Abstract
Background
Pacing the right ventricle is established practice, but there remains controversy as to the optimal site to preserve hemodynamic function.
Aims
To evaluate clinical and hemodynamic differences between apical and septal pacing in pacemaker-dependent patients.
Methods
Patients receiving their first pacemaker for advanced atrioventricular block, with the atria in sinus rhythm, were randomized to receive apical (Group A) or septal (Group S) ventricular leads. After implant, with the device programmed VVI 70 beats/min fixed rate, patients underwent a 6-minute walk test and a transthoracic echocardiogram. Then, DDDR was programmed at nominal settings. The same tests were performed at 6 months and 12 months follow-up. If ventricular pacing was less than 98%, the patient was excluded.
Results
A total of 142 patients were included in the study. During the study year, 71 (50%) were excluded for not fulfilling the condition of 98% ventricular pacing. Groups A and S had 34 and 37 patients, respectively. Age and gender were similar in the groups. At implant, QRS duration was significantly greater in Group A (158 ms) than Group S (146 ms; P = 0.018), and the QRS axis was different: –74.5° in Group A and 1° in Group S (P < 0.001). At 1 year, the 6-minute walk improved significantly in both groups: Group A 15% (P = 0.048) and Group S 24% (P = 0.001). Left ventricular ejection fraction (LVEF) increased from 0.57 to 0.61 (P = 0.008) in Group S, without significant change in Group A.
Conclusions
After 1 year, pacemaker-dependent patients with septal ventricular leads have better clinical and functional (LVEF) outcome.
Keywords: right ventricular pacing, pacemaker dependency, septal pacing, apical pacing, 6-minute walk, echocardiography
Background
Much has been said regarding the optimal right ventricular (RV) pacing site.1–4 On one hand, there is the irrefutable proof of time: apical pacing has been used for over four decades without substantial damage or even beneficial5,6 outcome in terms of heart function in patients who started with normal left ventricles; however, RV septal pacing has been argued to stimulate a more efficient ventricular contraction.7,8
The natural activation through the His-Purkinje system is, of course, the best way to depolarize the ventricular mass under normal circumstances,8,9 irrespective of conduction or contractile disturbances. Any device that artificially depolarizes the heart will have some deleterious physiological effects.10,11
The physiological rationale behind pacing the septum rather than the apex is based on initiating the ventricular depolarization in the RV septal wall, across the base of the mitral septal papillary muscle, where the first activation vector normally starts.12,13 By doing so, the QRS duration will be shorter than that with pacing from the apex, and the ventricular contraction—in theory—will be more efficient. Pacing from the apex has a more “desynchronizing effect”8 than pacing from the interventricular septum.
Pacemaker dependency is another crucial element to consider in artificial pacing. In nonpacemaker-dependent patients, the less stimulation, the better physiologic outcome.14,15 The pacing site becomes an increasing problem when the patient requires pacing for a considerable part of the time.16
Material and Methods
After the Institutional Review Board approved the protocol, and written informed consent was obtained, patients were randomized to receive a septal or apical ventricular lead.
All patients underwent their first pacemaker implant, using active fixation ventricular leads in the septum and passive fixation in the apex, for documented complete atrioventricular (AV) block. All patients’ atria were in sinus rhythm; none was in atrial fibrillation (AF). All the apical and septal positions were radiographically documented in relation to anatomic landmarks. Figure1 illustrates the range of positions considered to be septal. No attempt was made to achieve right ventricular outflow tract (RVOT) pacing.17,18 Once pacing was established, measurements of paced QRS duration and axis in the frontal plane leads were obtained.17
Figure 1.
