Abstract
Purpose
To summarise post-licensure safety surveillance over more than 4 years of routine use of the human papillomavirus-16/18-AS04-adjuvanted vaccine (HPV-16/18 vaccine: Cervarix®, GlaxoSmithKline, Belgium).
Methods
We describe global post-licensure passive surveillance data based on routine pharmacovigilance from 18 May 2007 until 17 November 2011 and enhanced surveillance implemented during the 2-year national immunisation programme in the UK (school years 2008–2010).
Results
Spontaneous reports from countries worldwide showed a similar pattern for the most frequently reported adverse events after HPV-16/18 vaccination. No patterns or trends were observed for potential immune-mediated diseases after vaccination. Observed incidences of Bell's palsy and confirmed Guillain–Barré syndrome were within the expected range in the general population. Outcomes of pregnancy in women who were inadvertently exposed to HPV-16/18 vaccine during pregnancy, were in line with published reports for similar populations. Enhanced surveillance of adverse events in the UK triggered a review of cases of anaphylaxis, angioedema and syncope reports, leading to an update to the prescribing information.
Conclusion
Collaborative partnerships between industry and national regulatory agencies facilitated rapid notification and transfer of safety information, allowing for rapid responses in the event of a safety signal of adverse event of concern. More than 4 years of post-licensure experience may provide confidence to providers and the public about the safety profile of HPV-16/18 vaccine in routine use. The safety profile appears to be consistent with pre-licensure data reporting that HPV-16/18 vaccine has an acceptable benefit–risk profile in adolescent girls and women.
Keywords: human papillomavirus vaccine, post-licensure surveillance, adverse drug reactions, potential immune-mediated diseases, pregnancy, AS04, pharmacoepidemiology
INTRODUCTION
Cervarix® (HPV-16/18 vaccine, GlaxoSmithKline [GSK], Belgium) is a human papillomavirus (HPV) vaccine formulated with the AS04 immunostimulatory adjuvant and is currently licensed in at least 129 countries. The safety of HPV vaccines has been demonstrated in clinical trials and in a range of post-licensure activities, recently reviewed by Macartney et al.1 Collection and evaluation of safety data during the early period of introduction of a new vaccine to the market is pivotal for the early detection and investigation of signals potentially related to vaccination. GSK follows a systematic approach for the identification of potential safety signals that is applied to all marketed products globally. All relevant data sources are interrogated when evaluating potential safety signals, including data from clinical and epidemiological studies, as well as pre-clinical information. This process includes systematic and regular review of individual case reports, aggregate safety data and relevant literature. Disproportionality analyses using an empirical Bayes data mining algorithm (Multi-Item Gamma Poisson Shrinker) are conducted when appropriate. Actions following evaluation and categorisation of a risk may include continuing routine pharmacovigilance, additional analyses, detailed epidemiological studies, changes to the product label and risk minimisation. Reports of adverse drug reactions (ADRs) are received by GSK through spontaneous reporting via worldwide sources that include medical personnel, regulatory authorities, individuals, pharmacists and literature sources. Adverse events (AEs) are coded in the database using the International Conference on Harmonisation Medical Dictionary for Regulatory Activities (MedDRA).2
At GSK, a safety signal is based on the definition by the Council for International Organizations of Medical Sciences-IV3: a report/reports of an event with an unknown causal relationship to vaccination that is recognised as worthy of further exploration and continued surveillance. As part of this process, AEs of interest identified during pre-licensure clinical development are monitored. For HPV-16/18 vaccine, AEs of interest include the new onset and exacerbation of potential immune-mediated diseases (pIMDs) after vaccination, and pregnancy outcomes associated with unintended vaccine exposure during pregnancy. The pIMDs are of interest because of the theoretical concern of acquiring a vaccine-induced disease of possible autoimmune aetiology in susceptible individuals after vaccination with a product containing an adjuvant system.4 Pregnancy outcomes are of interest because the target population includes women of child-bearing age. Pre-licensure studies were not designed to evaluate the safety of HPV-16/18 vaccine in pregnant women; thus, the data are currently insufficient to recommend vaccination during pregnancy.5
This safety review considered all ADR reports from spontaneous reporting and clinical trials reported to GSK from the launch of HPV-16/18 vaccine (18 May 2007) until the data lock point of 17 November 2011. This safety review complements a pooled analysis of safety data collected during the conduct of clinical trials.6
Common adverse reactions reported since launch
The 10 most frequent AEs (presented as MedDRA Preferred Terms [PTs]) reported after vaccination with HPV-16/18 vaccine are consistently reported across countries worldwide and are dominated by cases from five countries with longstanding national immunisation programmes, representing 80% (8916/11 145) of all reports since launch (Table 1). Varying frequencies of each event in individual countries reflect the known different local reporting awareness and reporting practices.7,8 Overall, the results shown in Table 1 are comparable to post-marketing safety surveillance conducted in the UK,9 Italy and the Netherlands.10,11 The majority (86%) of events were non-serious and are described in the global product label.5
Distribution of the 10 most frequent adverse drug reactions (ADRs) in countries where HPV-16/18 vaccine has been used the longest in national immunisation programmes
| Rate per 100 000 doses distributed | ||||||
|---|---|---|---|---|---|---|
| Country | Global | UK | Netherlands | Spain | Italy | Japan |
| Total ADR reporting rate | 20.79 | 62.93 | 20.98 | 147.25 | 82.81 | |
| Event Preferred Terms | ||||||
| Injection site pain | 9.18 | 25.30 | 2.76 | 81.90 | 13.13 | |
| Pyrexia | 6.61 | 1.18 | 22.23 | 2.52 | 20.75 | 18.36 |
| Headache | 6.50 | 2.67 | 23.00 | 2.76 | 43.35 | 7.66 |
| Nausea | 4.14 | 1.66 | 18.86 | 1.20 | 11.00 | 8.35 |
| Dizziness | 3.33 | 2.52 | 15.18 | 2.64 | ||
| Injection site swelling | 2.29 | 18.90 | ||||
| Malaise* | 2.21 | 1.97 | 8.74 | 1.32 | ||
| Pallor* | 2.10 | 7.13 | 8.65 | |||
| Myalgia | 2.08 | 12.33 | ||||
| Syncope | 1.94 | 1.77 | 1.68 | 5.65 | ||
| Vomiting | 1.22 | 1.08 | ||||
| Inappropriate schedule of drug administration | 2.04 | |||||
| Abdominal pain | 8.82 | 0.96 | 6.80 | |||
| Injection site erythema† | 10.41 | |||||
| Asthenia† | 8.35 | |||||
| Injection site pruritus† | 7.83 | |||||
| Maternal exposure during pregnancy | 1.38 | |||||
| Pain in extremity | 1.65 | |||||
| Product quality issue | 1.32 | |||||
| Injection site inflammation† | 8.36 | |||||
| Pain | 7.51 | |||||
| Presyncope* | 6.80 | |||||
| Loss of consciousness* | 6.76 | |||||
| Fall* | 4.93 | |||||
| Feeling abnormal* | 4.55 | |||||
Bold text indicates recognised adverse events in the product information for Cervarix®.
