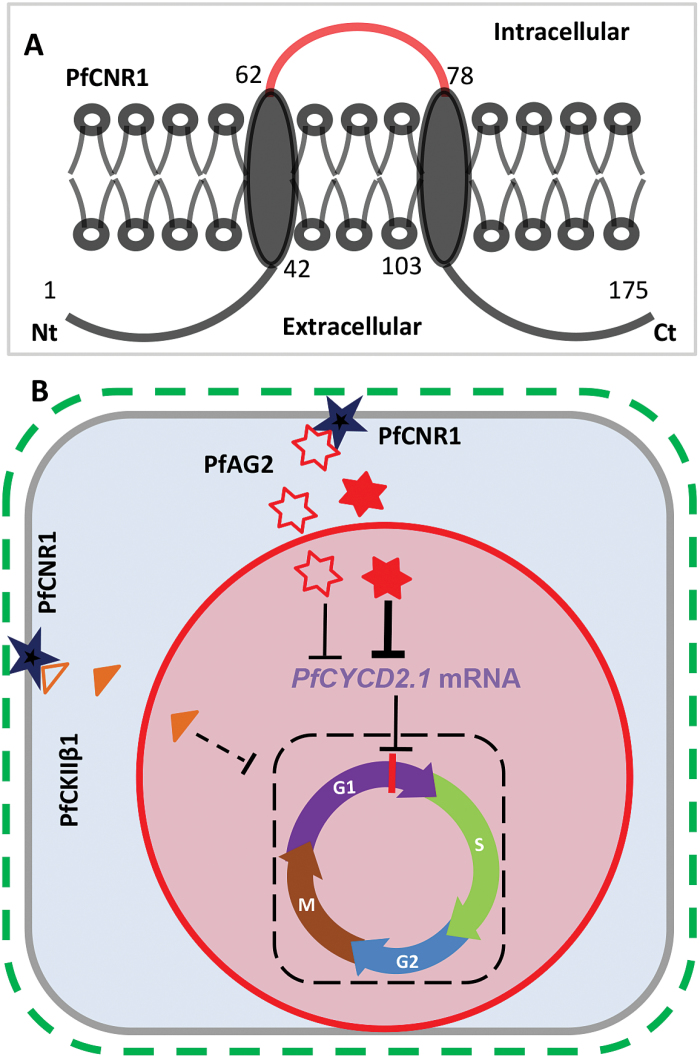
Similar to PfCNR1, FW2.2 and its homologous proteins in soybean and rice are suggested to be localized to the plasma membrane (Xu et al., 2013; Cong and Tanksley, 2006; Libault et al., 2010). These proteins were proposed to have one to two transmembrane domains in the PLAC8 domain, and the variation of the number of transmembrane domains between these proteins may lead to a different organization of the core domain relative to the membrane, and thus they may help mediate protein–protein interactions or be involved in the cellular signalling process (Libault and Stacey, 2010). In our work, based on computational and experimental approaches, we have proposed a detailed model for PfCNR1 subcellular localization (Fig. 7A). Both the N and C termini are extracellular and only the middle 16 aa section (P section) is intracellular. However, the fact that these CNR/FW2.2 proteins regulate cell division is intriguing. They may facilitate the transport of ions such as cadmium and calcium across membranes, but how regulation of ion transport would lead to changes in cell division is unknown (Song et al., 2004; Nakagawa et al., 2007; van der Knaap et al., 2014). We assumed that the FW2.2/CNR proteins need to interact with other proteins for signal transduction to direct cell division in the nuclei. A large portion of the protein is extracellular, which could perceive the external signal for cell division control, while the intracellular portion could mediate the interaction with proper protein partners. For example, tomato FW2.2 interacts with CKIIβ1 (Cong and Tanksley, 2006). CKIIβ1 plays a critical role in cell growth and proliferation in yeast (Roussou and Draetta, 1994), thereby suggesting an unconfirmed CKIIβ1-mediated model. The P section determined in PfCNR1 may mediate its interaction with specific interacting partners and thus determine its biological functionality. The CKIIβ1-mediated cell division pathway might be present in Physalis species, since PfCNR1 interacted with PfCKIIβ1 in P. floridana. We also found that PfCNR1 interacted with the MADS-domain protein PfAG2 and that PfAG2 could bind to a CArG-box in the PfCYCD2;1 promoter and repress the PfCYCD2;1 expression. Therefore, the expression of PfCYCD2;1, a key gene for the G1/S transition phase in the cell cycle (Mizukami, 2001; Oh et al., 2008; Talengera et al., 2012), was negatively correlated with PfCNR1 expression levels. In this regulatory pathway, binding the PfCYCD2;1 promoter by PfAG2 in the nuclei is essential. The interaction of PfCNR1 with PfAG2 is not required, but it enhances the repressive activity on PfCYCD2;1 expression. The intracellular 16 aa peptide is required for the interaction of PfCNR1 and PfAG2. This PfAG2-mediated regulation of PfCYCD2;1 represents a novel alternative regulatory pathway for the PfCNR1-like proteins integrated into the cell cycle. The proposed pathway seems to be independent of the PfCKIIβ1-mediated pathway, since PfAG2 did not interact with PfCKIIβ1, and since manipulating PfCKIIβ1 expression did not affect LUC expression driven by the PfCYCD2;1 promoter. In both regulatory pathways, certain biochemical modifications may be ascribed to PfAG2 or PfCKIIβ1 through their interactions with PfCNR1, which requires further investigation. Nonetheless, our work enriches the details of the PfAG2-mediated working model for PfCNR1-directed cell division (Fig. 7B). The MADS-domain proteins are major determinants of floral organ identity, and a molecular link between floral organ identity and growth is missing (Dornelas et al., 2011). PfAG2 encodes a closely related homologue of the Arabidopsis homeotic C-function transcription factor AG, which specifies carpel identity (Yanofsky et al., 1990). Thus, our work may provide a first glimpse at the molecular link between ovary identity and growth. To what extent the CNR–AG–Cyclin pathway is conserved in angiosperms is not yet clear. Expansion of the related gene family with subsequent divergence during evolution might complicate the situation, but comparative and in planta functional analyses of these molecular interactions among the FW2.2-like genes, the AG-like genes, and the Cyclin-like genes will clarify this. Since ectopically overexpressing the PfCNR1-like genes could affect multiple organ sizes, as in maize (Guo et al., 2010) and P. floridana, our work suggests a working model for a cell membrane-anchored protein that modulates cell division and thereby governs organ size determination in plants.