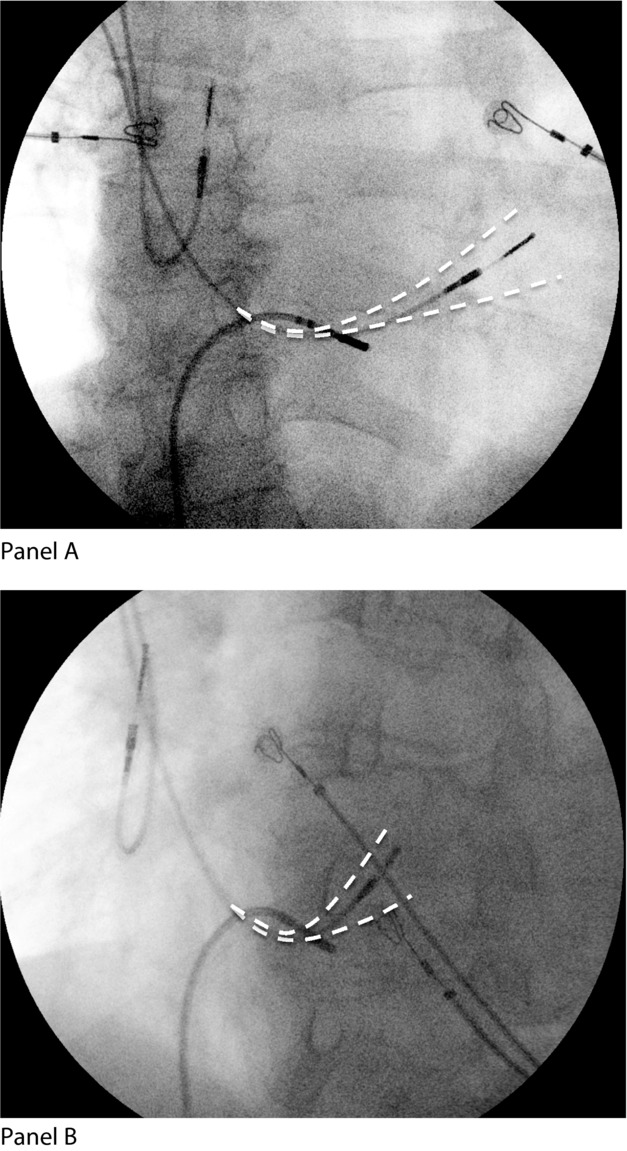
Right ventricular lead placement. This chest radiograph shows a typical septal pacing position in right anterior oblique (RAO) in Panel (A), and left anterior oblique (LAO) in Panel (B) projections with acceptable ranges of position drawn in. No leads were positioned outside these limits.
At the end of the first week after implant, a transthoracic echocardiogram was obtained (Model HD11XE, Phillips Healthcare, Eindhoven, the Netherlands). Only one operator acquired all the echocardiographic images and calculated all parameters. This operator's intraobserver reproducibility was 3–4%. During this period, all patients also had a 6-minute walk test.
Pacing Protocol
Once the generator was implanted, it was programmed as VVI (ventricular pacing only) at a fixed rate of 70 beats/min. This was determined to be a control phase, as far as possible to normalize the different clinical states of those entering the trial, before undertaking the echo and the 6-minute walk.
After the echo was obtained and the 6-minute walk performed, the pacemaker was reprogrammed as DDDR (dual chamber pacing and the rate response mechanism ON) with default pulse output. The AV interval was programmed at nominal values: 120 ms for sensed P waves and 150 ms for paced atrial activation.
Patients were then evaluated at the third month, to reduce the generator's output to three times threshold value. Six months after implant, a second echocardiogram was performed in addition to another 6-minute walk test. Finally, 6 months later (1 year after implant), the same two examinations were repeated (Fig.2).
Figure 2.
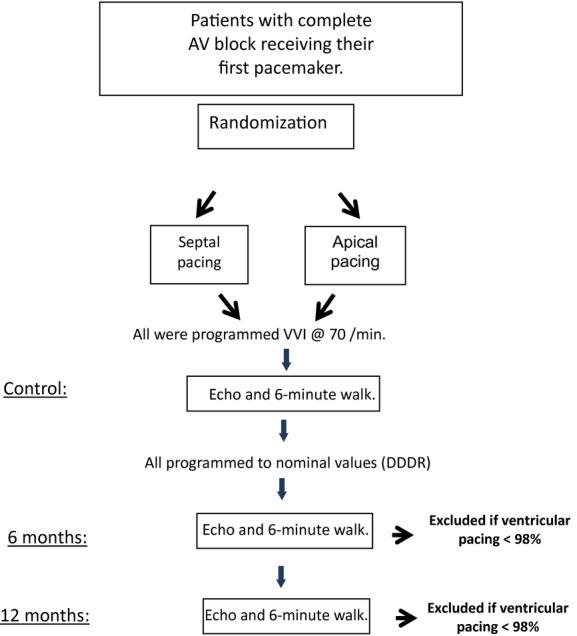
Study flow chart. AV = atrioventricular; Echo = echocardiogram.
