Abstract
Purpose
The increase in online purchasing of medications raises safety concerns regarding teratogenic drugs. The use of the teratogenic drug ‘isotretinoin’ for women of childbearing age requires strict adherence to the Pregnancy Prevention Programme (PPP), a risk minimisation measure imposed on prescribers and users. We sought to determine how readily consumers can purchase isotretinoin online and the associated safety procedures and information.
Methods
A descriptive cross-sectional survey was conducted of 50 e-pharmacies identified from commonly used search engines. E-pharmacy characteristics and isotretinoin PPP specific criteria were evaluated. Purchases of isotretinoin from seven e-pharmacies not bearing authentication logos and not requiring a prescription were assessed for PPP policy adherence, purchasing procedures and compound quality.
Results
Forty-three (86%) of the e-pharmacies did not have an authentication seal/logo. Isotretinoin could be purchased from 42 sites without a valid prescription. Information on isotretinoin causing birth defects was lacking in 25 of the 50 sites, on not taking isotretinoin in pregnancy in 24 sites and not taking isotretinoin if planning or at risk of a pregnancy in 33 sites. Of the eight attempted purchases, seven arrived, all without any patient information leaflet. All were verified as isotretinoin.
Conclusion
The Internet provides a loophole for purchasing of medications known to cause congenital abnormalities, which needs to be addressed by medicines regulatory agencies worldwide. The current PPP for isotretinoin may be failing to protect mothers and babies from preventable harm—clinicians need to be aware of this, and the public needs to be educated about the potential risks.
Keywords: isotretinoin, e-pharmacies, pregnancy prevention programmes, online purchasing, pharmacoepidemiology
INTRODUCTION
The increase in online purchasing of medication raises numerous safety concerns,1 particularly for teratogenic drugs—medications which if taken in early pregnancy can cause birth defects. Isotretinoin (brand name Accutane®/Roaccutane®) is a commonly used and effective drug for the treatment of severe acne vulgaris that has not responded to other treatments.2–4 However, it is known to cause birth defects.5,6 Pregnancy is an absolute contraindication to treatment with isotretinoin as harm to the fetus may occur before the pregnancy is known, and the teratogenic potential remains up to 1 month following stoppage.2,3 There is a 25-fold relative risk for birth defects with a characteristic pattern of craniofacial, cardiac, thymic and central nervous system malformations among babies of women exposed to isotretinoin in early pregnancy.6
The use of the drug for women of childbearing age requires strict adherence to a Pregnancy Prevention Programme (PPP), a risk minimisation measure imposed on prescribers and users as a condition of licensing.2,3 PPPs were first introduced in 1988 with the aim of preventing isotretinoin-exposed pregnancies. Since then, studies revealing poor compliance and higher than acceptable pregnancy rates have led to progressively stricter PPPs in the USA and in Europe with the prescribing of isotretinoin limited to dermatologists and selected clinicians. A prerequisite of these programmes is that women of childbearing age must sign up to effective contraception and regular pregnancy tests.2,3 Doubt continues to be expressed about the effectiveness of PPPs,7 as a number of identified isotretinoin-exposed pregnancies are still being reported.8,9 Non-compliance among physicians and women has been highlighted as a problem.10,11 Honein and colleagues reported over 10 years ago that women obtained isotretinoin from sources other than registered prescribers.12
In the recent years, the online sales of pharmaceuticals have become a rapidly growing phenomenon, and sales continue to escalate.1,13 In 2004, the US Government Accountability Office recognised that consumers could purchase isotretinoin online without a prescription,14 and more recently, Lott and Kovarik reported the proliferation of websites purporting to sell dermatologic medications without prescription, including isotretinoin.15
In the context of a wider study to assess the implications of the Internet in relation to medication access and safety information for pregnancy (http://euromedicat.eu/) and in the light of the ongoing need to review the effectiveness of the PPP for isotretinoin, this paper reports a study to determine how readily consumers can purchase isotretinoin online and the associated safety procedures and information.
