Abstract
Hippocampal sclerosis (HS) is a common pathology encountered in mesial temporal lobe epilepsy (MTLE) as well as other epilepsy syndromes and in both surgical and post-mortem practice. The 2013 International League Against Epilepsy (ILAE) classification segregates HS into typical (type 1) and atypical (type 2 and 3) groups, based on the histological patterns of subfield neuronal loss and gliosis. In addition, granule cell reorganization and alterations of interneuronal populations, neuropeptide fibre networks and mossy fibre sprouting are distinctive features of HS associated with epilepsies; they can be useful diagnostic aids to discriminate from other causes of HS, as well as highlighting potential mechanisms of hippocampal epileptogenesis. The cause of HS remains elusive and may be multifactorial; the contribution of febrile seizures, genetic susceptibility, inflammatory and neurodevelopmental factors are discussed. Post-mortem based research in HS, as an addition to studies on surgical samples, has the added advantage of enabling the study of the wider network changes associated with HS, the long-term effects of epilepsy on the pathology and associated comorbidities. It is likely that HS is heterogeneous in aspects of its cause, epileptogenetic mechanisms, network alterations and response to medical and surgical treatments. Future neuropathological studies will contribute to better recognition and understanding of these clinical and patho-aetiological subtypes of HS.
Keywords: hippocampal sclerosis, neuropathology, temporal lobe epilepsy
Introduction
The hippocampus is the most widely studied brain region in both human and experimental epilepsy. Sclerosis of the hippocampus in epilepsy was first noted as far back as 1825 1 followed by the first detailed study of the segmental patterns of neuronal loss in a series of 90 post-mortem (PM) cases, published by Sommer in 1880 (2, for historical review see 3). In the modern era of epilepsy surgical programmes for the management of drug-resistant epilepsy, hippocampal sclerosis (HS) is one of the commonest pathologies 4. HS is particularly associated with the syndrome of mesial temporal lobe epilepsy (MTLE) but can be seen at PM in other epilepsy syndromes. It's incidence in large epilepsy surgical series varies from 33.6% 5 to 66% 6 and in PM series, HS is identified in between 30.5% and 45% of all epilepsy syndromes and in 56% with the syndrome of MTLE 7,8. Neuropathology research in MTLE/HS has been based largely on human surgical tissues, often in parallel with observations in the numerous experimental models of temporal lobe epilepsy (TLE). It has been directed into causes of HS, the cellular and molecular alterations that render the hippocampus epileptogenic, the identification of biomarkers that could be predictive of outcome following surgery as well as correlation with other comorbidities associated with seizures. This review aims to summarize some of the recent advances in this field which may be of relevance to the diagnostic neuropathologist.
HS criteria, classification and patterns
There have been several schemes to classify subtypes of HS, based on the subfield distribution as well as extent of, hippocampal neuronal loss and gliosis. Other terms used interchangeably for HS include Ammon's Horn sclerosis (strictly refers to neuronal loss in CA1–4 and not the dentate gyrus) and mesial temporal sclerosis (which implies more extended sclerosis of extrahippocampal tissues, such as the amygdala and parahippocampal gyrus). A recent consensus classification system, validated through the neuropathology taskforce of the International League Against Epilepsy (ILAE) 9, aims to incorporate aspects of all previous schemes 10–15 (Table 1), through implementing a reproducible, semiquantitative scale for hippocampal subfield neuronal loss. The main advantages of the ILAE system is that it is based on standard stains (Table 2), so can be universally adopted in any centre, it clearly segregates ‘atypical’ (type 2 and 3) from ‘classical’ HS (type 1) and will reduce over-interpretation of endplate gliosis alone as HS. This new classification relies on patterns of neuronal loss and gliosis as objective measures of sclerosis and does not incorporate other alterations (e.g. mossy fibre sprouting, interneuronal alterations – see below) which may be more difficult to reproduce between laboratories. The obvious benefits of a single system is that it will enable comparisons of data sets between centres and emergence of accurate pathological and electroclinical correlations as well as with advanced magnetic resonance imaging (MRI) sequences 19. At present, for example, there is some evidence that HS patterns could be predictive of seizure history or outcome (as detailed in Table 2); the atypical HS patterns have been associated with poorer seizure-free outcomes 11. There is no evidence to support the proposal that type 2 and 3 evolve to type 1 HS over time. Implementation of this clear framework over the next years, integrated with clinical, genetic and imaging data will enable these issues to be addressed and clearer characterization of different subtypes of medial TLE with distinct aetiologies, network alterations and prognosis, in particular the likelihood of a long-term seizure-free outcome following surgical removal 20.
Table 1.
Summary of the various classification schemes adopted for the patterns of hippocampal sclerosis (HS) in epilepsy in recent eras

Table 2.
Panel of stains useful in the interpretation of HS in epilepsy; those shown in bold are used in the 2013 ILAE classification of HS 9
| Histological preparation | Application | Methods/technical tips, limitation and pitfalls |
|---|---|---|
| Luxol fast blue/cresyl violet | Assessment of neuronal loss; these stains may be used interchangeably. NeuN is preferable for assessment of GCD. Neurofilament can highlight additional neuronal hypertrophy in CA4. Synaptophysin has also been used in the assessment of MFS, but it is not specific for mossy fibre pathway |
NeuN is fixation sensitive and gives less consistent staining in post-mortem tissues* Thicker sections (>10 μm ) also recommended for assessment of cyto-architecture with NeuN |
| NeuN | ||
| Synaptophysin | ||
| Map2 | ||
| Neurofilament | ||
| GFAP | Assessment of gliosis, patterns and distribution | Over-interpretation of endplate gliosis as sclerosis |
| GFAP-delta | ||
| CD34 | ||
| Timm method 16 | Assessment of mossy fibre re-organization | Timm stain: requires fixation of hippocampal slice from fresh specimen in buffered 1.2% sodium sulphide solution |
| Dynorphin ZnT3 |
Dynorphin: Thicker sections (>10 μm) recommended for better visualization of MFS | |
| Parvalbumin | Assessment of interneuronal groups. Antibody clones used in illustrations in current paper: calbindin D-28K (1:10 000, Swant, Switzerland ) Calretinin (polyclonal, 1:2000; Sigma, Saint Louis, MO, USA) NPY ( 1:4000, Sigma) Parvalbumin, (1:300 Swant, Switzerland) |
Parvalbumin can be fixation sensitive and give less consistent staining in post-mortem tissues* |
| Calbindin | ||
| Calretinin | ||
| Neuropeptide Y (NPY) |
The histological hallmarks of HS have long been recognized since the earliest PM and surgical series 2,21. Distinctive histological features, which may aid in the discrimination from other causes of HS (e.g. neurodegeneration or hypoxia-ischaemia) include the sharp cut-off between the sclerotic CA1 and the spared neurones of the subiculum (Figure 1A–C), the typical fibrous, contracted gliosis of CA1 (attesting to the chronicity), granule cell dispersion (GCD) (Figure 1D,E), and scattered hypertrophic neurones (particularly in CA4) (Figure 1F) with cytoplasmic distention by microtubules and neurofilaments and increased dendritic complexity 22,23 (see Table 3). The diagnostic confirmation of HS relies to some extent on the provision by the surgeon of ‘en-bloc’ resections where the hippocampal subfield continuity is intact. In small, fragmented or poorly orientated specimens, confirmation of HS can be challenging 19, but supported through implementation of a small panel of special stains (Table 2). In anterior temporal lobectomy surgical procedures, an average 2 cm of the hippocampal body is resected 27,28. The pattern of sclerosis tends to be uniform along the longitudinal axis within a single surgical specimen in MTLE/HS 18 although variability, in the extent (Figure 1G–J) or distribution of subfield neuronal loss is occasionally seen; for example the pattern may represent type 1 HS at one coronal level and type 2 HS in another. The clinical significance of patchy vs. complete subfield damage in resected specimens is, as yet, uncertain 9.