Adverse events reported in the context of syncope.
Synonyms for listed events in the product label.
Analysis of adverse events of interest
Potential immune-mediated diseases
An in-house Standard MedDRA Query corresponding to a pre-defined list of diseases classified by GSK as pIMDs (Table 2) was developed to search GSK's global safety database for reports of pIMDs.
Suggested list of potential immune-mediated disorders of interest (reproduced from Tavares Da Silva et al.14)*
| Neuro-inflammatory disorders | Musculoskeletal disorders | Skin disorders |
|---|---|---|
| Cranial nerve inflammatory disorders, including paralyses/paresis (e.g. Bell's palsy) | Systemic lupus erythematosus | Psoriasis |
| Optic neuritis | Systemic sclerosis (with limited or diffuse cutaneous involvement) | Vitiligo |
| Multiple sclerosis | Dermatomyositis | Erythema nodosum |
| Transverse myelitis | Polymyositis | Autoimmune bullous skin diseases (including pemphigus, pemphigoid and dermatitis herpetiformis) |
| Acute disseminated encephalomyelitis including site-specific variants: encephalitis, encephalomyelitis, myelitis, myeloradiculoneuritis, cerebellitis | Anti-synthetase syndrome | Cutaneous lupus erythematosus |
| Myasthenia gravis (including Lambert–Eaton myasthenic syndrome) | Rheumatoid arthritis | Alopecia areata |
| Immune-mediated peripheral neuropathies and plexopathies (including Guillain–Barré syndrome, Miller Fisher syndrome and other variants: chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy and polyneuropathies associated with monoclonal gammopathy) | Juvenile chronic arthritis (including Still's disease) | Lichen planus |
| Narcolepsy | Polymyalgia rheumatica | Sweet's syndrome |
| Spondyloarthritis, including ankylosing spondylitis, reactive arthritis (Reiter's syndrome) and undifferentiated spondyloarthritis | Morphoea | |
| Psoriatic arthropathy | ||
| Relapsing polychondritis | ||
| Mixed connective tissue disorder | ||
| Liver disorders | Gastrointestinal disorders | Metabolic and endocrine disorders |
| Autoimmune hepatitis | Crohn's disease | Autoimmune thyroiditis (including Hashimoto thyroiditis) |
| Primary biliary cirrhosis | Ulcerative colitis | Grave's or Basedow's disease |
| Primary sclerosing cholangitis | Ulcerative proctitis | Diabetes mellitus type I |
| Autoimmune cholangitis | Celiac disease | Addison's disease |
| Vasculitides | Others | |
| Large vessels vasculitis including giant cell arteritis such as Takayasu's arteritis and temporal arteritis | Autoimmune haemolytic anaemia | |
| Autoimmune thrombocytopenia | ||
| Medium sized and/or small vessels vasculitis including polyarteritis nodosa, Kawasaki's disease, microscopic polyangiitis, Wegener's granulomatosis, Churg–Strauss syndrome (allergic granulomatous angiitis), Buerger's disease (thromboangiitis obliterans), necrotizing vasculitis and anti-neutrophil cytoplasmic antibody (ANCA) positive vasculitis (type unspecified), Henoch–Schonlein purpura, Behcet's syndrome, leukocytoclastic vasculitis | Antiphospholipid syndrome | |
| Pernicious anaemia | ||
| Autoimmune glomerulonephritis (including IgA nephropathy, glomerulonephritis rapidly progressive, membranous glomerulonephritis, membranoproliferative glomerulonephritis and mesangioproliferative glomerulonephritis) | ||
| Uveitis | ||
| Autoimmune myocarditis/cardiomyopathy | ||
| Sarcoidosis | ||
| Stevens–Johnson syndrome | ||
| Sjögren's syndrome | ||
| Idiopathic pulmonary fibrosis | ||
| Goodpasture syndrome | ||
| Raynaud's phenomenon | ||
Note that this table is not intended to be exhaustive but is indicative of the type of conditions that could be included as adverse events of special interest in clinical trials.