METHODS
The first phase of the study involved a cross-sectional descriptive survey identifying e-pharmacies (also commonly known as Internet or online pharmacies) accessible from the UK (September 2011) purporting to sell isotretinoin and evaluating each site in terms of e-pharmacy characteristics and isotretinoin PPP specific safety criteria. The second phase involved purchasing isotretinoin from a randomly selected subset of the e-pharmacies not bearing authentication logos, assessing these sites for PPP policy adherence and purchasing procedures and analysing the samples to check for chemical authenticity.
Search strategy
The term ‘buy isotretinoin’ was entered into the five most common search engines in order of ranking used by laypersons16—Google.com, Yahoo.com, Bing.com, Ask.com and Teoma.com. Eligible sites were e-pharmacies accessible in the UK claiming to sell isotretinoin. Websites that were duplicate matches within a search engine or identified from a previous search engine, had a non-functional link, were purely an advertising site, inaccessible or a portal for other sites or not in English were excluded. As the majority of users tend only to examine the first 10 results they obtain from a search engine,17 the first 10 eligible non-excluded e-pharmacy sites sequentially found within each of the search engines were selected for evaluation. In total, 242 sites were accessed by this process before the target sample of 10 different e-pharmacies (50 in total) from each search engine was achieved.
Evaluation of e-pharmacy characteristics
A Web-based IP address lookup location tool was used to trace the geographical location of each e-pharmacy. For the purposes of this study, an e-pharmacy was classified as ‘legitimate’ if it had an authentic accreditation seal/logo (e.g. Verified Internet Pharmacy Practice Sites Programme (VIPP),18 Canadian International Pharmacy Association19 or General Pharmaceutical Council20). Such seals/logos assure consumers that the pharmacy provider is licensed, reputable and adheres to stringent safety protocols. As rogue Internet pharmacies have been known to hijack accreditation logos and place them on their website,21 authenticities were verified if the seal had a hyperlink that led the user to a verification Web page.
We developed a survey instrument to evaluate the quality of the e-pharmacies using items from the DARTS (Date, Author, References, Type and Sponsor) tool22—a tool for assessing the quality of online medicine information and requirements outlined in the VIPP19 and the PharmacyChecker verification programme.23 Items from DISCERN,24a checklist to help users of consumer health information judge the quality of written information (e.g. Did the site describe risks of treatment?), and the Health on the Net Foundation25 standards for evaluating the credibility of the sources (e.g. transparency—was there a contact email address?) were also incorporated. The readability of the information was not assessed.
Evaluation of isotretinoin Pregnancy Prevention Programme specific criteria
The content coverage of the information specific to isotretinoin and pregnancy was assessed for accuracy and completeness using 14 items derived from PPPs approved by the Food and Drug Administration (iPLEDGE) and Medicines and Healthcare Products Regulatory Agency (MHRA) for dispensing isotretinoin to patients at risk of pregnancy.2,3 Two of the authors independently (BML, BW) reviewed and evaluated each of the 50 websites to determine whether each criterion was ‘fully met’, ‘partially met’ or ‘not met’ (i.e. provided no information). Information was classified as ‘poor’ quality if the site did not mention that isotretinoin may cause severe birth defects or did not mention that it should not be taken in pregnancy or planning or at risk of a pregnancy.
Purchasing process
Within funds available for the research, a purchasing request was submitted to the first eight illegitimate e-pharmacies (within the list of 50 surveyed), which reported selling Accutane in 20 mg capsules, did not require a prescription, did not require the consumer to register as a member and each had a different payment IP addresses. A specially set-up prepaid credit card, email account and an off-campus delivery address were used for purchasing the samples, for protection of the researcher and for reducing the risk of refusal by the e-pharmacy.
Medications were assessed for price, conditions of purchase [on the basis of European Council Directive (2001/83/EC criteria)]26 and basic quality. Quality and purity of the purchased medications were assessed by one of the authors (DU) using high-performance liquid chromatography with diode array detection. Prior to be being sent for analysis, the seven purchased samples and a ‘baseline standard control’, that is, known genuine sample from a local hospital pharmacy, were labelled A–H so the assessor was blinded to the products and their source.