Fig. 7.
PfCNR1-related models. (A) PfCNR1 membrane localization pattern. Both the N-terminal (Nt) and C-terminal (Ct) regions are extracellular. The intracellular portion (P) of PfCNR1 that mediated interaction with PfAG2 is highlighted in red. (B) A novel working model of PfCNR1 regulating cell cycle. The proposed PfCNR1 activity in the cell cycle is shown in a stylized cell. The green dashed box indicates the cell wall. The grey box indicates the cell membrane. The red circle indicates the nuclear membrane and the black dashed box inside depicts the four phases of the cell cycle (G1, S, G2, and M). The red line on the G1 phase indicates a check point of the cell cycle. The black star on the cell membrane is PfCNR1. The outlined triangle is PfCKIIβ1, and the yellow-filled triangle represents PfCKIIβ1, which is putatively modified by interacting with PfCNR1. The red outlined star represents PfAG2 and the red-filled star is PfAG2, which is putatively modified by interacting with PfCNR1. The PfCKIIβ1- and PfAG2-mediated pathways seem to be independent since PfCKIIβ1 did nor interact with PfAG2 or replace PfAG2. The black T-line indicates the repression effect of the upstream genes. PfAG2 represses PfCYCD2;1 expression, and the modified PfAG2 after interacting with PfCNR1 enhances the repression. The interacting and regulatory pathway of PfCNR1–PfAG2–PfCYCD2;1 for cell division established in the present work is indicated by solid T-lines while the dashed T-line indicates the repression effect of the PfCNR1–PfCKIIβ1 pathway that needs to be substantiated.
Altering expression is essential in PfCNR1-like function recruitment
GmFWL1 is uniquely expressed in roots and nodules, and affects nodule organogenesis (Libault et al., 2010). However, expression specification of FW2.2-like genes often is associated with organ size variation in various plant species including tomato, maize, rice, cherry, avocado, and melon (Frary et al., 2000; Dahan et al., 2010; Guo et al., 2010; Guo and Simmons, 2011; Franceschi et al., 2013; Xu et al., 2013; Monforte et al., 2014). Interestingly, FW2.2 is expressed early in floral development and controls carpel cell number (Frary et al., 2000). This gene negatively regulates tomato fruit size via allelic variation in gene expression rather than variation in the FW2.2 protein sequences (Cong et al., 2002; Liu et al., 2003). Expression of the larger fruit allele has an early and short duration, whereas expression from the smaller fruit allele peaks later and persists for a long period. Therefore, the heterochronic expression of FW2.2 is involved in tomato domestication (Cong et al., 2002). PfCNR1 was highly expressed in ovaries, ovules, and floral calyces, controlling multiple post-floral organ sizes including berries, seeds, and ICSs. Moreover, PfCNR1 transcript levels in the ovaries were negatively correlated with PfCYCD2;1 expression, mature fruit weight, and 100-seed weight among the Physalis species. This suggests a recruitment of the PfCNR1–PfAG2–PfCYCD2;1 function during the evolution of fruit sizes within Physalis species. Unlike FW2.2 (Frary et al., 2000; Cong et al., 2002), expression of the smaller berry PfCNR1 allele peaked earlier and was higher than that of the larger berry allele, and hence both heterochronic expression and differential mRNA levels of the PfCNR1 alleles may contribute to the evolution and development of berry size in Physalis species.