A total of 147 spontaneous reports (147 subjects) were identified, encompassing 163 pIMD PTs. All subjects had received vaccination with either HPV-16/18 vaccine or an unspecified HPV vaccine. The majority of vaccinees (122/148) were young (10–19 years). The reported events were distributed over a range of body systems and disease categories (Table 3). In 137 events in which the time-to-onset was available, the onset of the event was reported as within 1 week after vaccination for 62 events and within 1 month for 107 events (Figure 1). When available, pIMDs were medically assessed using Brighton Collaboration standard case definitions, which, although designed to facilitate collection of high quality safety information across many research settings, are frequently less useful for cases reported through passive surveillance where diagnostic certainty is often low.12 However, many of these reports were poorly documented and therefore considered unassessable (Table 3). Many pIMDs have multiple potential aetiologies, including associations with a genetic predisposition, infective triggers and other confounding factors. Hence, assessment of diagnostic certainty and a potential causality with vaccination could not be performed for most of the reported cases.13,14
Reporting rate of the 10 most frequently reported potential immune-mediated diseases reported since launch until 18 May 2007 to 17 November 2011
| Event system organ class | Event preferred term | Number of cases | Diagnostic certainty | Reporting rate per 100 000 doses distributed since launch |
|---|---|---|---|---|
| Nervous system disorders | VIIth nerve paralysis | 19 | Insufficient data: 5 | 0.066 |
| Alternative cause: 2 | ||||
| Nervous system disorders | Guillain–Barré syndrome | 14 | 4 cases BC level 1–3 | 0.048 |
| Skin and subcutaneous tissue disorders | Erythema multiforme | 8 | Insufficient data: 3 | 0.028 |
| Alternative cause: 1 | ||||
| Nervous system disorders | Optic neuritis | 8 | Insufficient data: 4 | 0.028 |
| Alternative cause: 1 | ||||
| Blood and lymphatic system disorders | Idiopathic thrombocytopenic purpura | 7 | Insufficient data: 1 | 0.024 |
| Alternative cause: 1 | ||||
| Musculoskeletal and connective tissue disorders | Systemic lupus erythematosus | 7 | Insufficient data: 2 | 0.024 |
| Alternative cause: 2 | ||||
| Nervous system disorders | Encephalitis | 6 | 2 cases BC level 1–3 | 0.021 |
| Nervous system disorders | Paralysis | 6 | * | 0.021 |
| Nervous system disorders | Multiple sclerosis | 5 | 3 cases met criteria‡ | 0.017 |
| Musculoskeletal and connective tissue disorders | Rheumatoid arthritis | 5 | Insufficient data: 2 | 0.017 |
| Alternative cause: 1 |
Insufficient data = information provided was insufficient to confirm the diagnosis.
Alternative cause = either other vaccines or drugs could have been implicated, or an alternative and biologically plausible cause was also suspected or reported.
BC = Brighton Collaboration definitions for Guillain–Barré syndrome17 and encephalitis38 were level 1–3 (meets the case definition), level 4 (insufficient evidence to meet the case definition) and level 5 (diagnosis excluded).
Occurred in the context of other syndromes.
McDonald criteria for multiple sclerosis.39
Figure 1.
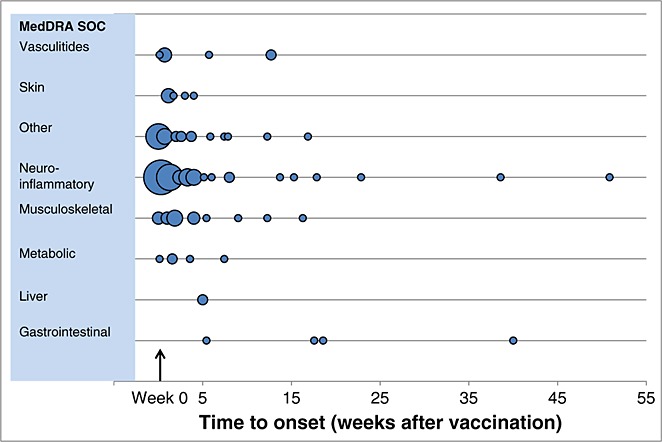
Time to onset of potential immune-mediated diseases after vaccination with any dose of HPV-16/18 vaccine (for 137 Preferred Terms in which time-to-onset data were available). Notes: Bubble size is the proportion to the number of reports in any given week (range 1 to 24 cases). Not shown: one case under the neuro-inflammatory system organ class that occurred 189 weeks after vaccination. Other = erythema multiforme; uveitis; Raynaud's phenomenon; Stevens–Johnson syndrome; antiphospholipid syndrome; idiopathic thrombocytopenic purpura; IgA nephropathy; glomerulonephritis rapidly progressive
An observed-to-expected analysis was performed for Guillain–Barré syndrome and Bell's palsy (the two most frequently reported pIMDs), to determine whether the observed number of reports was (or was not) greater than expected within a pre-defined risk period, under the null hypothesis of no association between vaccination and onset of the event (Table 4). The background rates were obtained from literature sources considered to be representative of the vaccinated populations for HPV-16/18 vaccine15,16 and were age stratified.