The lead placement was blinded to the patient and to the physician conducting the clinical follow-up.
Statistical Analysis
The sample size was estimated with a confidence level of 95% and error margin of 5%. We inserted International data18 into the following equation:
where Z (1.96) is the value for a 95% level of confidence, e (0.05) is error margin, and P (0.04) is the prevalence reported for the world population.
The population needed to achieve statistical strength was 59 subjects. Anticipating high rates of dropout because of the prospective exclusion criterion (below), we included 142 patients.
In order to determine which test had to be carried out, the data were analyzed to define if it had “Gaussian” characteristics with the Kolmogorov-Smirnov test.
We used the nonparametric Mann-Whitney U test to determine significant differences between variables that did not adjust to a normal distribution. For the normally distributed variables, the Student's t-test was used. To verify the significance, we performed a Monte Carlo (MC) test for 10,000 samples. This method was preferred over calculating Type I error and Power with classical theoretical distributions for asymptotic conditions, as clinical data are often not normally distributed.19
Patients
Patients receiving their first pacemaker for documented complete persistent AV block were randomized to septal or apical pacing. We used a “complete randomization” method (simple randomization) that is equivalent to a coin toss.20,21
Patients were included regardless of their age, gender, or underlying pathology. None had clinical evidence of severe congestive heart failure (CHF) as defined by New York Heart Association class IV.
Exclusion Criteria
All patients were followed-up every 3 months. They were excluded if ventricular pacing was less than 98% of the time, regardless of atrial rhythm (sinus or pacing). This was a prospective exclusion by protocol. An “underlying rhythm” test to ensure the persistence of complete AV block was performed at every visit. The application of the protocol led to late exclusion in 71 patients, but it determined that, as planned at the outset, only persistently pacemaker-dependent patients completed the study.
Methods
Ventricular leads were Medtronic® models Capsurefix® Novus 5076 for the screw-in (active) and Capsure® SP 5024 passive fixation (Medtronic Inc., Minneapolis, MN, USA), placed according to randomization in the septum or the RV apex. To achieve the midseptal position, a custom curved stylet was shaped as has been described,22,23 or by simply pulling down the lead from the pulmonary artery until it was parallel to the midseptal endocardium.24
The tip of the lead was verified to be in the midseptum using the left anterior oblique and right anterior oblique (RAO) projections (Fig.1).
The apical position was attained in the RAO projection, placing the lead as far and inferiorly as possible. No diaphragmatic stimulation was observed.
The generators used were Medtronic® model Kappa, series 400, 700, and 900 (Medtronic Inc.).
Results
A total of 142 patients had a pacemaker successfully implanted. Correct location was confirmed in all cases, and no dislodgements occurred. No major complications were observed during implant.
After 1 year, 71 patients registered >98% ventricular pacing: 34 were in Group A, and 37 in the septal (S) Group. The 71 excluded patients recovered some degree of AV conduction and hence had a pacing percentage of less than 98%.
Both groups of patients completing the follow-up were pacemaker dependent, and inhibition of pacing provoked symptomatic ventricular pauses or intense bradycardia.
Mean age of Group A was 72 ± 12, similar to the Group S, 69 ± 12 (P = ns). Likewise, 40% of patients in Group A were male, and 49.7% in Group S (P = ns).
Parameters Obtained during Implant
Of both groups, 12 had a femoral temporary pacing lead placed during the procedure (seven in Group A and five in Group S).
For this reason, only 27 in Group A had a spontaneous R wave with a median value of 12.1 mV, compared with 32 patients with an R wave of 8.9 mV in Group S (P = ns).
Other acute values such as impedance (765 Ω for Group A and 778 Ω for Group S) and threshold (0.6 V for Group A and 0.5 for Group S) were not significantly different between groups (P = ns).