Figure 1.
Typical pathology features of hippocampal sclerosis in epilepsy. (A) Luxol fast blue/cresyl violet-stained preparation of a surgical sample with subfield neuronal loss involving CA1 and CA4. (B) GFAP preparation confirming dense fibrillary gliosis in CA1 with a sharp cut-off point (arrows) with the adjacent subiculum (SBC) and (C) higher magnification detail of the abrupt transition of gliosis at the CA1/subiculum (arrows). (D) Granule cell dispersion as seen with cresyl violet and (E) with NeuN showing clusters of dispersed granule cells (inset shows reelin-positive interneurones and bipolar cells in the molecular layer). (F) Neurofilament-positive neurones with enlarged cell bodies in CA4 of the sclerotic hippocampus. (G) CA1 pyramidal cell layer in a patient with MTLE but no evidence of neuronal loss, (H) CA1 with evidence of partial neuronal loss from mid zone and, (I) CA1 with severe neuronal depletion and collapse of the layer. (J) Section of the pes hippocampus from a patients with confirmed classical ILAE type 1 sclerosis in the body; in the pes neuronal loss is limited to the endplate in this case as an illustration of variability in the pattern of atrophy that may occur along the longitudinal hippocampal axis. Bar is equivalent to approximately 1 mm (A, B, J), 250 micrometers (C, F, G, H, I) and 100 micrometers (D, E).
Table 3.
Neuropathological features that may aid in the distinction of hippocampal sclerosis in epilepsy (HS-e) from other cause of HS 24–26
| Histopathological features predictive of HS-e | ||
|---|---|---|
| Highly associated HS-e | Not-predictive | Less likely HS-e |
| Dense fibrillary gliosis in CA1 CA4 neuronal loss Sharp demarcation between neuronal loss and gliosis in CA1 and the subiculum Granule cell dispersion Mossy fibre sprouting Interneuronal network reorganization Hypertrophic (NF-positive) neurones in CA4 (and other subfields) |
CA2 sparing Neuronal loss and gliosis in CA1 is focal and not affecting all the subfield Variability of HS sclerosis along the longitudinal axis Bilateral HS Loss of calbindin expression in granule cells Microglial activation |
Patchy, widespread cellular ‘reactive’ gliosis; less subfield restricted Neuronal loss in CA1 extends into subiculum Non-subfield specific distribution of neuronal loss Identification of neuronal inclusions with IHC (e.g. Tau, p62 and TDP43) |
Granule cell dispersion (GCD)
Although there is no agreed definition for GCD in HS (granule cell layer thickness >10 cells 29 or 120 μm 30 have both been proposed), it is a common and striking feature in 40–50% of cases 29,31. The extent, as well as pattern of GCD, including ‘bi-laminar’ patterns or clusters of granule cells in the molecular layer (Figure 1E), may vary within cases and alternate with regions of granule cell loss. Extensive GCD is virtually pathognomonic of seizure-induced hippocampal changes and is typically seen in the context of hippocampal neuronal loss, particularly of CA4 13,32, suggesting that is an acquired process rather than a pre-existing abnormality. There is evidence linking the presence of GCD with early onset of epilepsy and febrile seizures (<4 years) as well as longer duration of epilepsy 31. There is conflicting data regarding whether GCD signifies a good outcome following surgery 13,31,32.There has been considerable interest in granule cell reorganization in HS from the perspective of its potential contribution to pro-epileptogenic circuitry, reflection of interference with ongoing neurogenesis and correlation with memory disorders associated with epilepsy.
Neuronal migration during development is normally associated with immature neurones. There are two schools of thought in HS: that GCD represents neuronal ‘heterotopia’ of newly generated neurones following aberrant neurogenesis or that it results from abnormal migration of mature neurones, both influenced by seizures. There is abundant evidence from experimental models that seizures influence rates of granule cell neurogenesis, with new neurones migrating to abnormal or ectopic positions and furthermore integrating into networks and acquiring pro-epileptogenic physiological properties 33–38. A model of febrile seizures recently reported that altered migration of newly generated granule cells was enhanced by excitatory gamma-aminobutyric acid (GABA) signalling during seizures 39. In contrast, following kainic acid-induced seizures, time-lapse studies argue for dispersion of fully differentiated granule cells 40, migrating by a process of ‘somatic translocation’, a mechanism which involves shifting of the cell body into an apical dendrite 41.
It has proven more difficult to explore and validate any altered rates of neurogenesis or the migratory mechanisms that have contributed to GCD in TLE at the ‘end stage’ of the disease process, in either surgical or PM tissues 42–44. Morphological studies of dispersed cells in HS tissues have confirmed wider branching angles of the apical dendrites and more frequent recurrent basal dendrites, which could support that somatic translocation had occurred 45. Local deficiency of reelin protein and loss of reelin-expressing cells has been implicated as orchestrating the process of GCD (Figure 1E) 40,46,47. Studies of calbindin expression patterns, a marker of granule cell maturity, have also demonstrated differential expression in the dentate gyrus in HS with more dispersed cells calbindin positive, while more basal cells are calbindin negative; one interpretation of this is that a neo-migration of mature granule cell neurones has occurred 48–52 (Figure 2A–C). Important clinicopathological correlations include that a reduction of calbindin-positive granule cells 53, loss of granule cells in the internal limb of the dentate gyrus 54 as well as loss of regenerative capacity have all been associated with memory impairment arising in association with TLE 55. This highlights the important contribution of granule cell pathology to comorbidities in epilepsy.
Figure 2.