Observed versus expected* analysis for Guillain–Barré syndrome cases reported in the UK according to Brighton Collaboration Diagnostic Level17
| Age group (years) | Report source | Rate per 100 000 person years | |||
|---|---|---|---|---|---|
| Observed [95%CI] | Expected16 | ||||
| Level 1–3 | Level 1–4 | Level 1–5 | |||
| All ages | Global | 0.13 [0.04; 0.34] | 0.30 [0.14; 0.57] | 0.34 [0.16; 0.62] | 1.22 |
| UK | 0.23 [0.03; 0.82] | 0.68 [0.25; 1.48] | 0.68 [0.25; 1.48] | ||
| 0–14 | Global | 0.00 [0.00; 0.19 | 0.19 [0.04; 0.55] | 0.25 [0.07; 0.64] | 0.42 |
| UK | 0.00 [0.00; 0.64] | 0.42 [0.05; 1.53] | 0.42 [0.05; 1.53] | ||
| 15–24 | Global | 0.39 [0.11; 1.00] | 0.58 [0.21; 1.27] | 0.58 [0.21; 1.27] | 1.08 |
| UK | 0.50 [0.06; 1.81] | 1.00 [0.27; 2.56] | 1.00 [0.27; 2.56] | ||
Diagnostic level 1–3: meets the criteria of Guillain–Barré syndrome (GBS).
Diagnostic level 1–4: includes cases with insufficient evidence to meet the criteria of GBS.
Diagnostic level 1–5: all reported cases including those where a diagnosis of GBS can be reasonably excluded.
The expected number of adverse events after vaccination was calculated using the following formula: number of expected events (Ne) equals the age-specific background incidence rate (Inc) multiplied by the number of doses of vaccine administered (Nd) (calculated for each age group from the age distribution of all spontaneous reports received until the data lock point where the age was known) multiplied by the pre-determined risk period (Risk period) (Ne = Inc × Nd × Risk period).
For Guillain–Barré syndrome, all reports were reviewed based on Brighton Collaboration diagnostic levels,17 considering a risk period of 42 days. The observed incidence rate was below the expected rate within each age stratum for all levels of diagnostic certainty criteria (Table 4). Because half of the cases were reported from the UK, a sensitivity analysis was performed for UK cases (Table 4). Regardless of diagnostic level (1–3, 1–4 and 1–5), the observed incidence rates were equal to or lower than the expected rates for all age strata (Table 4).
Given that most of the Bell's palsy cases were reported in Europe, an observed versus expected analysis was performed with an assumed risk period of 30 days after vaccination. The observed number of reports of Bell's palsy in Europe was below the expected number of reports in the background population measured by Rowlands in the UK (Table 5).15 These analyses are limited by the few number of reported cases, uncertainty around exact age of vaccination, the risk of under-reporting or reporting bias, incomplete clinical details, lack of a control group and uncertainty around the background incidence rates: we used a UK analysis with data available in comparable age ranges.15 However, studies from the USA suggest inter-country/inter-study variation.18,19
Observed versus expected* analysis for Bell's palsy cases reported in Europe
| Sensitivity analysis | Time to onset | N observed [95%CI] | N expected* (Rowlands et al.15) |
|---|---|---|---|
| 100/100 | 7 | 10 [4.79; 18.39] | 34 |
| 100/100 | 30 | 13 [6.91; 22.23] | 147 |
| 75/75 | 7 | 12 [6.19; 20.96] | 26 |
| 75/75 | 30 | 16 [9.14; 25.99] | 110 |
The expected number of cases was calculated for the age distribution from cases in the safety database for the reference study by Rowland et al.15 In the sensitivity analysis, it is assumed that there is 25% of under-reporting and that 75% of the doses distributed have been administered.
Vaccination coverage information among women vaccinated in each age strata in Rowlands et al.15 is not available. Therefore, the proportion of all Cervarix® spontaneous cases reported in Europe within each age stratum was used to weight each age stratum provided in the reference. Adjusted background incidence rates (representative of the vaccinated population) were calculated by taking the weighted average of the reference incidence rate. This adjusted background incidence rate is used to make the comparison with the observed incidence rate.
Pregnancy outcomes
GlaxoSmithKline receives pregnancy exposure reports from all countries as part of routine passive safety surveillance and via Pregnancy Registries established in the UK (2008, managed by Public Health England) and in the USA (2009, managed by GSK).
Pregnancy outcomes were monitored among cases encoded with the MedDRA PT ‘vaccine exposure during pregnancy’. Intrauterine deaths were defined as spontaneous abortions in pregnancies of <22 weeks gestation and stillbirths of ≥22 weeks gestation.20 Each pregnancy outcome was classified according to the presence/absence of congenital anomalies defined as any morphological, functional and/or biochemical developmental disturbance in the embryo or foetus whether detected at birth, or not.20 Congenital anomalies included birth defects identified by prenatal ultrasound, amniocentesis or examination of the products of conception after elective or spontaneous abortion. Live-born infants with only transient or infectious conditions, or biochemical abnormalities, were classified as free of birth defects unless there was a possibility that the condition reflected an unrecognised birth defect. Consistency in the application of definitions of birth defects was achieved using the Centers for Disease Control and Prevention Metropolitan Atlanta Congenital Defects Program as a reference.21
Pregnancy data were collected as part of routine global pharmacovigilance. Nevertheless, the global post-licensure data are dominated by reports from the UK, reflecting the reporting registry in place in that country. Full methods and details of data obtained through the UK Vaccine in Pregnancy surveillance are described elsewhere (PHE personal communication, manuscript in preparation).
As of 17 November 2011, there were a total of 487 prospective pregnancy reports of which 81 were ongoing and 173 were lost to follow-up. Around 52% of reports were from the UK and US registries. Among the 233 prospective pregnancy reports with a known pregnancy outcome, 226 reports were within the pre-defined risk period (Table 6): there were 189 live births (of which 17 were classified with congenital anomalies); 19 elective terminations for social or medical reasons; 17 spontaneous abortions; and one stillbirth.