During pacing, the QRS duration was longer in Group A patients (158 ms) than Group S (146 ms; P = ns). The QRS axes were highly statistically different: –74.5° in the Group A and 1° for the septal position (P < 0.001; Table1).
Table I.
Data at Implant
| Group A | Group S | P Value | |
|---|---|---|---|
| Age ± SD | 72 ±12 | 69 ± 12 | |
| Gender (% males) | 40.3 | 49 | |
| R-wave amplitude (mV)a (IR) | 12.1 (8.5) | 8.9 (6.2) | 0.15 |
| Impedance (Ω)a (IR) | 765 (288) | 778 (269) | |
| Threshold (V)a (IR) | 0.6 (0.4) | 0.5 (0.3) | |
| QRS axis (°)a (IR) | –74.5 (29) | 1 (90) | <0.001 |
| QRS duration (ms)a (IR) | 158 (29.5) | 146 (45.5) | 0.018 |
Values presented as medians.
IR = interquartile range; SD = standard deviation.
Acute Results Obtained during the First Week after Implant
These are the results while the patients had the device programmed in VVI mode at 70 beats/min.
For both groups, the 6-minute walk was possible in 32 and 36 patients in groups A and S, respectively. In Group A, the distance was 383 ± 177 m and 386 ± 114 m the Group S (Table2).
Table II.
Data at the First Week after Implant
| Group A | Group S | P Value | |
|---|---|---|---|
| 6-minute walk (m) ± SD | 383 ± 177 | 386 ± 114 | |
| Fractional shortening (%) ± SD | 0.37 ± 0.09 | 0.38 ± 0.1 | |
| LVESV (mL) ± SD | 35.6 ± 27.1 | 33.7 ± 26.2 | |
| LVEDV (mL) ± SD | 70.6 ± 34.0 | 66.2 ± 32.1 | |
| LVEDD (mm)a (IR) | 50 (8) | 45 (7) | 0.028 |
| LVESD (mm) ± SD | 31.7 ± 8.9 | 29.8 ± 8.1 | |
| LAD (mm) ± SD | 38.9 ± 5.4 | 38.8 ± 7.0 | |
| LVEF ± SD | 0.52 ± 0.1 | 0.57 ± 0.1 |
Values presented as medians.
IR = interquartile range; LAD = left atrial dimension; LVEDD = left ventricular end-diastolic dimension; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimension; LVESV = left ventricular end-systolic volume; SD = standard deviation.
The remaining data were acquired from the transthoracic echocardiogram: fractional shortening, left ventricular end-systolic volume, and left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic dimension were very similar in the two groups (Table2). The only parameter that showed significant difference between groups was left ventricular end-diastolic dimension (P = 0.028).
Control values for left ventricular ejection fraction (LVEF) were 0.52 ± 0.1 for Group A, and 0.57 ± 0.1 for Group S (P = ns).
Follow-Up
Patients were subjected to the same clinical examinations (6-minute walk and echocardiogram) as in the acute phase after 6 months and 1 year. At 6 months, there were no significant differences between groups or with respect to control values.
After 1 year, we compared changes between groups (Table3) and within each group (Table4). Comparing control values with 1-year results, the distance covered during the 6-minute walk, Group A patients increased from 383 ± 177 to 452 m (18% P = 0.018), though in Group S, the increment was from 386 ± 114 to 480 m (24% P = 0.002; MC = 0.002, 95% confidence interval [CI] = 0.001–0.003).
Table III.
One Year Follow-Up
| Group A | Group S | P Value | |
|---|---|---|---|
| 6-minute walk (m)a (IR) | 452 (133.3) | 480 (94.8) | |
| Fractional shortening (%) ± SD | 0.38 ± 0.09 | 0.37 ± 0.08 | |
| LVESV (mL) ± SD | 31.8 ± 20.7 | 32.6 ± 24.7 | |
| LVEDV (mL) ± SD | 61.9 ± 22.2 | 67.6 ± 29.9 | |
| LVEDD (mm) ± SD | 46.9 ± 6.2 | 45.4 ± 9.4 | |
| LVESD (mm) ± SD | 28.9 ± 5.8 | 28.8 ± 5.3 | |
| LAD (mm) ± SD | 37 ± 8.5 | 37 ± 6.4 | |
| LVEFa (IR) | 0.54 (0.11) | 0.61 (0.07) | 0.001 |
Values presented as medians.