In the domestication of tomato fruit size, FW2.2 accounts for the first key step, while increasing the carpel number by FAS and LC is considered the second step (Cong et al., 2008; Muños et al., 2011). QTL analyses have revealed that Solanaceous crops might share a common genetic basis for domestication as in tomato, eggplant, and pepper (Doganlar et al., 2002; Paran and van der Knaap, 2007; Wang et al., 2008). No differences were observed in the carpel number (two locules) in Physalis species; thus, Physalis domestication might be different from tomato. Nonetheless, recruiting a PfCNR1-like function for a different cell number in the ovary might be the first crucial step in the evolution of different species with a different fruit size as an adaption for seed dispersal. Besides, human selection might act on the particular species for the berry yields. P. philadelphica (tomatillo) is a domesticated species (Montes Hernández and Aguirre Rivera, 1994), and the berry size varied from about 1.2 to 11.2g (Wang et al., 2012). The cell number in ovaries, a consequence of recruiting a PfCNR1 function, seemed to be comparable among tomatillo accessions. However, the berry size variation in the species might correlate with differences in cell expansion of the developing berries, which would suggest an involvement of cell expansion regulators in determining tomatillo berry size. Very recently, Physalis Organ Size1 (POS1) encoding a putative regulatory proteins with double cytokinin response factor (CRF)– APETALA2 (AP2) domains was found to act as a promoter of cell expansion, and expression variation of this gene correlates to natural variation of berry size in P. philadelphica (Wang et al., 2014). Further studies can investigate the genetic interaction of the PfCNR1 and POS1 genes in the control of berry size in Physalis species.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Median transverse sections of ovaries and berries.
Supplementary Fig. S2. A neighbour-joining (NJ) tree of FW2.2-related genes.
Supplementary Fig. S3. Expression of PfCNR1-like genes during flower and fruit development.
Supplementary Fig. S4. VIGS of the PfCNR1 gene family in Physalis floridana.
Supplementary Fig. S5. Transgenic analyses of PfCNR1 in Physalis floridana.
Supplementary Fig. S6. Transient PfCNR1 expression in plant cells.
Supplementary Fig. S7. Characterizations of the putative PfCNR1-interacting proteins.
Supplementary Fig. S8. Promoter alignment of SlCYCD2;1 and PfCYCD2;1.
Supplementary Fig. S9. Yeast one-hybrid assays between PfSEP1 and the indicated DNA fragments.
Supplementary Fig. S10. LUC expression driven by the PfCYCD2;1 promoter in Physalis leaf protoplasts.
Supplementary Table S1. Physalis resources used in the present work.
Supplementary Table S2. Primers used in the present work.
Supplementary Table S3. Variation in cell number in the ovaries and berries during development.
Supplementary Table S4. Variation of cell size in the ovaries and the berries during development.
Supplementary Table S5. Correlation between PfCNR1-like expression and ovary cell activities.
Supplementary Table S6. Variation in sequences and expressions, and their correlations with organ size within Physalis species.
Supplementary Table S7. Phenotypic variation of 35S:PfCNR1-RNAi and 35S:PfCNR1 transgenic Physalis plants.
Supplementary Table S8. Cells in 35S:PfCNR1-RNAi and 35S:PfCNR1 transgenic Physalis plants.
Acknowledgements
The assistance of Dr Li Wang and Miss Jing Li in field experiments is acknowledged. We also thank Dr Lingli He, Dr Man Zhao, and Mr Zhe Cai for their help in phylogenetic and statistical analyses. This work was supported by grants 30870175 and 91331103 from the National Natural Science Foundation of China and by the Hundred Talents Project of the Chinese Academy of Sciences.