Prospective pregnancy reports with a known pregnancy outcome, for women exposed to HPV-16/18 vaccine within the pre-defined risk period* (N = 226)
| Trimester of exposure | Outcome classified with no congenital abnormality | Outcome classified with a congenital abnormality | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Live birth | Stillbirth | Elective termination | Spontaneous abortion | Live birth | Stillbirth | Elective termination | Spontaneous abortion | ||
| Within 60 days prior to pregnancy onset | 51† | 1 | 3 | 7 | 4 | 0 | 0 | 0 | 66 |
| First trimester | 103† | 0 | 14 | 8 | 12 | 0 | 0 | 0 | 137 |
| Second trimester | 9‡ | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 11 |
| Third trimester | 2‡ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Unknown trimester | 7 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 10 |
| Total | 172 | 1 | 19 | 17 | 17§¶ | 0 | 0 | 0 | 226 |
Risk period defined as vaccination with Cervarix® within 60 days prior to pregnancy onset up to whole duration of pregnancy. Multi-exposures during pregnancy:
Three cases were identified as having been exposed to Cervarix® more than once within 60 days and during the first trimester.
One case was identified as having been exposed to Cervarix® more than once during the second and third trimesters.
For one case, the congenital anomaly was observed 1 year after birth.
Five were classified as major structural defects including cardiac (2), palate (1), abdominal wall (1) and genital (1) structural defects, and 12 were classified as minor or non-structural anomalies.
Of these 226 pregnancy reports, 216 women were vaccinated with HPV-16/18 vaccine within a pre-defined risk period,6 defined as vaccination with HPV-16/18 within 60 days prior to the estimated date of conception (66 women), or had unintended vaccination during pregnancy (150 women). The majority of unintended vaccinations during pregnancy occurred during the first trimester (Table 6). Five of the 189 live infants (2.6%) were classified as having a major structural congenital anomaly.22 The remaining 12 reported congenital anomalies were minor or non-structural. There was no particular pattern of anomalies suggestive of a possible teratogenic effect. In nine of the 17 live births with congenital anomalies, maternal vaccination occurred either before the estimated pregnancy onset (four reports) or during the first trimester but within the first 14 days after conception (peri-conceptual period in which hazardous exposures usually cause embryonic death rather than injury23,24) (Table 6). The rate of major congenital anomalies was within the expected background population rate of 2–3%.25,26 No trends were observed, and the rate of spontaneous abortion is in line with reported rates in the UK and USA.27–30
Events with fatal outcome
GlaxoSmithKline received a total of five reports with a fatal outcome from subjects who received HPV-16/18 vaccine in the post-licensure setting during the study period. None were assessed by the reporter as causally related to vaccination. The causes of death were associated with the subjects' medical conditions and/or other factors, as follows: streptococcus group A septicaemia (UK), underlying malignancy (UK), suspected snake bite (India), severe anaemia associated with malaria infection (India) and an inherited catecholaminergic polymorphic ventricular tachycardia (Japan).
Post-licensure data leading to a change to the prescribing information
As data accumulate during the early stages of market introduction, notably in settings of mass immunisation programmes where high vaccine uptake is expected in a short period, a systematic, regular review of aggregate safety data is essential to detect any safety signals and determine whether changes are needed to the prescribing information. In 2009, an aggregate review of all reports of anaphylaxis, angioedema and syncope reports was triggered by a signal detected based on passive AE reporting in the UK. The observed incidence rate was 3/1 000 000 doses for anaphylaxis, 8/1 000 000 doses for angioedema and 32/1 000 000 doses for syncope. Medical assessment of the individual cases determined that these AEs could be reasonably assumed to be causally associated with HPV-16/18-vaccine administration because of the short onset time following vaccination for many cases and the lack of other apparent/stated alternative explanations. Thus, the product prescribing information was updated to include these events based on post-licensure experience.
SAFETY SURVEILLANCE IN NATIONAL IMMUNISATION PROGRAMMES
Human papillomavirus-16/18 vaccine is used routinely in national immunisation programmes in the UK, the Netherlands, Japan, Malaysia, Mexico, Argentina, South Korea, and in regions of Spain and Italy. Regional safety surveillance activities initiated in Italy assessed AEs within 7 days after HPV vaccination using a standard questionnaire.10 No serious AEs were reported by the 4643 study subjects. No safety concerns were identified, and the authors noted that the good safety profile of HPV-16/18 vaccine had been confirmed. Here, we report the results of enhanced safety monitoring initiated by national regulatory and public health agencies in the UK and the Netherlands.
National immunisation in the UK: 2-year experience with human papillomavirus-16/18 vaccine
Human papillomavirus universal immunisation using HPV-16/18 vaccine was initiated in the UK in September 2008 for 12–13 year old girls, with a catch-up programme that included girls/women 14–18 years of age. Coverage with the complete three-dose regimen was 81% in 2010.31
Based on experience with ADR reporting after the introduction of universal immunisation against meningococcal serogroup C, the Medicines and Healthcare products Regulatory Agency (MHRA) and Departments of Health anticipated a large volume of ADR reports soon after HPV-16/18-vaccine introduction. Thus, it was necessary to develop procedures and processes to handle the expected large numbers of AE reports.