For abbreviations please see Table2.
Table IV.
Changes at Apical versus Septal Site
| Apical Changes | Septal Changes | |||||
|---|---|---|---|---|---|---|
| Initial | Final | P Value | Initial | Final | P Value | |
| 6-minute walk (m) | 383 ± 177 | 452a | 0.018 | 386 ± 114 | 480a | 0.002 |
| Fractional shortening | 0.37 ± 0.09 | 0.38 ± 0.09 | 0.38 ± 0.1 | 0.37 ± 0.08 | ||
| LVESV (mL) | 35.6 ± 27.1 | 31.8 ± 20.7 | 33.7 ± 26.2 | 32.6 ± 24.7 | ||
| LVEDV (mL) | 70.6 ± 34.0 | 61.9 ± 22.2 | 66.2 ± 32.1 | 67.6 ± 29.9 | ||
| LVEDD (mm) | 50a | 46.9 ± 6.2 | 45a | 45.4 ± 9.4 | ||
| LVESD (mm) | 31.7 ± 8.9 | 28.9 ± 5.8 | 29.8 ± 8.1 | 28.8 ± 5.3 | ||
| LAD (mm) | 38.9 ± 5.4 | 37 ± 8.5 | 38.8 ± 7.0 | 37 ± 6.4 | ||
| LVEF | 0.52 ± 0.1 | 0.54a | 0.33 | 0.57 ± 0.1 | 0.61a | 0.004 |
At the end of the follow-up period, the difference in LVEF between the groups was significant (S: 0.61, A: 0.54; P = 0.001; MC = 0.002, 95% CI = 0.001–0.003; Fig. 3). Likewise, LVEF increased seven percentage points in the Group S (0.57 ± 0.1 to 0.61, P = 0.004; MC = 0.002, 95% CI = 0.001–0.002) compared with the nonsignificant change (P = 0.33) from 0.52 ± 0.1 to 0.54 in Group A patients. None of the other parameters was statistically different.
Figure 3.
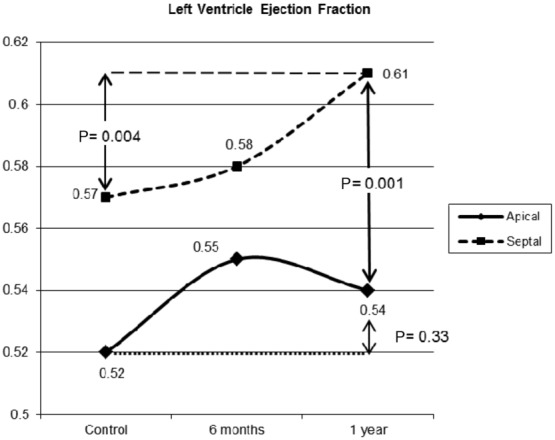
Ejection fraction changes. This figure shows the left ventricular ejection fraction plotted for the apical and septal groups at control, 6 months and 1 year.
Discussion
This study has shown in patients with ventricular pacemaker dependency using a randomized double-blind single-center prospective design, that there is significant improvement in LVEF and 6-minute walk distance, with septal RV pacing—over a period of 1 year—compared with RV apical pacing.
Perhaps, the most important feature of this study is the high degree of pacemaker dependency. Most reports on the differences between septal and apical pacing have not considered this facet,1–3,25–27 which remains important because the ideal pacing site has not been definitively demonstrated. This failure to demonstrate the ideal pacing site may, at least in part, be due to intermittent AV block with its implied percentage of normal or near-normal AV conduction. The main prospective exclusion criterion was ventricular pacing for less than 98% of the time throughout the follow-up period. Not only were patients being paced almost all the time, but also the “underlying rhythm” test demonstrated they did not have AV conduction that could compete with the pacemaker. This was not manipulated by programming short AV intervals. Hence, the clinical progress of the patients in this study was a consequence of ventricular pacing only. Thus, the influence of normally conducted beats was eliminated.
The use of active or passive fixation ventricular leads22,28,29 was not associated with dislodgements or differences in any of the acute values during implant.