RESULTS
E-pharmacy characteristics
In this study, 58% (n = 29) of the websites originated from Western Europe, 32% (n = 16) from North America and 8% (n = 4) from Eastern Europe. The origin of one site could not be identified. The majority of the sites (n = 45, 90%) had a generic registration such as .com (n = 38), .net (n = 4) or .org (n = 3) domain. The remaining five websites had a country coded suffix (e.g. .uk, .us) domain. Almost half (n = 21, 42%) of the e-pharmacies had home and purchasing pages hosted on servers in different countries. Five had ‘Canadian’ in the title of their Web page, but the IP address indicated a domain location in various countries of Europe.
Eight sites displayed a pharmacy accreditation seal; however, one of these was not authentic. Therefore, seven (14%) were eligible to be classified as a ‘legitimate’ e-pharmacies. The majority of the e-pharmacies (n = 47, 94%) displayed some form of medication quality assurance policy or statement such as ‘all generic pills are FDA approved’, but 34 (68%) provided no indication as to where the medication was manufactured. Fifteen (30%) stated the medication was manufactured in India and one in the UK.
Table 1 provides an overview of risk reduction measures evident from reviewing each website. Displaying an ‘authentic’ accreditation seal did not necessarily mean there were procedures in place to minimise the risk of harm for consumers purchasing this medication online.
Risk reduction procedures for purchasing isotretinoin displayed on the websites of 50 e-pharmacies
| Measure | Non-authenticated sites (n = 43) | Authenticated sites (n = 7) |
|---|---|---|
| Prescription requirements for isotretinoin | ||
| • None required | 32 | 1 |
| • ‘Prescription Policy’* | 9 | 0 |
| • Required from own healthcare practitioner | 0 | 6 |
| • ‘May be required’ | 2 | 0 |
| Asked to complete medical questionnaire | ||
| • No | 34 | 5 |
| • Optional | 7 | 1 |
| • Compulsory | 7 | 1 |
| Restriction on quantity | ||
| • No limit stated | 40 | 1 |
| • As stated on prescription | 0 | 3 |
| • 1 month supply | 2 | 1 |
| • 3 months supply | 1 | 2 |
| Procedure for reporting adverse drug events | ||
| • Not mentioned | 24 | 4 |
| • Consumer contacts own physician | 18 | 3 |
| • Consumer or GP contacts Food and Drug Administration/Medicines and Healthcare products Regulatory Agency | 1 | 0 |
A hidden rider added to some websites, which stated that the consumer was confirming they ‘had a valid prescription for the drug’.
There were variations in prescription requirements (Table 1). Thirty-three sites including one authenticated site required no prescription at all. Some sites had a ‘Prescription Policy’ as a link in diminutive font size at the bottom of the Web page. These ‘Prescription Policies’ included a disclaimer statement should the consumer suffer any adverse effects from taking the medication and shifted the responsibility for the purchase from the supplier to the purchaser, evidenced in the following statement:
…by placing the order on our site the user customer confirms …they have a valid prescription for the drug they are ordering and their doctor has full knowledge of this and all medications they are taking.
(Website 27—URL: Canada)
Sixteen of the e-pharmacies asked the customer to complete a medical questionnaire; however, this was optional in half of these sites. The majority of sites (82%), including one with a VIPP logo, did not stipulate a limit to the number of capsules that could be purchased. Prices ranged from 50p to £3.67 per capsule. Purchasing of single capsules was possible, but in some cases, it was restricted to a minimum of 30.
Evaluation of pregnancy prevention information
Table 2 provides an evaluation of the content of the information provided in relation to isotretinoin and pregnancy. The Cronbach's alpha for the PPP specific criteria 14-item scale was 0.906 indicating high internal reliability. In the study, 78% of the sites provided information of ‘poor’ quality as judged by whether any of the first three criteria shown in Table 2 were not fully met. Only one site complied with all of the requirements regarding information, by providing a link to a PPP.