Axonal sprouting in hippocampal sclerosis (HS)/temporal lobe epilepsy (TLE). Mossy fibre sprouting identified with Timm staining in hippocampal sclerosis (HS) (A–C). In (A) the black silver granules are mainly confined to the subgranular zone and significant sprouting into the molecular layer (arrow) is not observed. In (B) there is focal sprouting in the molecular layer (arrow) and in (C) marked sprouting is shown with a dense band of zinc positive granules in the molecular layer. (D) Similar mossy fibre sprouting confirmed with dynorphin immunohistochemistry. (E) Extensive sprouting of neuropeptide Y-positive fibres is seen in the dentate gyrus compared with normal pattern (inset). (F) Sprouting of galanin fibres is shown in the gliotic dentate gyrus from a case with TLE and hippocampal sclerosis. In HS more intense synaptic pattern of staining was noted in the molecular layer of the dentate gyrus in a series compared with mTLE cases without HS (M. Thom, unpub. obs.). (G) The myeloarchitecture can appear abnormal in HS with a random arrangement of fibres permeating the dentate gyrus. (H) Calretinin immunohistochemistry with sprouting fibres through the molecular layer compared with the compact alignment of fibres embracing the dentate gyrus in the normal hippocampus (shown in inset). (I) Calbindin immunohistochemistry with sprouted fibres through the molecular layer in HS and loss of the normal expression in the granule cells. In all images the molecular layer is at the top of each figure and the subgranular zone at the bottom. Bar is equivalent to approximately 50 μm (A, B, C) and 100 μm in others.
Neuropathology studies of epileptogenic processes in HS
The term epileptogenesis encompasses the cascade of cellular events, following which a brain develops spontaneous seizures or epilepsy. There are obvious limitations and challenges in exploring these processes in human tissues of HS, usually at an advanced stage and subjected to the effects of anti-epileptic drugs. The obvious question has always been how a region with such dramatic depletion of neurones can generate seizure activity. It is plausible that the pro-epileptogenic pathophysiological mechanisms are separate from the sclerosing process. Nevertheless, there has been a valuable contribution from neuropathological studies of altered neuronal networks and connectivity that could underpin hippocampal hyper-excitability to parallel observed altered neuronal electrophysiological properties in slice cultures 56. Furthermore, unravelling the key epileptogenic events is an essential pathway towards identifying novel molecular therapeutic targets to intervene with these processes 57,58.
Mossy fibre sprouting
Axonal sprouting, a common process in the developing brain, is revived in adult tissues in response to seizures. Such plasticity may represent primarily a reparative response to hippocampal neuronal loss, but may ultimately be mal-adaptive and pro-epileptogenic 59. The mossy fibre pathway has been most widely studied in this respect. First observed in animal models, and subsequently in human HS 60, mossy fibre sprouting has been argued a critical component in the development of recurrent seizures in HS. In normal conditions, fewer than 1% of mossy fibres possess a recurrent axonal branch into the molecular layer but, in HS, extensive recurrent projection of mossy fibre collaterals into the molecular layer of the dentate gyrus occurs, to make excitatory synaptic contact 61 with apical dendrites and spines of granule cells in the inner molecular layer, essentially creating a local ‘short-circuit’ with a potential to synchronize neuronal groups. In tissues, mossy fibre sprouting is best demonstrated with Timm silver method (reaction with zinc, sequestered in synaptic vesicles) (Figure 2A–C) (Table 2), zinc transporter 3 16,62 or with immunohistochemistry for dynorphin A (an opioid neuropeptide present in granule cells and in the terminal fields of the mossy fibres) 63 (Figure 2D).
The extent of mossy fibre sprouting can vary between HS cases (Figure 2A–D). The process of sprouting is thought to be triggered early by both hippocampal seizure activity 37,64,65 and neuronal loss. There is some evidence that newly generated cells form a significant contribution to mossy fibre sprouting 36,52. Of note, it is absent or at the most mild in HS associated with neurodegeneration and dementia 24. In TLE patients with milder degrees of CA4 neuronal loss, mossy fibre sprouting may be less pronounced 66–68 as well as in HS with marked granule cell depletion. The molecular cues initiating or promoting sprouting include a role for mammalian target of rapamycin (mTOR) pathway activation; it has been shown that rapamycin administration reduces both sprouting and seizures experimentally 69,70 offering a potential novel treatment. There is also experimental literature to support that suppression of mossy fibre sprouting, for example with cyclohexamide, does not ameliorate seizures 71,72. In addition we have noted that sprouting remains sustained in elderly patients at PM with HS, even with spontaneous cessation of seizure activity for many years 73. These observations argue somewhat against a critical role of sprouting in hippocampal epileptogenesis. Therefore, although mossy fibre sprouting is a hallmark pathological feature of HS in epilepsy, any critical physiological contribution underpinning seizure activity remains to be established.
Interneuronal networks in HS
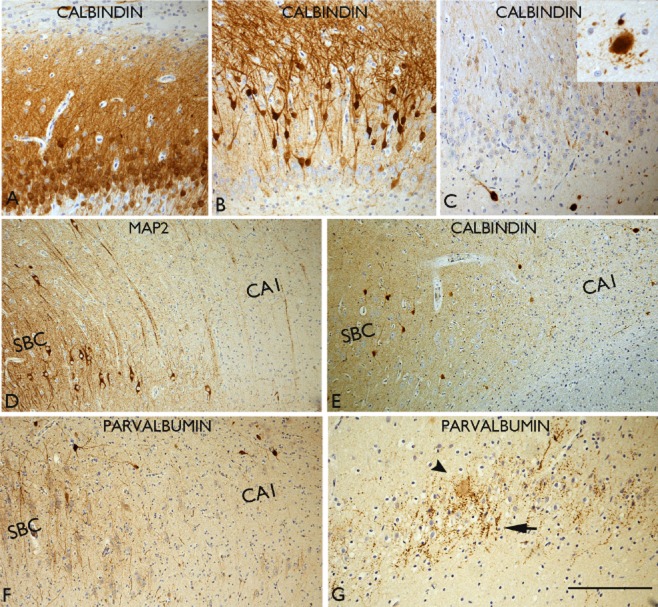
Although the ILAE classification of HS is based on patterns of principal pyramidal cell loss, stereotypical alterations to hippocampal interneuronal cell types also accompany this process. This has been studied more extensively in experimental models, where functional alterations in relation to cellular, network changes and epileptogenesis, can be directly correlated with histological changes to interneuronal groups 74. GABAergic cell types more extensively studied in human TLE/HS include those expressing calcium-binding proteins (calbindin, parvalbumin and calretinin) (Figures 2H,I and 3). Interneurones are also classified according to their morphology, localization and their target domain in the dentate gyrus and CA regions 75,76. In general they provide inhibition at the perisomatic or axon-initial segment of principle pyramidal neurones, for example parvalbumin-positive interneurones 50, or dendritic inhibition for single neurones or neuronal groups (including other interneurones), for example calretinin and neuropeptide Y (NPY)-expressing neurones 75,77. Both spatial and temporal variation in the vulnerabilities of specific interneuronal populations in response to seizures, is noted in experimental models 78.
Figure 3.