Data obtained after 1-year follow-up were analyzed with a Kolmogorov-Smirnov test to define the normality of the distribution determining selection of Student's t-test or Mann-Whitney U test for statistical significance.
The most remarkable disparities during implant were the R-wave amplitude and the QRS axis. For the R wave, little has previously been stated30: we found a persistent statistically nonsignificant lower R wave in the septal position (P = 0.15). This could reflect purely the confluent depolarization vectors at the apex or possibly a trend toward significance. The QRS axis pacing from the RV apex directs all vectors toward a left superior angle, although there are opinions opposing this statement.31 Our findings were that there was a highly significant difference in QRS vectors of septal and apical pacing.
There remains controversy over QRS duration. Some authors have described32,33 a faster depolarization with a beneficial hemodynamic consequence from septal pacing.31,33–35 The possible small variations in septal position of the pacing electrode36 could have some influence on the outcome. In these observations, the Group S had the QRS 12 ms shorter than those with apical stimulation (P = 0.018). This observation had no acute hemodynamic effect, but it may explain the favorable results we detected at 1 year.
Concerning the first week after implant, when patients had the device programmed in VVI mode at a fixed rate of 70 beats/min, we observed nonsignificant differences: LVEF was slightly better in the Group S (0.57 ± 0.1 vs 0.52 ± 0.1). The LVEDV was also modestly smaller in the Group S (66.2 ± 32.1 vs 70.6 ± 34.0).
Six months after implant, there were no significant improvements in any of the evaluated parameters. This coincides with the findings of others in acute and short-term studies.5,25
The most remarkable changes within a group and dissimilarities between the groups were seen after 1 year.
Considering the 6-minute walk, there were no differences between groups, although within both sets of patients we found important increments: those with the apical lead increased 18% (from 383 ± 177 m to 452 m), whereas the septal group had an increment of 24% (from 386 ± 114 m to 480 m). Although both groups had a significant rise, that of the septal group was clearly superior (Table4).
One possible reason for the remaining echocardiographic/anatomical parameters not improving may be the relatively brief follow-up period.5,37
Just as stated in our hypothesis, the LVEF showed a considerable increment within the septal group, and had an important divergence from apical pacing. Even though the absolute values are not substantially different, we consider that the septal curve shows a clear tendency toward a better LVEF compared with the changes in the apical pacing group.
Limitations
Although this is a single-center study with a relatively small number of patients, the absolute pacemaker dependency, we believe, makes this report a relevant contribution to the RV pacing site controversy.
The follow-up period was relatively brief, though sufficient to demonstrate significant differences between groups.
Conclusion
We have shown that after 1-year follow-up in persistently pacemaker-dependent patients, with no clinical evidence of severe CHF, midseptal ventricular lead placement is superior to the apical location. We observed significant improvements in both clinical (6-minute walk) and functional (LVEF) parameters.
References
- 1.Silva Junior O, Melo CS, Marra M, Correia D. Alternative endocardial sites for artificial cardiac stimulation. Arq Bras Cardiol. 2011;96:76–85. [PubMed] [Google Scholar]