Glossary
Abbreviations:
- BiFC
bimolecular fluorescence complementation
- cDNA
complementary DNA
- CNR
cell number regulator
- GFP
green fluorescence protein
- GUS
β-glucuronidase
- LUC
luciferase
- ORF
open reading frame
- RNAi
RNA interference
- TRV
tobacco rattle virus
- VIGS
virus-induced gene silencing
- YFP
yellow fluorescence protein.
References
- Carr SM, Irish VF. 1997. Floral homeotic gene expression defines developmental arrest stages in Brassica oleracea L. vars. botrytis and italica . Planta 201, 179–188. [DOI] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH. 2009. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis . Plant Cell 21, 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B, Barrero LS, Tanksley SD. 2008. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nature Genetics 40, 800–804. [DOI] [PubMed] [Google Scholar]
- Cong B, Liu J, Tanksley SD. 2002. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proceedings of the National Academy of Sciences, USA 99, 13606–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B, Tanksley SD. 2006. FW2.2 and cell cycle control in developing tomato fruit: a possible example of gene co-option in the evolution of a novel organ. Plant Molecular Biology 62, 867–880. [DOI] [PubMed] [Google Scholar]
- Dahan Y, Rosenfeld R, Zadiranov V, Irihimovitch V. 2010. A proposed conserved role for an avocado fw2.2-like gene as a negative regulator of fruit cell division. Planta 232, 663–676. [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JA. 2003. The plant cell cycle. Annual Review of Plant Biology 54, 235–264. [DOI] [PubMed] [Google Scholar]
- Doganlar S, Frary A, Daunay MC, Lester RN, Tanksley SD. 2002. Conservation of gene function in the Solanaceae as revealed by comparative mapping of domestication traits in eggplant. Genetics 161, 1713–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan J. 2000. Social controls on cell proliferation in plants. Current Opinion in Plant Biology 3, 482–487. [DOI] [PubMed] [Google Scholar]
- Dornelas MC, Patreze CM, Angenent GC, Immink RG. 2011. MADS: the missing link between identity and growth? Trends in Plant Science 16, 89–97. [DOI] [PubMed] [Google Scholar]
- Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martinez MC. 1999. Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. The Plant Journal 19, 655–666. [DOI] [PubMed] [Google Scholar]
- Franceschi PD, Stegmeir T, Cabrera A, et al. 2013. Cell number regulator genes in Prunus provide candidate genes for the control of fruit size in sweet and sour cherry. Molecular Breeding 32, 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frary A, Nesbitt TC, Grandillo S, Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tanksley SD. 2000. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289, 85–88. [DOI] [PubMed] [Google Scholar]
- Guo M, Rupe MA, Dieter JA, Zou JJ, Spielbauer D, Duncan KE, Howard RJ, Hou ZL, Simmons CR. 2010. Cell number regulator1 affects plant and organ size in maize: implications for crop yield enhancement and heterosis. Plant Cell 22, 1057–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Simmons CR. 2011. Cell number counts—the fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Science 181, 1–7. [DOI] [PubMed] [Google Scholar]
- Han Y, Jiang JF, Liu HL, Ma QB, Xu WZ, Xu YY, Xu ZH, Chong K. 2005. Overexpression of OsSIN, encoding a novel small protein, causes short internodes in Oryza sativa . Plant Science 169, 487–495. [Google Scholar]
- He CY, Münster T, Saedler H. 2004. On the origin of floral morphological novelties. FEBS Letters 567, 147–151. [DOI] [PubMed] [Google Scholar]
- He CY, Saedler H. 2005. Heterotopic expression of MPF2 is the key to the evolution of the Chinese lantern of Physalis, a morphological novelty in Solanaceae. Proceedings of the National Academy of Sciences, USA 102, 5779–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CY, Sommer H, Grosardt B, Huijser P, Saedler H. 2007. PFMAGO, a MAGO NASHI-like factor, interacts with the MADS-box protein MPF2 from Physalis floridana . Molecular Biology and Evolution 24, 1229–1241. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Melzer R, Theissen G. 2005. MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene 347, 183–198. [DOI] [PubMed] [Google Scholar]
- Libault M, Stacey G. 2010. Evolution of FW2.2-like (FWL) and PLAC8 genes in eukaryotes. Plant Signaling and Behavior 5, 1226–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Zhang XC, Govindarajulu M, Qiu J, Tong Y, Brechenmacher L, Berg RH, Hurley-Sommer A, Taylor CG, Stacey G. 2010. A member of the highly conserved FWL (tomato fw2.2-like) gene family is essential for soybean nodule organogenesis. The Plant Journal 62, 852–864. [DOI] [PubMed] [Google Scholar]