Evaluation of information provided on 50 e-pharmacy websites in relation to isotretinoin and pregnancy using Medicines and Healthcare products Regulatory Agency4 and iPLEDGE5 Pregnancy Prevention Programme criteria
| Pregnancy Prevention Programme Specific Criteria | Fully met n (%) | Partially met n (%) | Not met n (%) | |
|---|---|---|---|---|
| Non-authenticated sites (n = 43) | Authenticated sites (n = 7*) | |||
| Isotretinoin may cause severe birth defects | 24 (48) | 1 (2) | 23 (53) | 2 (29) |
| Not to be taken in pregnancy | 20 (40) | 6 (12) | 21 (49) | 3 (43) |
| Not to be taken if planning a pregnancy | 14 (28) | 3 (6) | 28 (65) | 5 (71) |
| Speak to a doctor before taking | 9 (18) | 8 (16) | 30 (70) | 3 (43) |
| Two negative pregnancy tests prior to starting therapy | 6 (12) | 4 (8) | 35 (81) | 5 (71) |
| Use two forms of contraception | 18 (36) | 1 (2) | 26 (60) | 5 (71) |
| Avoid progesterone only pill | 3 (6) | 13 (26) | 28 (65) | 6 (86) |
| Certain medicines e.g. St John's Wort may make contraceptives' less effective | 2 (4) | 2 (4) | 40 (93) | 6 (86) |
| Monthly pregnancy test | 7 (14) | 9 (18) | 28 (65) | 6 (86) |
| Not getting pregnant for at least one month after stopping treatment | 16 (32) | 1 (2) | 28 (65) | 5 (71) |
| Informing doctor straight away if should become pregnant while taking isotretinoin | 6 (12) | 3 (6) | 36 (84) | 5 (71) |
| Increases chance of miscarriage | 4 (8) | 1 (2) | 39 (91) | 6 (86) |
| Don't share medicine with anyone else, particularly females | 1 (2) | 13 (26) | 30 (70) | 6 (86) |
| Not donating blood for one month at least post stopping treatment | 17 (34) | 1 (2) | 26 (60) | 6 (86) |
One site provided a link to the Food and Drug Administration iPLEDGE programme and therefore met all criteria.
During the review process, statements provided on the site were observed and recorded. Some of these were misleading: with some openly encouraging online purchasing of isotretinoin/Accutane/Roaccutane (Table 3).
Table 3.
Statements from websites selling isotretinoin
| Buy Accutane without prescription, as there is no need to wait for your doctor if you can get cured easily getting online Accutane. |
| (Website 4 – URL Germany) |
| Accutane sale is absolutely and is regulated by FDA as well as other regulatory bodies. So if you ask where I can buy Accutane without woories aboutn its safety you can confidently buy Accutane .& If you have a problem with finding out wehre to buy Accutane and have diificulties getting a doctor prescription or you are ashamed to go to the physician fo a prescription list there is a way out for you. Online phamacy is a place where you can find not only cheap Accutane, but also Accutane without prescription. |
| (Website 21-URL: Latvia) |
| In the recent time of improved medical science as well as technology, you can aslo buy Accutane without prescription. In the past, you had to either visit a meidcal store or a pharmacist to get your medicines after showeing the doctor;s original prescription. It was a lot of hassle. But those days are gone now. You can buy Accutane without prescription from our site just with the click of a mouse. |
| (Website 36-URL: Russian) |
Online purchasing
From the eight purchasing requests, seven resulted in delivery of the product. One purchase was blocked by the credit card company as the company was named on their fraudulent blacklist. One of the sites required the purchaser to complete a medical questionnaire, for another, it was optional.
All seven purchased samples were received by mail and franked in India. A custom declaration form displaying minimal information was attached to each package received. Figure 1 illustrates an example of the packaging and presentation of the samples. None of the samples were enclosed in a box or container. Two samples were wrapped in bubble wrap, and one was taped between two pieces of light cardboard. An unexpected gift of four free tablets claiming to be Viagra (we did not test the compound) was received with one of the purchases (Figure 1).
Figure 1.