Educational material detailing types of AEs and how to prevent and report them was issued to primary care organisations. GSK collaborated closely with the MHRA to conduct enhanced safety surveillance over the 2-year period of the programme. Contact details within both organisations were defined for urgent escalation of potential safety issues. Information actively exchanged between GSK and the MHRA on a weekly basis included transmission of safety signal listings and results of comprehensive signal reviews performed by both parties. All suspected ADRs were posted weekly on the MHRA Internet webpage.
At least 4.5 million doses of HPV-16/18 vaccine were administered in the UK by July 2010. A total of 4703 reports including 10 410 events terms were reported to MHRA in association with HPV-16/18 vaccination over the 2 years of the programme.32 The most frequently reported events (37%) were related to recognised side effects listed in the prescribing information. Of these, 21% of reports were ‘psychogenic reactions’ such as syncope and panic attacks related to the vaccination procedure, which are relatively common in adolescents; 17% were injection-site reactions, and 11% were classified as allergic type reactions, including 11 cases that met the Brighton Collaboration criteria for a definition of anaphylaxis. The remaining cases in this category related to a wide range of generalised signs and symptoms suggestive of a possible allergic event, such as rashes and other skin reactions. At the end of the second year of enhanced safety surveillance, the MHRA concluded that the benefit–risk ratio of HPV-16/18 vaccine remained positive.32
The untimely death of an adolescent in the UK within hours of vaccination stimulated global media attention. Prior to determining the true cause of death and as a precautionary measure, GSK immediately and voluntarily quarantined remaining doses of the lot administered to the teenager, and recalled the lot on the day following the event. An in-depth manufacturing investigation was launched and revealed no quality issues with the vaccine. The initial reported diagnosis was a possible anaphylactic reaction, but post-mortem results showed that the girl had a serious underlying medical condition (an undiagnosed rare malignancy in the chest). Immediate responses by GSK, the MHRA and the UK Department of Health encouraged balanced and responsible reporting by journalists.
This collaboration enabled real-time, scientifically robust analysis of vaccine safety. Weekly publication of safety reports by the MHRA33,34 provided reassurance on events likely to be coincidental to vaccination and helped minimise unfounded concerns among parents and teenagers. The MHRA concluded that the active safety monitoring had proven to be a model on which to build a similar strategy for future major immunisation programmes in the UK.9,32
National immunisation in the Netherlands: 2-year experience with human papillomavirus-16/18 vaccine
In the Netherlands, safety monitoring is the initiative of the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment (RIVM). A catch-up campaign for all girls born from 1993 to 1996 continued from 2009 into 2010, and routine immunisation of 12-year-old girls began in 2010. Intensive safety surveillance undertaken in 2009 and 2010 employed three tools: reporting by staff of AEs occurring immediately after vaccination on pre-distributed forms; routine passive surveillance; and a web-based questionnaire administered to over 3000 girls after each dose.
During 2010, there were 130 reports of immediate reactions following 168 134 vaccinations.11 The most frequently reported immediate reactions were presyncope and syncope. No cases of anaphylaxis were noted, and the medical impact of the reported events was considered low. There were 129 spontaneous AE reports in 2010, and injection-site reactions, minor general illnesses and fainting were the most frequent causally related events.11 The results of the web-based survey indicated that local and general reactions were reported frequently after vaccination and were influenced by season, age of the subject and dose number. Most reactions were mild in nature and transient. The RIVM concluded that no unexpected AEs were found and that the results contributed to confidence in the safety of the product.11
CONCLUSION
Clinical studies conducted during vaccine clinical development are essential but usually too limited in size to detect rare AEs such as pIMDs. Moreover, temporal and geographical clustering of AEs occurring by chance can be misinterpreted by health professionals, the public and the media as being causally related to a vaccine, and have the potential to stop a vaccination campaign. Post-licensure safety monitoring and post-authorisation safety studies can assess safety outcomes in large populations but require enhanced data collection methods and effective signal detection and management strategies.35 Although AE reporting systems allow the evaluation of events at a population level, there are limitations to spontaneous AE data,36 including under-reporting, lack of denominator data (i.e. number of patients exposed as estimated by the number of doses distributed), misclassifications or miscoding, and biases related to length of time on the market and the reporting environment. In addition, whereas passive surveillance can identify safety signals that warrant further evaluation, the information provided is frequently insufficient to determine whether a causal association between the vaccine and the reported AE exists.
Most countries rely on passive reporting of AEs after vaccination, and reporting rates are therefore highly variable and influenced by the media, awareness/ease of use of the AE reporting system, cultural expectations and experience with the vaccine or with similar vaccines. So far, active and ongoing evaluation of safety in countries with high rates of HPV-16/18 vaccine use in mass immunisation programmes has not shown any safety concerns and has added to the confidence of healthcare providers in those countries. Cases resembling Complex Regional Pain Syndrome (CRPS; also known as reflex sympathetic dystrophy or causalgia) were reported after HPV vaccination in Japan (n = 24 with more than 8 million doses distributed),37 where both HPV-16/18 vaccine and quadrivalent HPV vaccine are used. CRPS is a chronic neuropathic pain disorder distinguished by significant autonomic features, typically developing in an extremity after acute tissue trauma. Although no causal association with vaccination has been established, Japan temporarily stopped actively promoting the use of both HPV vaccines on 14 June 2013. HPV vaccination remains part of the Japanese government-funded vaccine programme.37 Review and re-evaluation of available data is scheduled.
Collaborative approaches between industry and national authorities contribute to higher ADRs and pregnancy reporting rates, and facilitate rapid responses in the event of a signal. Such collaborations also aid in the interpretation and management of serious AEs that may give rise to public concern.