Interneuronal changes in hippocampal sclerosis in epilepsy (HS). (A) Normal calbindin pattern with labelling of the granule cells and apical dendrites. (B) Absent or loss of calbindin expression is noted in the basal cells while the dispersed cells are calbindin positive; this is a common pattern observed in HS with granule cell dispersion. (C) Virtual total loss of calbindin expression in all the granule cells; residual hilar calbindin-positive neurones as well as in CA1 (inset) can appear hypertrophic and dysmorphic with abnormal processes extending from the cell body. (D) The sharp transition between the neuronal loss in CA1 and the preserved subicular (SBC) neurones is illustrated with MAP2 staining. (E) Calbindin labelling in the same case as (D) shows relative loss of interneurones and their arborizations in the CA1 as with parvalbumin (F). Increased perisomatic labelling and terminals in the dentate gyrus, as with parvalbumin in an HS case. (G) Complex parvalbumin terminals are seen in the dentate gyrus granule cell layer in HS. Bar is equivalent to approximately 75 μm.
The predominant qualitative and quantitative change reported in human HS studies include loss of protein expression or reduction in interneuronal number 50,79,80 (Figure 3D–F). Morphological changes include cell hypertrophy (Figure 3C), abnormal dendritic projections with altered distribution of spines 81–83 and axonal sprouting (Figure 2E,F,G,H,I). Axonal sprouting is the most readily demonstrated qualitative interneuronal change in routine diagnostic practice and can be a helpful aid, particularly in a fragmented HS specimen as well as in investigations of the aetiology of HS in PM samples 24,25,73 (Tables 2 and 3). It is exemplified in the dentate gyrus with calretinin, where expansion of the axonal plexus from the inner to the outer molecular layer may be observed (Figure 2H) 25, 81. Sprouting of calbindin-positive axonal networks through the dentate gyrus (Figure 2L) may also be evident in tissue sections, when unmasked by the loss of the normal calbindin expression in the dentate granule cells and dendrites, a common occurrence in HS (Figure 2C). Increased complexity of parvalbumin-positive chandelier cells and terminals in the pyramidal cell layer and the dentate gyrus has also been noted 50 (Figure 3G). Sprouting of these inhibitory networks is often observed to parallel sprouting of excitatory networks (the mossy fibres) in established HS 24,25,59,73. Many interneuronal alterations likely represent adaptive or compensatory responses to seizures but, nevertheless, may contribute to sustained excitatory/inhibitory imbalances as well as synchronization of local neuronal networks 74,84,85. As yet there is limited information regarding any differences in interneuronal changes that characterize ILAE HS subtypes. There is also experimental evidence, from stereological acquired data, that there is not necessarily a direct relationship between the extent of interneuronal loss and the severity of clinical seizures 86 which would also be important to further explore in MTLE subtypes in human tissues.
Neuropeptides in HS
Neuropeptides colocalize with inhibitory neurones, are stored in large dense vesicles and when released, have longer half-lives than neurotransmitters, thus modulating neuronal or network activity over longer periods 87. NPY, somatostatin, galanin and dynorphin have endogenous anti-convulsant properties with protective effects against epilepsy, whereas substance P has a pro-epileptic effect 87. Many neuropeptides show high expression in hippocampal structures, particularly NPY 88. Experimental studies confirm release and increased synthesis of NPY and somatostatin following seizures 89–91. Reorganization of NPY fibre networks is the best studied and argued to ‘define’ the epileptogenic hippocampus 12,67,92. In the normal hippocampus, NPY-expressing cells are readily seen in the hilus of CA4 with a dense plexus of axons in the outer molecular layer, that synapse with granule cell dendrites 93 (Figure 2E). In HS, loss of NPY neurones and extensive sprouting and beading of NPY axons is noted, extending through the granule cell layer and into the molecular layer 67,92,94 (Figure 3E). This NPY-sprouting typically parallels mossy fibre sprouting and is thought to act by blocking the synchronization of granule cells through these recurrent mossy collaterals 95. NPY-sprouting has been reported in other (non-MTLE) epilepsy syndromes and in the absence of significant HS 73 but is significantly less pronounced, and often absent, in HS associated with dementia/neurodegeneration 24; as such it appears to represent a good histological marker for the epileptogenic hippocampus 95. Recently gene therapy studies using viral vectors to induce hippocampal NPY over-expression have resulted in decreased seizure frequency and remains an attractive treatment strategy 96. In addition, it has been recently recognized that NPY also regulates hippocampal neurogenesis, indicating roles beyond seizure modulation 97 but that may be relevant to cognitive decline in epilepsy.
Studies of somatostatin-expressing neurones in HS, similar to NPY, have demonstrated a reduction in cell number and radial sprouting of fibres in the dentate gyrus 67,92. Galanin is a small peptide of 29 amino acids with high concentrations in the adjacent amygdala 87. Its effects in the hippocampus are mediated by receptors GalR1 and GalR2 98 where it exerts a presynaptic inhibitory effect on glutamatergic transmission and is another promising treatment target 99. Alteration of galanin networks and receptor expression in human HS tissues, compared with other neuropeptides, remain less studied (Figure 2F). Substance P has been shown to have pro-epileptic effects with increased expression shown in status epilepticus 87. Studies of substance-P receptor expressing interneurones in HS have been carried out which have demonstrated a reduction of interneurones in CA1 and the hilus in addition to increased multipolar cells in the molecular layer; morphological changes were also noted including increased complexity of dendritic branches 81 and synaptic reorganization 100. These studies all reinforce the widespread alteration of neuropeptidergic systems in HS; as endogenous neuromodulators and a potential new therapeutic option, these systems, including the distribution of synthesizing neurones and their receptors, is likely to be more extensively studied in the future.
Neurotransmitters in HS
Abnormalities in the regulation of synaptic neurotransmission in HS can occur at any level from protein synthesis, release, to receptor distribution and assembly and subsequent signalling cascades 101. Monogenic epilepsy syndromes commonly involve mutations in ion channel or neurotransmitter receptor proteins 102. The genetics of partial epilepsies, including TLE are likely to be complex 103; familial forms of TLE with HS are recognized and some of the candidate genes also involve ion channels 104 as well as in sporadic TLE 105. For example, a recent genome-wide association study has linked a sodium channel gene cluster of SCN1a in patients with HS and a history of febrile seizures 106. On this theme, abnormalities in the expression and distribution of voltage-dependent potassium channels have been shown in tissue sections in HS 107 (Figure 4A). Altered GABAA neurotransmitter receptor expression, distribution and assembly has also been reported in HS; these mediate fast postsynaptic inhibition 108, and alterations are particularly noted in the dentate gyrus where the granule cells display remarkable plasticity 109,110. Extracellular GABA concentration is influenced by the rate of uptake of this neurotransmitter and altered levels of GABA transporters GAT-1 and GAT-3 have been shown in TLE 111. The endocannabinoid system also modulates glutamatergic and GABAergic synaptic transmission 112 with a proposed role for seizure potentiation in TLE 113. In tissue sections of HS, a decrease in cannabinoid type 1 receptor (CB1) and CB1 receptor-binding protein mRNA has been observed 114 but with increased levels associated with GABAergic fibres in the dentate gyrus 115, suggesting a primary modulation of GABAergic transmission (Figure 4B). There is also evidence for a switch from an inhibitory to excitatory action of GABA in epilepsy 116,117, mirroring its developmental function. This change is mediated by altered expression of neuronal cation-chloride cotransporters (KCC2 and NKCC1) 118. Changes in the relative expression of NKCC1 and KCC2 in pyramidal cells of hippocampal subfields has been shown in TLE/HS (Figure 4C) which may contribute to epileptiform activity 119,120.