- Liu J, Cong B, Tanksley SD. 2003. Generation and analysis of an artificial gene dosage series in tomato to study the mechanisms by which the cloned QTL fw2.2 controls fruit size. Plant Physiology 132, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y. 2001. A matter of size: developmental control of organ size in plants. Current Opinion in Plant Biology 4, 533–539. [DOI] [PubMed] [Google Scholar]
- Monforte AJ, Diaz AI, Cano-Delgado A, van der Knaap E. 2014. The genetic basis of fruit morphology in horticultural crops: lessons from tomato and melon. Journal of Experimental Botany 65, 4625–4637. [DOI] [PubMed] [Google Scholar]
- Montes Hernández S, Aguirre Rivera JR. 1994. Neglected crops: 1492 from a different perspective. In: Hernándo Bermejo JE, León J, eds. Plant production and protection, Series No. 26. Rome, Italy: FAO, 117–122. [Google Scholar]
- Moreno-Romero J, Carme Espunya M, Platara M, Ariño J, Carmen Martínez M. 2008. A role for protein kinase CK2 in plant development: evidence obtained using a dominant-negative mutant. The Plant Journal 55, 118–130. [DOI] [PubMed] [Google Scholar]
- Muños S, Ranc N, Botton E, et al. 2011. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL . Plant Physiology 156, 2244–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, et al. 2007. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proceedings of the National Academy of Sciences, USA 104, 3639–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SE, Kim SJ, Shic Kim YS, Park SH, Ha SH, Kim JK. 2008. Arabidopsis cyclin D2 expressed in rice forms a functional cyclin-dependent kinase complex that enhances seedling growth. Plant Biotechnology Reporter 2, 227–231. [Google Scholar]
- Paran I, van der Knaap E. 2007. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. Journal of Experimental Botany 58, 3841–3852. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Lorenz P, Ansorge W, Pyerin W. 1994. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. Journal of Biological Chemistry 269, 6986–6991. [PubMed] [Google Scholar]
- Roussou I, Draetta G. 1994. The Schizosaccharomyces pombe casein kinase II α and β subunits: evolutionary conservation and positive role of the β subunit. Molecular and Cellular Biology 14, 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha NP. 1951. Chromosome number and morphology in Physalis . Current Science 20, 70–71. [PubMed] [Google Scholar]
- Song W-Y, Martinoia E, Lee J, Kim D, Kim D-Y, Vogt E, Shim D, Choi KS, Hwang I, Lee Y. 2004. A novel family of Cys-rich membrane proteins mediates cadmium resistance in Arabidopsis . Plant Physiology 135, 1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talengera D, Beemster GTS, Tushemereirwe WK, Kunert K. 2012. Isolation and characterisation of a banana CYCD2;1 gene and its over-expression enhances root growth. African Journal of Biotechnology 11, 10328–10339. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Chakrabarti M, Chu YH, et al. 2014. What lies beyond the eye: the molecular mechanisms regulating tomato fruit weight and shape. Frontiers in Plant Science 5, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang L, He LL, Li J, Zhao J, Li ZC, He CY. 2014. Regulatory change at Physalis Organ Size1 correlates to natural variation in tomatillo reproductive organ size. Nature Communications 5, 4271. [DOI] [PubMed] [Google Scholar]
- Wang L, Li ZC, He CY. 2012. Transcriptome-wide mining of the differently expressed transcripts for natural variation of floral organ size in Physalis philadelphica . Journal of Experimental Botany 63, 6457–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Diehl A, Wu F, Vrebalov J, Giovannoni J, Siepel A, Tanksley SD. 2008. Sequencing and comparative analysis of a conserved syntenic segment in the Solanaceae. Genetics 180, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xiong W, Cao B, Yan T, Luo T, Fan T, Luo M. 2013. Molecular characterization and functional analysis of “fruit-weight2.2-like” gene family in rice. Planta 238, 643–655. [DOI] [PubMed] [Google Scholar]
- Yamamoto YY, Deng XW. 1998. A new vector set for GAL4-dependent transactivation assay in plants. Plant Biotechnology 15, 217–220. [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. 1990. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Zhao J, Zhang SH, He CY. 2014. Efficient gene silencing mediated by tobacco rattle virus in an emerging model plant Physalis . PLoS ONE 9, e85534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Tian Y, Zhang JS, Zhao M, Gong PC, Riss S, Saedler R, He CY. 2013. The euAP1 protein MPF3 represses MPF2 to specify floral calyx identity and displays crucial roles in Chinese lantern development in Physalis . The Plant Cell 25, 2002–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.