- 2.Cano O, Osca J, Sancho-Tello MJ, Sanchez JM, Ortiz V, Castro JE, Salvador A, et al. Comparison of effectiveness of right ventricular septal pacing versus right ventricular apical pacing. Am J Cardiol. 2010;105:1426–1432. doi: 10.1016/j.amjcard.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cho GY, Kim MJ, Park JH, Kim HS, Youn HJ, Kim KH, Song JK. Comparison of ventricular dyssynchrony according to the position of right ventricular pacing electrode: A multi-center prospective echocardiographic study. J Cardiovasc Ultrasound. 2011;19:15–20. doi: 10.4250/jcu.2011.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolis AS. The deleterious consequences of right ventricular apical pacing: Time to seek alternate site pacing. Pacing Clin Electrophysiol. 2006;29:298–315. doi: 10.1111/j.1540-8159.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 5.Kypta A, Steinwender C, Kammler J, Leisch F, Hofmann R. Long-term outcomes in patients with atrioventricular block undergoing septal ventricular lead implantation compared with standard apical pacing. Europace. 2008;10:574–579. doi: 10.1093/europace/eun085. [DOI] [PubMed] [Google Scholar]
- 6.Ng AC, Allman C, Vidaic J, Tie H, Hopkins AP, Leung DY. Long-term impact of right ventricular septal versus apical pacing on left ventricular synchrony and function in patients with second- or third-degree heart block. Am J Cardiol. 2009;103:1096–1101. doi: 10.1016/j.amjcard.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Muto C, Ottaviano L, Canciello M, Carreras G, Calvanese R, Ascione L, Iengo R, et al. Effect of pacing the right ventricular mid-septum tract in patients with permanent atrial fibrillation and low ejection fraction. J Cardiovasc Electrophysiol. 2007;18:1032–1036. doi: 10.1111/j.1540-8167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Zhang Q, Chan JY, Xie JM, Fung JW, Yip GW, Lam YY, et al. Deleterious effect of right ventricular apical pacing on left ventricular diastolic function and the impact of pre-existing diastolic disease. Eur Heart J. 2011;32:1891–1899. doi: 10.1093/eurheartj/ehr118. [DOI] [PubMed] [Google Scholar]
- 9.Mabo P, Scherlag BJ, Munsif A, Otomo K, Lazzara R. A technique for stable His-bundle recording and pacing: Electrophysiological and hemodynamic correlates. Pacing Clin Electrophysiol. 1995;18:1894–1901. doi: 10.1111/j.1540-8159.1995.tb03838.x. [DOI] [PubMed] [Google Scholar]
- 10.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma AJ, Lemler MS, Zeltser IJ, Scott WA. Relation of right ventricular pacing site to left ventricular mechanical synchrony. Am J Cardiol. 2010;106:806–809. doi: 10.1016/j.amjcard.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Okayama H, Nishimura K, Saito M, Yoshii T, Hiasa G, Sumimoto T, et al. Right ventricular septal pacing preserves global left ventricular longitudinal function in comparison with apical pacing: Analysis of speckle tracking echocardiography. Circ J. 2011;75:1609–1615. doi: 10.1253/circj.cj-10-1138. [DOI] [PubMed] [Google Scholar]
- 14.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. J Am Med Assoc. 2002;288:3115–3123. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 15.Healey JS, Crystal E, Connolly SJ. Physiologic pacing: Where pacing mode selection reflects the indication. Heart. 2004;90:593–594. doi: 10.1136/hrt.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 17.Spodick DH, Frisella M, Apiyassawat S. QRS axis validation in clinical electrocardiography. Am J Cardiol. 2008;101:268–269. doi: 10.1016/j.amjcard.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 18.Kojic EM, Hardarson T, Sigfusson N, Sigvaldason H. The prevalence and prognosis of third-degree atrioventricular conduction block: The Reykjavik study. J Intern Med. 1999;246:81–86. doi: 10.1046/j.1365-2796.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- 19.Sawilowsky S, Fahoome G. Statistics via Monte Carlo simulation with Fortran. Rochester Hills, MI: JMASM; 2003. [Google Scholar]
- 20.Lachin JM. Properties of simple randomization in clinical trials. Control Clin Trials. 1988;9:312–326. doi: 10.1016/0197-2456(88)90046-3. [DOI] [PubMed] [Google Scholar]
- 21.Blackwell D, Hodges JL. Design for the control of selection bias. Ann Math Statist. 1957:449–460. [Google Scholar]