Figure 4.
Pathological alterations potentially contributing to epileptogenesis in hippocampal sclerosis (HS). (A) Prominent labelling of voltage gate potassium channel (KV1.1) in residual neurones in CA1 in hippocampal sclerosis; differences were noted in the patterns of expression between hippocampal subfields in HS compared with controls (M. Thom, unpub. obs.). (B) Prominent labelling of cannabinoid receptor is noted in the plexus in the molecular layer of the dentate gyrus in HS. (C) Intense labelling of CA1 neurones with cation-chloride cotransporter (NKCC) in HS. (D) In some cases of HS in epilepsy, particularly in adult onset cases or in the context of recent encephalitis, striking reactive and ‘balloon cell’ gliosis can be seen in the granule cell layer with a proportion of these cells showing membranous CD34 staining, mimicking a focal cortical dysplasia (inset). (E) Immunolabelling with isoform GFAP-delta highlights prominent numbers of small astroglia, particularly in the subgranular zone, in HS. (F) Immunostaining for gap junction protein connexin 43 (Cx43) demonstrated prominent labelling of astrocytic cells in the subgranular zone and occasionally in the molecular layer in a post-mortem HS case. (G) Expression of drug transporter protein, p-glycoprotein, is shown on capillaries within hippocampus. (H) Albumin staining demonstrating focal leakage from small capillary vessels in a case of HS/TLE. (I) Coexpression of vascular endothelial growth factor (VEGF) and hypoxic inducible factor 1 alpha (HIF1-alpha) is shown in pyramidal neurones in HS. Bar is equivalent to approximately 35 μm (A, B, D, E, I); 60 μm (C, E, F, H).
Astroglia and blood brain barrier dysfunction
Gliosis is a striking component of HS with chronic, fibrillary gliosis in CA1 and a radial gliosis in the dentate gyrus 43; gliosis involving CA4 is a less specific finding for HS, being a common finding in the absence of neuronal loss. A few case reports of HS have also noted a more cellular gliosis with CD34-positive ‘balloon cell’ like astrocytes in the dentate gyrus, reminiscent of those observed in focal cortical dysplasia (Figure 4D). These astroglia were associated with striking rarefaction of the dentate hilus 121 and were also reported arising in HS following a nonherpetic limbic encephalitis 122 and may indicate a different aetiology of the HS in epilepsy. Glial fibrillary acidic protein (GFAP)-delta, a developmentally regulated isoform, highlights small multinucleate glial cells, particularly in the subgranular zone of the dentate gyrus which colocalize with nestin in HS (Figure 4E) and may represent a specific subpopulation of glia with specific roles in this neurogenic niche 123; similar glial cell types were also noted in HS associated with dementia 24.
Over the last decade there has been a trend away from a ‘neurocentric’ focus with more directed investigations addressing the participation of astrocytes in the causation of seizures 124. There is a breadth of astrocytic function beyond their acting as supporting cells 125; dysfunctions of many of these processes may be of relevance to seizures in HS. Evidence for this includes impaired glutamate clearance by astrocytes and release in HS (via EAAT1 and EAAT and glutamine synthetase) 126 and impaired K+ clearance via inwardly rectifying potassium channels 127. Astrocytes are extensively coupled via gap junctions to form a functional syncytium, primarily expressing connexin 43. There have been conflicting findings, however, regarding the elevation of these proteins in hippocampal astrocytes in HS 127–129 (Figure 4F).
Abnormalities of the vasculature have been reported in HS, with proliferation of micro-vessels 130, vascular endothelial growth factor receptor expression and loss of blood-brain barrier integrity 131,132. Vascular leakage of proteins, including IgG 133 and albumin may contribute to neuronal dysfunction in epilepsy 134 (Figure 4H). Of note, other studies, demonstrated a reduced microvasculature in the sclerotic hippocampus 135,136. Over-expression of drug transporter proteins at the blood brain barrier in HS, such as p-glycoprotein and multidrug resistance-associated proteins, have been linked to treatment failure in HS, due to facilitated drug efflux preventing anti-epileptic drugs from reaching their target neurones 137,138 (Figure 4G).
Aetiology of HS
Initiating insult and genetic susceptibility factors
The aetiology of HS is still controversial and is likely to be multifactorial. It is widely considered an acquired pathology. Alfred Meyer's seminal hypothesis from the 1950s proposed that an initiating event, injury or insult the initial precipitating injury’ (IPI), particularly prolonged febrile convulsions early in life, primed the immature hippocampus for the subsequent development of HS 139,140. Seizures are known to damage the hippocampus, particularly prolonged seizures and status epilepticus 141–145. Experimentally, TLE has been induced following prolonged febrile seizures 146 and prospective studies, as the FEBSTAT study have confirmed HS (as defined by MRI criteria) in a minority of patients following febrile status epilepticus 147. However, as neither status epilepticus, prolonged febrile seizures nor repetitive generalized or partial seizures inevitably lead to HS in the majority of cases, there are likely other susceptibility factors. HS is considered a sporadic condition. Rare pedigrees provide evidence for a common genetic basis for febrile seizures and MTLE 148–150. Although genetic susceptibility determinants to nonfamilial or sporadic HS have, as yet, not been clearly defined 103,151–153, ApoEε4 genotype has been associated with increased risk of bilateral HS 154 and more recently, HS and febrile seizures were linked by common genetic variation around SCN1A gene 106.
Seizure-induced neuronal loss
Necrotic or apoptotic neurones are only very occasionally seen in surgical specimens of HS as evidence of ongoing neuronal death. It is generally regarded that seizure-induced neuronal injury and subsequent loss results from excitotoxic, glutamatergic neurotransmission, excessive Na+ and Ca2+, resulting in osmolytic stress and cellular free-radical production, culminating in necrosis of neurones 155. Molecular studies also support that activation of apoptotic cell death pathways (mediated by both intrinsic and extrinsic pathways) contribute to hippocampal neurone loss 155,156. Regarding the distribution of neuronal loss in HS, subfield specific regulation of microRNAs has been shown following seizures; these post-transcriptional regulators of gene expression may be critical for determining cell death pathways 157. Hypoxic inducible factor 1 alpha (HIF1-alpha) and vascular endothelial growth factor (VEGF) neuronal induction has been shown in HS cases at PM, with some evidence of correlation of seizure activity prior to death 158 (Figure 4I). HS can be observed in association with a second seizure-focus or epileptogenic pathology such as a low grade tumour/malformation, e.g. dysembryoplastic neuroepithelial tumour (DNT), cavernoma; the pattern of HS is often type 3 in such ‘dual pathologies’ 9 and mechanisms of this potentially ‘kindled’ hippocampal neuronal loss may differ from isolated HS. Indeed, in paediatric MTLE/HS series, a higher percentage of cases have dual pathologies compared with adult surgical series; this fact, together with hippocampal maturation, may be relevant to different patterns of neuronal loss and dentate gyrus abnormalities observed between age groups 159,160. Mitochondrial dysfunction has also been proposed to play a role in the underlying pathogenesis of HS 161.