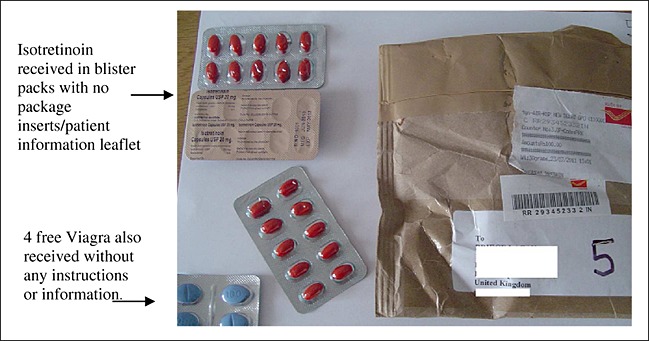
An illustration of how one sample was received
None of the purchases were accompanied with a manufacturer's ‘patient information sheet’. However, four of the samples did have a statement titled: ‘Warning to female patients’ written in minute text on the blister pack: ‘This medication may cause severe birth defects. You must not take this medicine if you are pregnant or may likely to become pregnant during treatment.’ Two of these four samples also had an ‘Avoid in Pregnancy’ logo on each of their blister packs. The remaining three samples contained no reference or warning in relation to pregnancy.
Analysis of online purchases
Table 4 provides details and analysis of the seven procured drugs. The medication ordered from each site was ‘Accutane’ 20 mg, but a range of generic and branded versions were received. The price per capsule of the purchased drugs was approximately twice the price of the same medication accessed locally in the UK (£0.72). All products received were within the expiration date. When the analyses of the purchased capsules were compared against a quality control sample and the British Pharmacopoeia27—standards for isotretinion capsules—none of the samples failed the identification, related substance and assay test.
E-pharmacy and product characteristics and results of analysis of medications purchased from eight illegitimate sites
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| URL location of website | Latvia | Netherlands | USA | Spain | Germany | UK | Russia |
| Source | India | India | India | India | India | India | India |
| Product name | Isotretinoin | Acnomor | Isotretinoin | Treat-A | Irotin | Acnomor | Irotin |
| Expiry date (months) | 8 | 10 | 8 | 1 | 10 | 10 | 5 |
| Price per capsule (£) | 1.89 | 1.32 | 1.86 | 1.45 | 1.40 | 1.45 | 2.56 |
| Acitretinoin | <LOQ* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Isotretinoin (mg) | ±20 | ±20 | ±20 | ±20 | ±20 | ±20 | ±20 |
| Tretinoin† (%) | — | <3 | <2 | — | <1 | <3 | <1 |
| MOB/POB‡ | Present | Present | Present | Present | Present | Present | Present |
<LOQ (limit of quantification) is not detectable.
Tretinoin: a derivative of vitamin A.
MOB, methylparahydroxybenzoate (antifungal and antibacterial agents used as a preservative in pharmaceutical preparations). POB, propylparahydroxybenzoate (an antimicrobial preservative).
DISCUSSION
This study demonstrates that women of childbearing age have the opportunity to purchase isotretinoin directly from websites that do not provide any form of risk assessment, pregnancy prevention advice or adequate warnings of the dangers associated with taking this medication. A prescription is not required for purchase at most sites. Women obtaining isotretinoin from e-pharmacy sites may be at risk of becoming pregnant and being exposed to a known and highly potent teratogen. Even among accredited sites, only one site provided, via a link, the full safety information required by PPPs.
The advent of e-pharmacies has changed the dynamics of the doctor/patient relationship, and the very nature of e-pharmacies allows consumers to bypass the safeguards provided by this relationship. PPPs mandate that isotretinoin can only be prescribed by, or under the supervision of, a specialist with expertise in the use of systemic retinoids for the treatment of severe forms of acne.2,3 The majority of the websites in this study allowed customers to bypass these regulations, allowing any person with access to the Internet to self-prescribe. PPPs also stipulate that women must take effective contraception and regular pregnancy tests, but Internet purchase constitutes a loophole in this risk minimisation measure as women are not required, and usually not even advised, to take these measures. We recommend that PPPs are urgently revised to take into account Internet access to isotretinoin. This issue was previously reported in 2005,14 and to date, there is no evidence of effective action having been taken. Moreover, two of the seven sites from which isotretinoin was purchased without prescription are still (mid 2013) advertising that they are selling isotretinoin, 18 months after, the MHRA were advised of this.
