Abstract
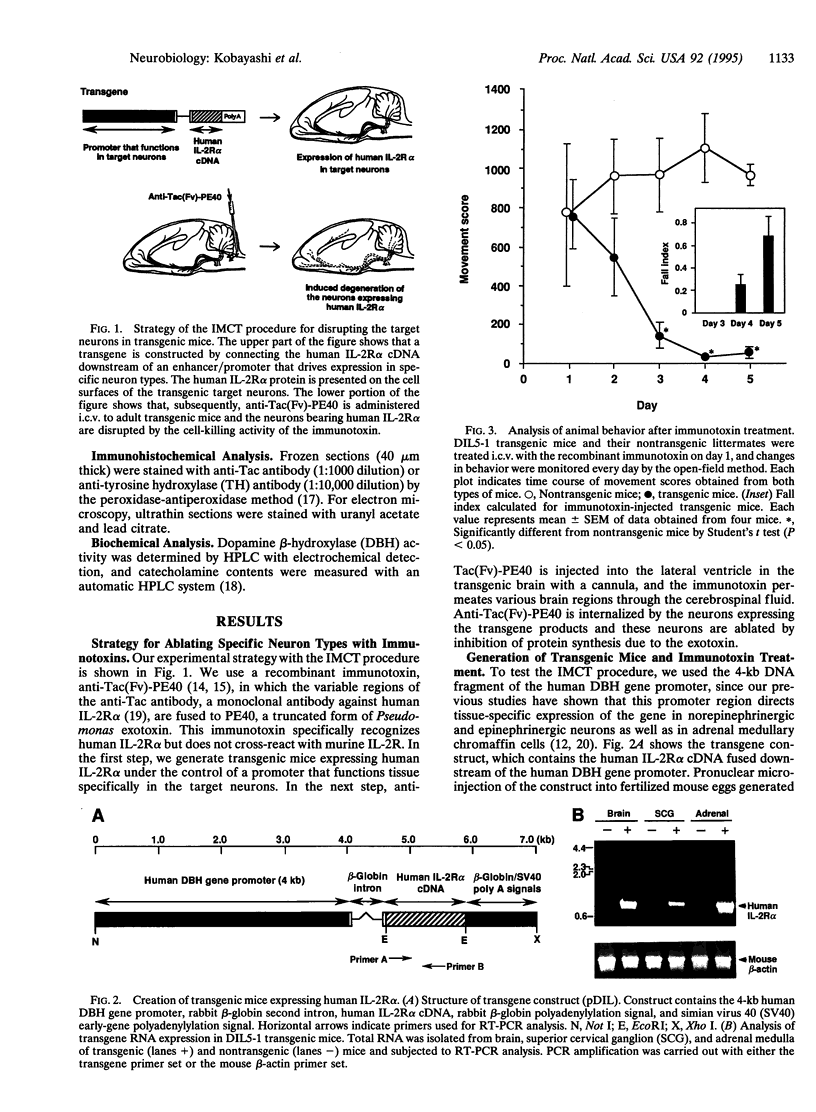
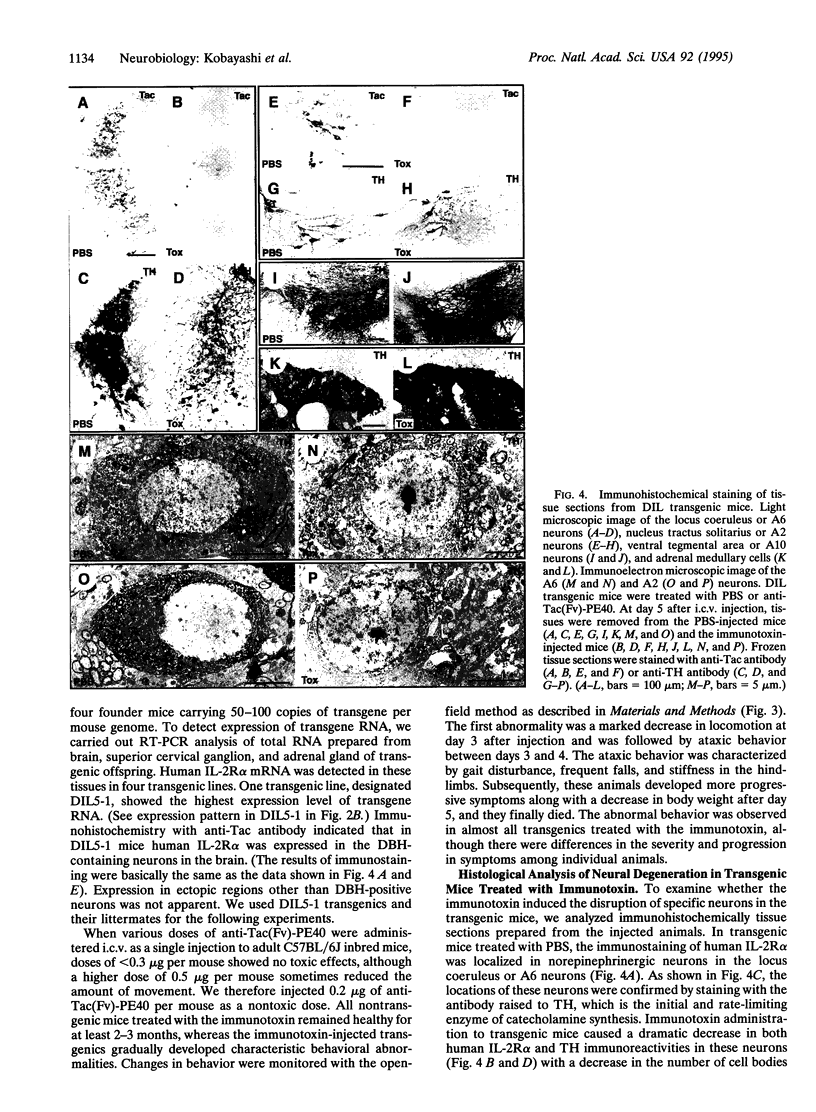
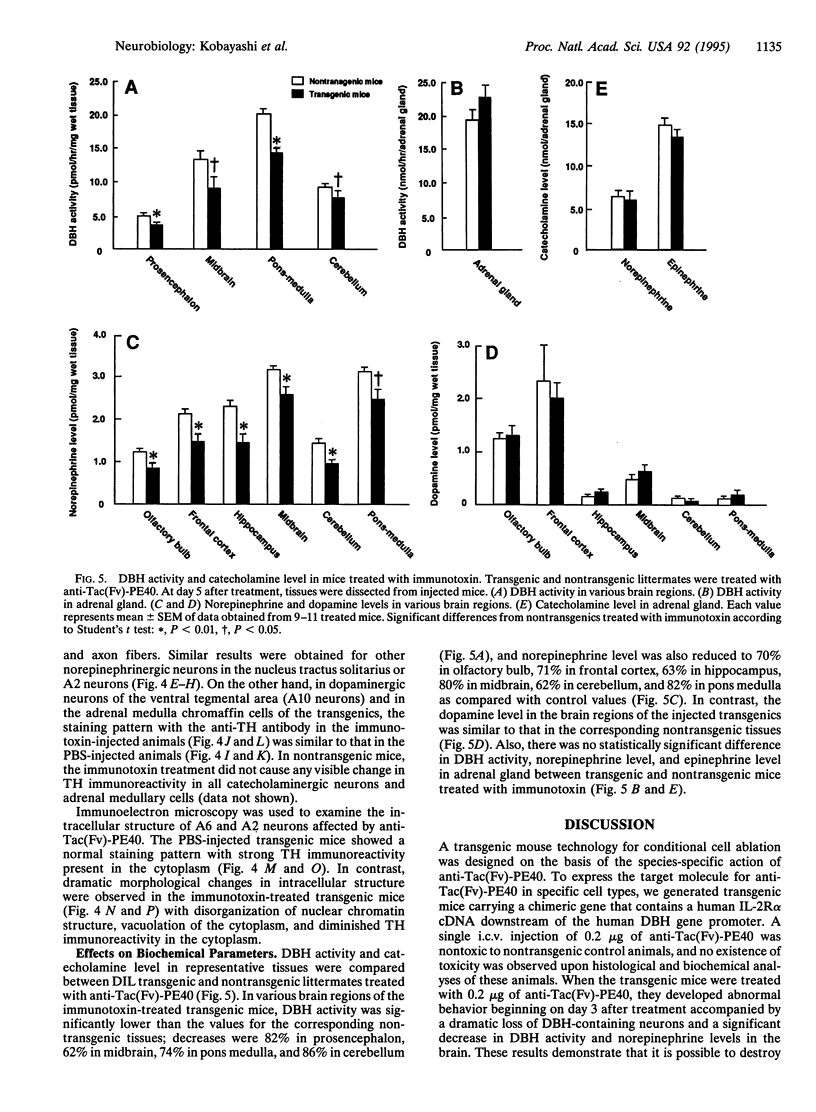
We have developed a transgenic approach, termed immunotoxin-mediated cell targeting (IMCT), to ablate conditionally selective neurons in the brain with the cytotoxic activity of immunotoxins. Transgenic mice were created that express the human interleukin 2 receptor alpha subunit (IL-2R alpha) under the control of the dopamine beta-hydroxylase (DBH) gene promoter. The animals were treated intracerebroventricularly with a recombinant immunotoxin, anti-Tac(Fv)-PE40, which selectively kills animal cells bearing human IL-2R alpha. The immunotoxin caused a characteristic behavioral abnormality only in the transgenic mice. This was accompanied by a dramatic loss of DBH-containing neurons and a significant decrease in DBH activity and norepinephrine levels in various regions of the brain. IMCT should provide a general technique to create animal models of human neurodegenerative disorders by targeting neurons or other cell types.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra J. K., FitzGerald D., Gately M., Chaudhary V. K., Pastan I. Anti-Tac(Fv)-PE40, a single chain antibody Pseudomonas fusion protein directed at interleukin 2 receptor bearing cells. J Biol Chem. 1990 Sep 5;265(25):15198–15202. [PubMed] [Google Scholar]
- Bloom F. E., Hoffer B. J., Siggins G. R. Studies on norepinephrine-containing afferents to Purkinje cells of art cerebellum. I. Localization of the fibers and their synapses. Brain Res. 1971 Feb 5;25(3):501–521. doi: 10.1016/0006-8993(71)90457-4. [DOI] [PubMed] [Google Scholar]