Inflammation
In HS, neuronophagia or focal infiltrates of microglia are occasional findings. There is evidence to support activation of both the innate and adaptive immune system, for example IL-1β and IL-1 receptor upregulation, has been noted in astrocytes, microglia and neurones in HS, and intercellular adhesion molecule 1 (ICAM-1) and kallikrein expression in glia 162,163. B and T cell infiltrates, however, are usually inconspicuous in tissue sections and mainly in a perivascular location 162–165. Inflammatory pathway activation in human TLE is supported by gene expression studies 166 and has been argued to be a driving force in disease progression 167. There is evidence that inflammation can perpetuate, augment or even initiate seizures 168,169. A potential diagnostic pitfall to consider is that localized active/chronic inflammation can be seen in the hippocampus in relation to prior invasive depth electrode recordings. However, the presence of prominent, more widespread inflammation in HS specimens, nevertheless, should always raise the possibility of an underlying or previous limbic or autoimmune encephalitis, particularly in adult onset epilepsy cases 170. Regarding underlying viral infection in HS, detection of human Herpes virus 6 infection was frequently reported in one HS/TLE series 171 but in another was only identified in patients with a history of a prior episode of limbic encephalitis 172.
Underlying maldevelopmental template
Hippocampal sclerosis can be observed in association with generalized or focal malformations of cortical development (MCD) as a dual pathology 173 and with focal cortical dysplasia (FCD) Type IIIa of the temporal lobe 174. There has been ongoing debate regarding if a pre-existing isolated hippocampal developmental abnormality could precede HS, and predispose to the development of sclerosis 29,175–178. A recent MRI study of family members of patients with TLE and HS confirmed smaller hippocampal volumes than normal, which may represent a developmental variant at risk for HS 179. Indeed recent genome-wide studies have also linked common genetic variants which associate with hippocampal volume 180,181; further study may identify how these genes influence hippocampal development and if they are of relevance to loss of hippocampal volume and vulnerability to HS in epilepsy. There is also ongoing debate regarding the relevance of hippocampal malrotation (also known as incomplete hippocampal inversion) to epilepsy, TLE, HS and febrile seizures 182. In malrotation, the pyramidal cell layer of the CA1/subiculum region typically appears preserved but hyper-convoluted. There is no evidence to support that this necessarily evolves into the typical picture of HS in patients with epilepsy 183,184 and there is also a lack of the mossy fibre sprouting, GCD and interneuronal re-organization that typify HS 175,183,185.
Studies of HS in PM tissues
Although the bulk of neuropathology research in the past decades has been carried out on surgical tissue with its obvious advantages optimally preserved tissues, homogeneous clinical cohorts and access to up-to-date electroencephalography (EEG) and neuroimaging studies we should not neglect the ongoing contribution that autopsy samples can provide in the study of HS in epilepsy. Primarily, PM tissues enable comparisons of HS occurring in epilepsy syndromes other than TLE (also known as secondary HS 7), the effects of a lifetime of seizures on the severity of hippocampal neuronal loss and its associated pathology 186, enable study of the bilaterality of HS 73, the extent of involvement along the entire length of the hippocampus 25 (Figure 5) and to address degeneration in wider networks that have been implied from quantitative MRI studies in TLE 20,187,188, in particular the amygdala, cortex 189, thalamus 190 and cerebellum 191. Neuropathology studies have, from the outset, recognized that more extensive pathology may accompany HS 15,21, the hippocampus being the epicentre of a wider process. Experimental data support widespread changes occurring following induced seizures 192 which could equally contribute to epileptogenesis. Involvement of wide networks has been shown in TLE 193,194 and functional and structural imaging of TLE indicates altered brain connections or connectome in TLE 195,196. As such, in recent years there has been a move away from a ‘hippocampocentric’ view of TLE 197,198 addressing contribution from other brain regions. The main studies have investigated (i) electrophysiological evidence to support origin of seizures from extrahippocampal structures 199,200; (ii) if wider disease offers the explanation for poor outcomes (in terms of seizure-freedom) following localized surgery; and (iii) if severity (or progression) of any extrahippocampal pathology correlates with comorbidities, such as cognitive decline.
Figure 5.
Variability of hippocampal sclerosis (HS) in post-mortem and surgical samples. (A) Surgical hippocampectomy specimens, which on histological examination correlated to (i) end folium gliosis with no evidence of sclerosis; (ii) ILAE Type 3 HS (end-folium sclerosis); (iii) ILAE type 2 HS (CA1 predominant sclerosis); and (iv) ILAE type 1 HS (classical hippocampal sclerosis). In all of the images the arrow indicates the pyramidal cell layer of CA1. (B) A 9.4T MRI image of a post-mortem hippocampus from a patient with longstanding epilepsy and ILAE type 2 (CA1 predominant pattern) of HS at this level (shown in C in a luxol fast blue/cresyl violet preparation), although in other levels the pattern was type 1 (classical). The MRI has the ability to identify subfields and white matter tracts and, indistinctly (arrowed), the dentate gyrus. Improved high fields sequences in the future may be able to identify and define patterns of HS pre-operatively. The MRI image was provided with courtesy of Dr Sofia Eriksson at the Department of Clinical and Experimental Epilepsy, UCL, Institute of Neurology. (D to I) Paired sections of hippocampus from one hemisphere labelled with (D to F) GFAP/counterstained with cresyl violet and (G to I) calretinin: at the level of the subthalamic nucleus (STNc; D, G), lateral geniculate nucleus (LGNc; E, H) and hippocampal tail (F, I). Classical pattern (ILAE type 1) HS is seen in the anterior levels with sprouting of calretinin-positive fibres visible in the dentate gyrus (arrow) at this low magnification; in the tail gliosis and neuronal loss is visible in the CA4 region of the hippocampal tail. Bar is equivalent to approximately 3 mm (D to I).
The cellular and pathological basis of structural changes in extrahippocampal regions associated with HS typically manifests as neuronal loss and gliosis with few studies exploring the contribution of interneuronal populations 189,201. It remains to be established whether any more extended ‘network’ changes arise as a result of the same initial insult causing HS, if they are subsequent to HS through retrograde/anterograde degeneration, or if they arise independently. In PM series it is also possible to explore the long-term effects of seizures on the brain and any predisposition to neurodegenerative disease or accelerated ageing processes in HS. It has been observed from a patient cohort closely followed for decades at the National Society for epilepsy, Chalfont centre, that patients with pathology proven HS/TLE at PM were less likely to go into terminal seizure remission with advanced age compared with other epilepsies 8. It has also been shown in this same PM cohort that accelerated tau accumulation in epilepsy was not associated with the severity of seizures and was also independent of the presence of HS but correlated with acquired and accumulative traumatic brain injury incurred from frequent seizures 202. Continued donation of brain tissues from patients with epilepsy to tissue bank resources for example the Epilepsy Society Brain and Tissue Bank at University College London (UCL) will enable further studies of HS in the future.