None of the purchased medicines were packaged according to the European council directive 2001/83/EC,25 which stipulates the sale of a medicinal product without a proper container is in contravention to labelling controls S.85(4). Regulators such as the MHRA state that the safe use of all medicines depends on users reading the patient information sheet, labelling and packaging carefully and accurately and being able to assimilate and act on the information presented. Four of the seven samples purchased for this study had minimal pregnancy warning on the blister pack, the other three none at all. None of the drugs had a patient information leaflet or any instructions enclosed on how the medication should be taken. Sites that required a prescription were prepared to accept a fax or email, which is in contravention of the recommendations of the General Pharmaceutical Council20 about what constitutes a valid prescription. According to the National Association of Boards of Pharmacy,18 99% of the Internet drug companies they review are not in compliance with state or federal regulations.28
Governments have been slow to address the Internet pharmacy problem. Because of the nature of the Internet in having no geographical boundaries, and the lack of harmonisation of regulatory policy at international level, products can be bought from almost any part of the world. From January 2013, the ‘new legislation on falsified medicines’ for Europe aims to improve the protection of public health by introducing new harmonised, pan-European rules to ensure that medicines are safe, particularly in being what they purport to be, and that the trade in medicines is rigorously controlled.29 Ironically perhaps, whereas much concern centres on falsification of Internet-purchased medicine, we were able to verify the compound quality as the true, but teratogenic, medication. Included in these European measures is the introduction of a common, European Union-wide logo to identify legal online pharmacies. It is envisaged that this will make it easier to distinguish between legal and illegal e-pharmacies throughout the European Union.29 However, logos and seals are not enough. People must be educated about their meaning and how to verify them (we found one false VIPP). The presence of a logo may give false security to those who purchase medicines online, and they may assume that they are engaging in safe practices—we found that even legitimate sites were not giving full safety information.
Our findings add additional evidence to substantiate the need to engage in wide ranging international measures to minimise harm associated with online purchasing of medications, including the potential harm of obtaining medications without prescription, and obtaining medications without full safety information and associated risk minimisation measures. This research shows that the special case of pregnancy—women already pregnant and women at risk of pregnancy should be specifically addressed in these measures, involving as it does both the woman and her unborn baby.
Whereas on the one hand, stricter control of online pharmacies is required; on the one hand, PPPs must recognise the potential for women to bypass the prescriber, intentionally or unintentionally. This means, for example, making young women aware by other means (social media, magazines) of the potential dangers of this form of acne medication, and also of the potential dangers of Internet medication purchase, and including these issues also in preconception care for all women.30 General practitioners, dermatologists and pharmacists need to reinforce these messages if they believe their patients may consider online purchase.
This study goes further than previous reports regarding isotretinoin by evaluating the safety information on the websites, exploring the ease of the purchasing process and verifying the quality and purity of the product purchased online. However, a number of limitations of this study should be recognised. The frequency with which isotretinoin is purchased from the Internet was not established nor the characteristics of women who purchase or their reasons for doing so. Furthermore, our results concern e-pharmacies accessible in the UK, and different situations may prevail in other countries, for example, in relation to e-pharmacy accessibility and customs policies and practices. No attempt was made to purchase samples from legitimate e-pharmacies. The laboratory analysis of the purchased medications was limited—the dissolution of the samples was not tested, and the assay was carried out on only one capsule per batch. However, some of these limitations can be seen as avenues for future research.
CONCLUSIONS
Increasing awareness of Internet-based pharmacies among health care providers is the first step for health care professionals to help limit the online purchasing of isotretinoin and other potentially teratogenic drugs. Health care providers should recognise that all consumers of health care are susceptible and should educate clients about the risks of purchasing medications over the Internet without medical supervision. In addition, increased awareness of the teratogenic potential of medications needs to be part of preconception care and public health interventions. This study evaluated the example of isotretinoin, a highly teratogenic drug, but the implications of Internet purchase relate also to drugs of unknown or lesser teratogenicity. Regulation of drug safety in pregnancy by medicines regulatory agencies must take into account the growing use of online pharmacies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
KEY POINTS.