- Bonnerot C., Grimber G., Briand P., Nicolas J. F. Patterns of expression of position-dependent integrated transgenes in mouse embryo. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6331–6335. doi: 10.1073/pnas.87.16.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Heyman R. A., Arias C., Sawchenko P. E., Evans R. M. Transgenic mice with inducible dwarfism. Nature. 1989 Jun 15;339(6225):538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman M. L., Clapoff S., Rossant J., Tsui L. C., Glode L. M., Maxwell I. H., Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987 Dec 11;238(4833):1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Davis G. C., Williams A. C., Markey S. P., Ebert M. H., Caine E. D., Reichert C. M., Kopin I. J. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979 Dec;1(3):249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- Evans G. A. Dissecting mouse development with toxigenics. Genes Dev. 1989 Mar;3(3):259–263. doi: 10.1101/gad.3.3.259. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Borrelli E., Lesley J., Anderson D., Richman D. D., Baird S. M., Hyman R., Evans R. M. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Bloom F. E. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res. 1971 Feb 5;25(3):523–534. doi: 10.1016/0006-8993(71)90458-6. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27(2):183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Morita S., Mizuguchi T., Sawada H., Yamada K., Nagatsu I., Fujita K., Nagatsu T. Functional and high level expression of human dopamine beta-hydroxylase in transgenic mice. J Biol Chem. 1994 Nov 25;269(47):29725–29731. [PubMed] [Google Scholar]