Post-licensure safety surveillance after more than 4 years of HPV-16/18 vaccine use confirms the acceptable benefit–risk of vaccination in adolescent girls and adult women. No safety concerns have been identified since the data lock point. GSK continues to closely monitor pIMDs and pregnancy outcomes, with no specific safety concern identified from more than 4 years of HPV-16/18 vaccine use in routine clinical practice.
TRADEMARKS
Cervarix is a registered trademark of the GlaxoSmithKline Group of Companies.
CONFLICT OF INTEREST
Maria-Genalin Angelo, Julia Zima, Fernanda Tavares Da Silva, Laurence Baril and Felix Arellano are all employed by GlaxoSmithKline Vaccines and hold shares in the company as part of their employee remuneration.
KEY POINTS.
Ongoing active review and analysis of safety data is routinely undertaken by GlaxoSmithKline Vaccines to detect and investigate potential safety signals arising through use of marketed vaccines. Post-licensure surveillance passive reports from countries around the world showed that the distribution of the most frequently recognised adverse reactions after HPV-16/18 vaccination was as anticipated.
An analysis of potentially immune-mediated diseases after vaccination showed no patterns or trends for concern. The observed incidences of VIIth nerve (facial) palsy and Guillain–Barré syndrome were within the overall range of expected background incidence rates in the general population.
Pregnancy outcomes of women who were vaccinated with HPV-16/18 vaccine during pregnancy were in line with published literature for similar populations.
Enhanced surveillance of adverse events following introduction of HPV-16/18 vaccine in national immunisation programmes in the UK and the Netherlands confirmed an acceptable safety profile, as evaluated in clinical trials.
The safety data of HPV-16/18 vaccine 5 years post-licensure confirm its acceptable benefit–risk profile in women of all ages.
ETHICS STATEMENT
For all clinical studies included in this analysis, written informed consent or assent was obtained from all participants or their parents, or both. The protocol of each study and other materials were approved by independent ethics committees or institutional review boards.
Acknowledgments
The authors thank all healthcare professionals, regulators and individuals who report adverse reactions after vaccination to GSK Vaccines and on whom post-licensure surveillance relies. We thank Marta Lopez for the pregnancy exposure data analysis, Benedicte Vallery and Anne-Françoise Delsaute for management of surveillance safety data, GSK-UK affiliates for operational support, and all employees of GSK Vaccines at the time of study conduct. We also thank Thomas Verstraeten who provided guidance and support in the overall functioning of surveillance systems during his term at GSK Vaccines. We thank the MHRA and the Department of Health in the UK for the joint collaboration in enhanced safety monitoring for Cervarix® over the 2-year mass immunisation programme, as well as the Health Protection Agency for establishing the Pregnancy Exposure Registry in the UK.
Writing support services were provided by Joanne Wolter (independent medical writer, Brisbane, Australia); editing and publication co-ordinating services were provided by Veronique Delpire and Mandy Payne (Words and Science, Brussels, Belgium). All costs related to the development of this manuscript were met by GlaxoSmithKline Biologicals SA.
GlaxoSmithKline Biologicals SA was the funding source and was involved in all surveillance activities and overall data management (collection, analysis and interpretation); GlaxoSmithKline also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
REFERENCES
- 1.Macartney KK, Chiu C, Georgousakis M, Brotherton JML. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;36:393–412. doi: 10.1007/s40264-013-0039-5. [DOI] [PubMed] [Google Scholar]
- 2.Medical Dictionary for Regulatory Activities Maintenance and Support Services Organization. 2012. http://www.meddramsso.com/ (accessed 21 November 2012)
- 3.Report of CIOMS Working Group IV. Benefit–risk balance for marketed drugs: evaluating safety signals. 1998. http://www.cioms.ch/publications/g4-benefit-risk.pdf (accessed 21 November 2012)
- 4.Verstraeten T, Descamps D, David M-P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26:6630–6638. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Cervarix Summary of Product Characteristics. 2012. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000721/human_med_000694.jsp&mid=WC0b01ac058001d124 (accessed 21 November 2012)
- 6.Angelo M-G, David M-P, Zima J, et al. Pooled analysis of large and long-term safety data from the human papillomavirus (HPV)-16/18-AS04-adjuvanted vaccine clinical trial programme. (in press). [DOI] [PMC free article] [PubMed]
- 7.Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. The European Pharmacovigilance Research Group. Eur J Clin Pharmacol. 1997;52:423–427. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Fung M, Hornbuckle K, Muniz E. Impact of geographic and cross-cultural differences on spontaneous adverse events reporting. Drug Inf J. 1999;33:921–931. [Google Scholar]
- 9.Medicines and Healthcare products Regulatory Agency (MHRA) Public assessment report. Cervarix (HPV vaccine): update on UK safety experience at the end of 4 years use in the HPV routine immunisation programme. 2012. December http://www.mhra.gov.uk/PrintPreview/DefaultSplashPP/CON023340?ResultCount=10&DynamicListQuery=&DynamicListSortBy=xCreationDate&DynamicListSortOrder=Desc&DynamicListTitle=&PageNumber=1&Title=Human%20papillomavirus%20 (HPV)%20vaccine (accessed 17 January 2013)