Outcomes and the future
Although, benign forms of TLE and HS exist with infrequent and well controlled seizures 203 many patients with HS are medication-resistant and surgical intervention, following a series of investigations such as imaging, functional tests and video-EEG 204, may offer the best treatment option at present. Temporal lobe resection techniques have evolved, specifically with more limited resections, aiming to preserve function without compromising seizure outcome. Of the selected patients who undergo surgical treatments, approximately two-thirds will remain seizure-free in the first 2–3 years with around 57% seizure-free outcome at 5 years 6. The cause for surgical failure and poor outcomes in a proportion if carefully selected patients, is as yet, unclear.
In summary, there is accumulating evidence that HS in epilepsy is likely to be heterogenous in many aspects, including its aetiology, genetics, epigenetics, networks involved, patterns of neuronal loss as well as responses to drugs and surgical treatments 20. Neuropathology- and tissue-based studies in the future, are likely to continue to contribute to the ongoing identification of diagnostic and prognostic biomarkers as well as to further our understanding of the causes and ultimately, the prevention of this pathology.
Acknowledgments
I would like to acknowledge the contribution of Joan Liu and Cheryl Reeves (supported by MRC grant Grant Ref: MR/J01270X/1) to this work. The PM tissues for many of the quoted studies are part of the Epilepsy Society Brain and Tissue Bank, based at UCL (http://www.epilepsysociety.org.uk/brainbank). Acknowledgements are due to Paul Johns, Rianne van der Pijl who collaborated with some of the unpublished studies included in this review as well as Lillian Martinian for the majority of the published studies. I would also like to particularly thank Sofia Eriksson of the Department Of Clinical And Experimental Epilepsy at UCL, Institute of Neurology for permission to use the MRI in-vivo image of the PM specimen from an unpublished study. This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. There are no conflicts of interest to declare.
References
- 1.Bouchet C, Cazauvieilh CA. De l'épilepsie considerée dans ses rapports avec l'aliénation mentale. Recherche sur la nature et le siège de ces deux maladies. Arch Gen Med. 1825:510–542. [Google Scholar]
- 2.Sommer W. Erkrankung desAmmonshornes als aetiologisches Moment der Epilepsie. Arch Psychiatr Nervenkr. 1880:361–375. [Google Scholar]
- 3.Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–572. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15:34–39. doi: 10.1016/j.yebeh.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Blumcke I, Coras R, Miyata H, Ozkara C. Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 2012;22:402–411. doi: 10.1111/j.1750-3639.2012.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 7.Meencke HJ, Veith G, Lund S. Bilateral hippocampal sclerosis and secondary epileptogenesis. Epilepsy Res Suppl. 1996;12:335–342. [PubMed] [Google Scholar]
- 8.Novy J, Belluzzo M, Caboclo LO, Catarino CB, Yogarajah M, Martinian L, Peacock JL, Bell GS, Koepp MJ, Thom M, Sander JW, Sisodiya SM. The lifelong course of chronic epilepsy: the Chalfont experience. Brain. 2013;136:3187–3199. doi: 10.1093/brain/awt117. [DOI] [PubMed] [Google Scholar]
- 9.Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshe SL, Oz B, Ozkara C, Perucca E, Sisodiya S, Wiebe S, Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54:1315–1329. doi: 10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- 10.Bruton CJ. The Neuropathology of Temporal Lobe Epilepsy. Oxford: Oxford University Press; 1988. [Google Scholar]
- 11.Blumcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstock J, Stefan H, Hildebrandt M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol (Berl) 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- 13.Thom M, Liagkouras I, Elliot KJ, Martinian L, Harkness W, McEvoy A, Caboclo LO, Sisodiya SM. Reliability of patterns of hippocampal sclerosis as predictors of postsurgical outcome. Epilepsia. 2010;51:1801–1808. doi: 10.1111/j.1528-1167.2010.02681.x. [DOI] [PubMed] [Google Scholar]
- 14.Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37:982–990. doi: 10.1227/00006123-199511000-00019. discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 15.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 16.Jaarsma D, Korf J. A novel non-perfusion Timm method for human brain tissue. J Neurosci Methods. 1990;35:125–131. doi: 10.1016/0165-0270(90)90102-l. [DOI] [PubMed] [Google Scholar]
- 17.Liu JY, Martinian L, Thom M, Sisodiya SM. Immunolabeling recovery in archival, post-mortem, human brain tissue using modified antigen retrieval and the catalyzed signal amplification system. J Neurosci Methods. 2010;190:49–56. doi: 10.1016/j.jneumeth.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Thom M, Sisodiya SM, Beckett A, Martinian L, Lin WR, Harkness W, Mitchell TN, Craig J, Duncan J, Scaravilli F. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002;61:510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- 19.Jackson GD. Epilepsy: hippocampal sclerosis – are we speaking the same language? Nat Rev Neurol. 2013;9:548–549. doi: 10.1038/nrneurol.2013.173. [DOI] [PubMed] [Google Scholar]
- 20.Bonilha L, Martz GU, Glazier SS, Edwards JC. Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia. 2012;53:1–6. doi: 10.1111/j.1528-1167.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- 21.Cavanagh JB, Meyer A. Aetiological aspects of Ammon's horn sclerosis associated with temporal lobe epilepsy. Br Med J. 1956;2:1403–1407. doi: 10.1136/bmj.2.5006.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumcke I, Zuschratter W, Schewe JC, Suter B, Lie AA, Riederer BM, Meyer B, Schramm J, Elger CE, Wiestler OD. Cellular pathology of hilar neurons in Ammon's horn sclerosis. J Comp Neurol. 1999;414:437–453. doi: 10.1002/(sici)1096-9861(19991129)414:4<437::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Ryufuku M, Toyoshima Y, Kitaura H, Zheng Y, Fu YJ, Miyahara H, Murakami H, Masuda H, Kameyama S, Takahashi H, Kakita A. Hypertrophy of hippocampal end folium neurons in patients with mesial temporal lobe epilepsy. Neuropathology. 2011;31:476–485. doi: 10.1111/j.1440-1789.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 24.Bandopadhyay R, Liu JY, Sisodiya SM, Thom M. A comparative study of the dentate gyrus in hippocampal sclerosis in epilepsy and dementia. Neuropathol Appl Neurobiol. 2014;40:177–190. doi: 10.1111/nan.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thom M, Liagkouras I, Martinian L, Liu J, Catarino CB, Sisodiya SM. Variability of sclerosis along the longitudinal hippocampal axis in epilepsy: a post mortem study. Epilepsy Res. 2012;102:45–59. doi: 10.1016/j.eplepsyres.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyata H, Hori T, Vinters HV. Surgical pathology of epilepsy-associated non-neoplastic cerebral lesions: a brief introduction with special reference to hippocampal sclerosis and focal cortical dysplasia. Neuropathology. 2013;33:442–458. doi: 10.1111/neup.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296–1307. doi: 10.1111/j.1528-1167.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- 28.Schramm J, Lehmann TN, Zentner J, Mueller CA, Scorzin J, Fimmers R, Meencke HJ, Schulze-Bonhage A, Elger CE. Randomized controlled trial of 2.5-cm versus 3.5-cm mesial temporal resection-part 2: volumetric resection extent and subgroup analyses. Acta Neurochir (Wien) 2011;153:221–228. doi: 10.1007/s00701-010-0901-5. [DOI] [PubMed] [Google Scholar]