Isotretinoin can be bought online without a prescription.
The majority of websites do not warn women of the teratogenicity of isotretinoin or the need to prevent pregnancy.
Isotretinoin available online has been chemically verified as isotretinoin but does not conform to pharmaceutical standards with regard to packaging and product information.
Online purchase is a new problem regarding safety of drug use in pregnancy, which needs to be tackled urgently.
ETHICS STATEMENT
Both phases of the study received ethics permission from the University of Ulster Institute of Nursing and the Health Research Ethics Filter Committee. To comply with standards for the safe and secure handling of medications, an inventory of all medications purchased was kept, and medications received were stored in the Pharmacy Department of the University of Ulster. Following online purchase, the research team informed the Medicines and Healthcare products Regulatory Agency of the specific websites from which the researcher (BML) was able to purchase isotretinoin without a prescription.
Acknowledgments
This study is part of the EUROmediCAT research project (www.euromedicat.eu), which has been supported by the European Commission under the Seventh Framework Programme through the key action: HEALTH.2010.4.2–3, Adverse Drug Reaction Research under grant agreement no. 260598.
The authors would like to acknowledge the input from the EUROmediCAT Steering Group members who independently reviewed this paper.
AUTHOR CONTRIBUTIONS
B. L. contributed to the design; collected, analysed and interpreted the data from the Internet sites and wrote the first draft. H. D. and M. S. conceptualised the research idea, contributed to the design, interpreted the data and commented on all drafts. B. W. analysed the Internet sites and commented on the draft. D. U. analysed the medication samples and commented on the draft.
REFERENCES
- 1.Orizio G, Merla A, Schulz PJ, Gelatti U. Quality of online pharmacies and websites selling prescription drugs: a systematic review. J Med Internet Res. 2011;13:e74. doi: 10.2196/jmir.1795. doi: 10.2196/jmir.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medicines and Healthcare products Regulatory Agency (MHRA) Isotretinoin for severe acne. Available at: http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Product-specificinformationandadvice-G-L/Isotretinoinforsevereacne/index.htm [9 September 2013]
- 3.Food and Drug Administration (FDA) iPLEDGE information. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm094307.htm [9 September 2013]
- 4.Goldfield MJD, Cox NH, Bower A, et al. Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. Br J Dermatol. 2010;162:837–841. doi: 10.1111/j.1365-2133.2010.09836.x. doi: 10.1111/j.1365-2133.2010.09836.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosa RW. Teratogenicity of isotretinoin. Lancet. 1983;2:513. doi: 10.1016/s0140-6736(83)90538-x. doi: 10.1016/S0140-6736(83)90538-X. [DOI] [PubMed] [Google Scholar]
- 6.Lammer EJ, Chen DT, Hoar RM, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 7.Crijns HJ, Straus SM, Gispen-de Wied C, de Jong-van den Berg LT. Compliance with pregnancy prevention programmes of isotretinoin in Europe: a systematic review. Br J Dermatol. 2011;164:238–244. doi: 10.1111/j.1365-2133.2010.09976.x. doi: 10.1111/j.1365-2133.2010.09976.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer C, Meister R, Weber-Schoendorfer C. Isotretinoin exposure and pregnancy outcome: an observational study of the Berlin Institute for clinical teratology and Drug Risk Assessment in Pregnancy. Arch Gynecol Obstet. 2010;281:221–227. doi: 10.1007/s00404-009-1112-2. doi: 10.1007/s00404-009-1112-2. [DOI] [PubMed] [Google Scholar]