- 10.Gasparini R, Bonanni P, Levi M, et al. Safety and tolerability of bivalent HPV vaccine: an Italian post-licensure study. Hum Vaccin. 2011;7(Suppl):136–146. doi: 10.4161/hv.7.0.14576. [DOI] [PubMed] [Google Scholar]
- 11.van't Klooster T, Kemmeren J, Vermeer-de-Bondt P, et al. Adverse events following vaccination against human papillomavirus: results of the 2010 campaign in the Netherlands. 2011. http://www.rivm.nl/en/Library/Scientific/Reports/2011/december/Adverse_events_following_vaccination_against_human_papillomavirus_Results_of_the_2010_campaign_in_the_Netherlands?sp=cml2bXE9ZmFsc2U7c2VhcmNoYmFzZT01OTAzMDtyaXZtcT1mYWxzZTs=&pagenr=5904 (accessed 10 September 2012)
- 12.Bonhoeffer J, Heininger U, Kohl K, et al. Standardized case definitions of adverse events following immunization (AEFI) Vaccine. 2004;22:547–550. doi: 10.1016/s0264-410x(03)00511-5. [DOI] [PubMed] [Google Scholar]
- 13.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 14.Tavares da Silva F, De Keyser F, Lambert P-H, et al. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31(14):1870–1876. doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell's palsy in the UK. Eur J Neurol. 2002;9:63–67. doi: 10.1046/j.1468-1331.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 16.Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain–Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med. 2006;166:1301–1304. doi: 10.1001/archinte.166.12.1301. [DOI] [PubMed] [Google Scholar]
- 17.Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain–Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Siegrist C-A, Lewis EM, Eskola J, Evans SJW, Black SB. Human papilloma virus immunization in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J. 2007;26:979–984. doi: 10.1097/INF.0b013e318149dfea. [DOI] [PubMed] [Google Scholar]
- 19.Savettieri G, Salemi G, Rocca WA, et al. Incidence and lifetime prevalence of Bell's palsy in two Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Acta Neurol Scand. 1996;94:71–75. doi: 10.1111/j.1600-0404.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency (Committee for Medicinal Products for Human Use) London: EMEA; 2005. Guideline on the exposure to medicinal products during pregnancy: need for post-authorisation data. [Google Scholar]
- 21.Birth defects and genetic diseases branch 6-digit code for reportable congenital anomalies. 2013. http://www.cdc.gov/ncbddd/birthdefects/documents/macdpcode0807.pdf (accessed 05 Sept )
- 22.Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res Part A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 23.Bánhidy F, Lowry RB, Czeizel AE. Risk and benefit of drug use during pregnancy. Int J Med Sci. 2005;2:100–106. doi: 10.7150/ijms.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polifka JE, Friedman JM. Medical genetics: 1. Clinical teratology in the age of genomics. CMAJ. 2002;167:265–273. [PMC free article] [PubMed] [Google Scholar]
- 25.Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–364. doi: 10.1007/978-90-481-9485-8_20. [DOI] [PubMed] [Google Scholar]
- 26.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res Part A Clin Mol Teratol. 2007;79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 27.Saraiya M, Berg CJ, Shulman H, Green CA, Atrash HK. Estimates of the annual number of clinically recognized pregnancies in the United States, 1981–1991. Am J Epidemiol. 1999;149:1025–1029. doi: 10.1093/oxfordjournals.aje.a009747. [DOI] [PubMed] [Google Scholar]
- 28.Seamark C. Design or accident? The natural history of teenage pregnancy. J R Soc Med. 2001;94:282–285. doi: 10.1177/014107680109400607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura SJ, Abma JC, Mosher WD, Henshaw SK. Estimated pregnancy rates by outcome for the United States, 1990–2004. Natl Vital Stat Rep. 2008;56:1–25. 28. [PubMed] [Google Scholar]
- 30.Devine S, West S, Andrews E, et al. The identification of pregnancies within the general practice research database. Pharmacoepidemiol Drug Saf. 2010;19:45–50. doi: 10.1002/pds.1862. [DOI] [PubMed] [Google Scholar]
- 31.The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. 2010. http://www.eurosurveillance.org/viewarticle.aspx?ArticleId=19730 (accessed 7 September 2012) [DOI] [PubMed]
- 32.Medicines and Healthcare products Regulatory Agency (MHRA) MHRA Public Assessment Report. Cervarix (HPV vaccine): update on UK safety covering the first two years of the HPV immunisation programme. 2010. October http://www.mhra.gov.uk/PrintPreview/DefaultSplashPP/CON023340?ResultCount=10&DynamicListQuery=&DynamicListSortBy=xCreationDate&DynamicListSortOrder=Desc&DynamicListTitle=&PageNumber=1&Title=Human%20papillomavirus%20 (HPV)%20vaccine (accessed 7 September 2012)
- 33.Medicines and Healthcare products Regulatory Agency (MHRA) YellowCard. Helping to make medicines safer. 2012. http://yellowcard.mhra.gov.uk/ (accessed 17 January 2012)
- 34.Medicines and Healthcare products Regulatory Agency (MHRA) Human papillomavirus (HPV) vaccine. 2013. http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Product-specificinformationandadvice-G-L/HumanpapillomavirusHPVvaccine/index.htm (accessed 17 January )
- 35.Wise L, Parkinson J, Raine J, Breckenridge A. New approaches to drug safety: a pharmacovigilance tool kit. Nat Rev Drug Discov. 2009;8:779–782. doi: 10.1038/nrd3002. [DOI] [PubMed] [Google Scholar]
- 36.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998;20:C40–C44. doi: 10.1016/s0149-2918(98)80007-6. [DOI] [PubMed] [Google Scholar]
- 37.Global Advisory Committee on Vaccine Safety. Wkly Epidemiol Rec. 2013;88:301–312. [PubMed] [Google Scholar]
- 38.Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 39.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]