- Kobayashi K., Sasaoka T., Morita S., Nagatsu I., Iguchi A., Kurosawa Y., Fujita K., Nomura T., Kimura M., Katsuki M. Genetic alteration of catecholamine specificity in transgenic mice. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1631–1635. doi: 10.1073/pnas.89.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Mason S. T. Noradrenaline in the brain: progress in theories of behavioural function. Prog Neurobiol. 1981;16(3-4):263–303. doi: 10.1016/0301-0082(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Matsui K., Wada K., Kwak S. Ataxia-ameliorating effects of YM-14673, a potent analog of thyrotropin releasing hormone, in ataxic mutant mice. Eur J Pharmacol. 1994 Mar 21;254(3):295–297. doi: 10.1016/0014-2999(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Morita S., Kobayashi K., Mizuguchi T., Yamada K., Nagatsu I., Titani K., Fujita K., Hidaka H., Nagatsu T. The 5'-flanking region of the human dopamine beta-hydroxylase gene promotes neuron subtype-specific gene expression in the central nervous system of transgenic mice. Brain Res Mol Brain Res. 1993 Mar;17(3-4):239–244. doi: 10.1016/0169-328x(93)90007-c. [DOI] [PubMed] [Google Scholar]
- Nagatsu I., Komori K., Takeuchi T., Sakai M., Yamada K., Karasawa N. Transient tyrosine hydroxylase-immunoreactive neurons in the region of the anterior olfactory nucleus of pre- and postnatal mice do not contain dopamine. Brain Res. 1990 Mar 12;511(1):55–62. doi: 10.1016/0006-8993(90)90224-y. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Pastan I., Chaudhary V., FitzGerald D. J. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Crenshaw E. B., 3rd, Lira S. A., Simmons D. M., Swanson L. W., Rosenfeld M. G. Neuronal expression of chimeric genes in transgenic mice. Neuron. 1988 Jun;1(4):311–320. doi: 10.1016/0896-6273(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]