- 9.Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;16:117–125. doi: 10.1016/j.jaad.2010.09.017. doi: 10.1016/j.jaad.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Teichert M, Visser LE, Dufour M, et al. Isotretinoin use and compliance with the Dutch Pregnancy Prevention Programme: a retrospective cohort study in females of reproductive age using pharmacy dispensing data. Drug Saf. 2010;33:b315–b326. doi: 10.2165/11319190-000000000-00000. doi: 10.2165/11319190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Crijns HJMJ, van Rein N, Gispen-de Wied CC, et al. Prescriptive contraceptive use among isotretinoin users in The Netherlands in comparison with non-users: a drug utilisation study. Pharmacoepidemiol Drug Saf. 2012;21:1060–1066. doi: 10.1002/pds.3200. doi: 10.1002/pds.3200. [DOI] [PubMed] [Google Scholar]
- 12.Honein MA, Paulozii LJ, Erickson JD. Continued occurrence of Accutane-exposed pregnancies. Teratology. 2001;64:142–147. doi: 10.1002/tera.1057. doi: 10.1002/tera.1057. [DOI] [PubMed] [Google Scholar]
- 13.Ghodse H. Watching Internet pharmacies. Br J Psychiatry. 2010;196:169–170. doi: 10.1192/bjp.bp.109.072413. doi: 10.1192/bjp.bp.109.072413. [DOI] [PubMed] [Google Scholar]
- 14.GAO (United States General Accounting Office) Internet pharmacies: some pose safety risks for consumers. 2004. Available at: http://www.gao.gov/new.items/d04820.pdf [9 September 2013]
- 15.Lott JP, Kovarik CL. Availability of oral isotretinoin and terbinafine on the Internet. J Am Acad Dermatol. 2010;62:153–154. doi: 10.1016/j.jaad.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Stat Counter. 2011. Available at: http://statcounter.com/ [9 September 2013]
- 17.Kagan M. 100 Awesome marketing stats, charts and graphs [data] 2011. Available at: http://blog.hubspot.com/blog/tabid/6307/bid/14416/100-Awesome-Marketing-Stats-Charts-Graphs-Data.aspx [9 September 2013]
- 18.National Association of Boards of Pharmacy. Available at: http://vipps.nabp.net/ [9 September 2013]
- 19.Canadian International Pharmacy Association (CIPA) Available at: http://www.cipa.com/ [9 September 2013]
- 20.General Pharmaceutical Council (GPhC) Available at: http://www.pharmacyregulation.org/ [9 September 2013]
- 21.Canadian International Pharmacy Association. CIPA fights rogue Internet pharmacies displaying CIPA seal without authorization. 2011. Available at: http://www.cipa.com/news/cipa-fights-rogue-Internet-pharmacies-displaying-cipa-seal-without-authorization/#more-314 [9 September 2013]
- 22.Närhi U, Pohjanoksa-Mäntylä M, Karjalainen A, et al. The DARTS tool for assessing online medicines information. Pharm World Sci. 2008;30:898–906. doi: 10.1007/s11096-008-9249-9. [DOI] [PubMed] [Google Scholar]
- 23.PharmacyChecker Verification Program. Available at: https://www.pharmacychecker.com/sealprogram/choose.asp [9 September 2013]
- 24.Charnock D, Shepperd S. DISCERN on the Internet: workshop participants' views and experiences. Health Educ Res. 2004;19:440–446. doi: 10.1093/her/cyg046. doi: 10.1093/her/cyg046. [DOI] [PubMed] [Google Scholar]
- 25.Health on Net (HON) 2011. Available at: http://www.hon.ch/ [9 September 2013]
- 26.European Council Directive (2001/83/EC) Directive of the European Parliament and of the council on the community code relating to medicinal products for human use. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0083:EN:NOT [9 September 2013]
- 27.British Pharmacopoeia. The only official source of British pharmaceutical standards. 2012. Available at: http://www.pharmacopoeia.co.uk/ [9 September 2013]
- 28.Bihari M. Online pharmacies: how can I find an online pharmacy that is honest? 2008. Available at: http://drugs.about.com/od/costofdrugs/a/trust_online_rx.htm [9 September 2013]
- 29.European Commission. Medicinal products for human use: falsified medicines. 2011. Available at: http://ec.europa.eu/health/files/eudralex/vol-1/dir_2011_62/dir_2011_62_en.pdf [9 September 2013]
- 30.Centres for Disease Control and Prevention (CDC) Preconception health and care. 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/at-a-glance-4-11-06.pdf [9 September 2013]