- 22.Rosso R, Teh AW, Medi C, Hung TT, Balasubramaniam R, Mond HG. Right ventricular septal pacing: The success of stylet-driven active-fixation leads. Pacing Clin Electrophysiol. 2010;33:49–53. doi: 10.1111/j.1540-8159.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaye G, Stambler BS, Yee R. Search for the optimal right ventricular pacing site: Design and implementation of three randomized multicenter clinical trials. Pacing Clin Electrophysiol. 2009;32:426–433. doi: 10.1111/j.1540-8159.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosso R, Medi C, Teh AW, Hung TT, Feldman A, Lee G, Mond HG. Right ventricular septal pacing: A comparative study of outflow tract and mid ventricular sites. Pacing Clin Electrophysiol. 2010;33:1169–1173. doi: 10.1111/j.1540-8159.2010.02836.x. [DOI] [PubMed] [Google Scholar]
- 25.Victor F, Leclercq C, Mabo P, Pavin D, Deviller A, de Place C, Pezard P, et al. Optimal right ventricular pacing site in chronically implanted patients: A prospective randomized crossover comparison of apical and outflow tract pacing. J Am Coll Cardiol. 1999;33:311–316. doi: 10.1016/s0735-1097(98)00589-0. [DOI] [PubMed] [Google Scholar]
- 26.Medi C, Mond HG. Right ventricular outflow tract septal pacing: Long-term follow-up of ventricular lead performance. Pacing Clin Electrophysiol. 2009;32:172–176. doi: 10.1111/j.1540-8159.2008.02199.x. [DOI] [PubMed] [Google Scholar]
- 27.Frohlig G, Schwaab B, Kindermann M. Selective site pacing: The right ventricular approach. Pacing Clin Electrophysiol. 2004;27(Pt 2):855–861. doi: 10.1111/j.1540-8159.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 28.Mond HG. The road to right ventricular septal pacing: Techniques and tools. Pacing Clin Electrophysiol. 2010;33:888–898. doi: 10.1111/j.1540-8159.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 29.Mond HG, Feldman A, Kumar S, Rosso R, Hung TT, Pang B. Alternate site right ventricular pacing: Defining template scoring. Pacing Clin Electrophysiol. 2011;34:1080–1086. doi: 10.1111/j.1540-8159.2011.03129.x. [DOI] [PubMed] [Google Scholar]
- 30.Okmen E, Erdinler I, Oguz E, Akyol A, Turek O, Cam N, Ulufer T. An electrocardiographic algorithm for determining the location of pacemaker electrode in patients with right bundle branch block configuration during permanent ventricular pacing. Angiology. 2006;57:623–630. doi: 10.1177/0003319706293146. [DOI] [PubMed] [Google Scholar]
- 31.Burri H, Park CI, Zimmermann M, Gentil-Baron P, Stettler C, Sunthorn H, Domenichini G, et al. Utility of the surface electrocardiogram for confirming right ventricular septal pacing: Validation using electroanatomical mapping. Europace. 2011;13:82–86. doi: 10.1093/europace/euq332. [DOI] [PubMed] [Google Scholar]
- 32.Su Y, Pan W, Gong X, Cui J, Shu X, Ge J. Relationships between paced QRS duration and left cardiac structures and function. Acta Cardiol. 2009;64:231–238. doi: 10.2143/AC.64.2.2036143. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, Mine T, Kanemori T, Ohyanagi M, Masuyama T. Effect of right ventricular pacing site on QRS width. Asian Cardiovasc Thorac Ann. 2011;19:339–345. doi: 10.1177/0218492311422485. [DOI] [PubMed] [Google Scholar]
- 34.Padeletti L, Lieberman R, Schreuder J, Michelucci A, Collella A, Pieragnoli P, Ricciardi G, et al. Acute effects of His bundle pacing versus left ventricular and right ventricular pacing on left ventricular function. Am J Cardiol. 2007;100:1556–1560. doi: 10.1016/j.amjcard.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 35.Takemoto Y, Hasebe H, Osaka T, Yokoyama E, Kushiyama Y, Suzuki T, Kuroda Y, et al. Right ventricular septal pacing preserves long-term left ventricular function via minimizing pacing-induced left ventricular dyssynchrony in patients with normal baseline QRS duration. Circ J. 2009;73:1829–1835. doi: 10.1253/circj.cj-09-0256. [DOI] [PubMed] [Google Scholar]
- 36.Balt JC, van Hemel NM, Wellens HJ, de Voogt WG. Radiological and electrocardiographic characterization of right ventricular outflow tract pacing. Europace. 2010;12:1739–1744. doi: 10.1093/europace/euq341. [DOI] [PubMed] [Google Scholar]
- 37.Vlay SC. Right ventricular outflow tract pacing: Practical and beneficial. A 9-year experience of 460 consecutive implants. Pacing Clin Electrophysiol. 2006;29:1055–1062. doi: 10.1111/j.1540-8159.2006.00498.x. [DOI] [PubMed] [Google Scholar]