- 29.Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 30.Lurton D, El Bahh B, Sundstrom L, Rougier A. Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy. J Neurol Sci. 1998;154:133–136. doi: 10.1016/s0022-510x(97)00220-7. [DOI] [PubMed] [Google Scholar]
- 31.Blumcke I, Kistner I, Clusmann H, Schramm J, Becker AJ, Elger CE, Bien CG, Merschhemke M, Meencke HJ, Lehmann T, Buchfelder M, Weigel D, Buslei R, Stefan H, Pauli E, Hildebrandt M. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol. 2009;117:535–544. doi: 10.1007/s00401-009-0512-5. [DOI] [PubMed] [Google Scholar]
- 32.da Costa Neves RS, Jardim AP, Caboclo LO, Lancellotti C, Marinho TF, Hamad AP, Marinho M, Centeno R, Cavalheiro EA, Scorza CA, Targas Yacubian EM. Granule cell dispersion is not a predictor of surgical outcome in temporal lobe epilepsy with mesial temporal sclerosis. Clin Neuropathol. 2013;32:24–30. doi: 10.5414/NP300509. [DOI] [PubMed] [Google Scholar]
- 33.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Res Brain Res Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2011;519:2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy BL, Pun RY, Yin H, Faulkner CR, Loepke AW, Danzer SC. Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci. 2011;31:105–117. doi: 10.1523/JNEUROSCI.2728-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, Matsuki N, Ikegaya Y. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med. 2012;18:1271–1278. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- 40.Chai X, Munzner G, Zhao S, Tinnes S, Kowalski J, Haussler U, Young C, Haas CA, Frotscher M. Epilepsy-induced motility of differentiated neurons. Cereb Cortex. 2013 doi: 10.1093/cercor/bht067. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Murphy BL, Danzer SC. Somatic translocation: a novel mechanism of granule cell dendritic dysmorphogenesis and dispersion. J Neurosci. 2011;31:2959–2964. doi: 10.1523/JNEUROSCI.3381-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thom M, Martinian L, Williams G, Stoeber K, Sisodiya SM. Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J Neuropathol Exp Neurol. 2005;64:194–201. doi: 10.1093/jnen/64.3.194. [DOI] [PubMed] [Google Scholar]
- 43.Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, Frotscher M, Haas CA. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007;203:320–332. doi: 10.1016/j.expneurol.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Engel T, Schindler CK, Sanz-Rodriguez A, Conroy RM, Meller R, Simon RP, Henshall DC. Expression of neurogenesis genes in human temporal lobe epilepsy with hippocampal sclerosis. Int J Physiol Pathophysiol Pharmacol. 2011;3:38–47. [PMC free article] [PubMed] [Google Scholar]
- 45.Freiman TM, Eismann-Schweimler J, Frotscher M. Granule cell dispersion in temporal lobe epilepsy is associated with changes in dendritic orientation and spine distribution. Exp Neurol. 2011;229:332–338. doi: 10.1016/j.expneurol.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–640. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- 47.Haas CA, Frotscher M. Reelin deficiency causes granule cell dispersion in epilepsy. Exp Brain Res. 2010;200:141–149. doi: 10.1007/s00221-009-1948-5. [DOI] [PubMed] [Google Scholar]
- 48.Abraham H, Richter Z, Gyimesi C, Horvath Z, Janszky J, Doczi T, Seress L. Degree and pattern of calbindin immunoreactivity in granule cells of the dentate gyrus differ in mesial temporal sclerosis, cortical malformation- and tumor-related epilepsies. Brain Res. 2011;1399:66–78. doi: 10.1016/j.brainres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Abraham H, Veszpremi B, Kravjak A, Kovacs K, Gomori E, Seress L. Ontogeny of calbindin immunoreactivity in the human hippocampal formation with a special emphasis on granule cells of the dentate gyrus. Int J Dev Neurosci. 2009;27:115–127. doi: 10.1016/j.ijdevneu.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Arellano JI, Munoz A, Ballesteros-Yanez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127:45–64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- 51.Magloczky Z, Halasz P, Vajda J, Czirjak S, Freund TF. Loss of Calbindin-D28K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience. 1997;76:377–385. doi: 10.1016/s0306-4522(96)00440-x. [DOI] [PubMed] [Google Scholar]
- 52.Martinian L, Catarino CB, Thompson P, Sisodiya SM, Thom M. Calbindin D28K expression in relation to granule cell dispersion, mossy fibre sprouting and memory impairment in hippocampal sclerosis: a surgical and post mortem series. Epilepsy Res. 2012;98:14–24. doi: 10.1016/j.eplepsyres.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Karadi K, Janszky J, Gyimesi C, Horvath Z, Lucza T, Doczi T, Kallai J, Abraham H. Correlation between calbindin expression in granule cells of the resected hippocampal dentate gyrus and verbal memory in temporal lobe epilepsy. Epilepsy Behav. 2012;25:110–119. doi: 10.1016/j.yebeh.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Pauli E, Hildebrandt M, Romstock J, Stefan H, Blumcke I. Deficient memory acquisition in temporal lobe epilepsy is predicted by hippocampal granule cell loss. Neurology. 2006;67:1383–1389. doi: 10.1212/01.wnl.0000239828.36651.73. [DOI] [PubMed] [Google Scholar]
- 55.Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blumcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133:3359–3372. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- 56.Stegen M, Kirchheim F, Hanuschkin A, Staszewski O, Veh RW, Wolfart J. Adaptive intrinsic plasticity in human dentate gyrus granule cells during temporal lobe epilepsy. Cereb Cortex. 2012;22:2087–2101. doi: 10.1093/cercor/bhr294. [DOI] [PubMed] [Google Scholar]
- 57.Loscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 59.Magloczky Z. Sprouting in human temporal lobe epilepsy: excitatory pathways and axons of interneurons. Epilepsy Res. 2010;89:52–59. doi: 10.1016/j.eplepsyres.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 61.Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol. 2003;458:272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- 62.Crevecoeur J, Kaminski RM, Rogister B, Foerch P, Vandenplas C, Neveux M, Mazzuferi M, Kroonen J, Poulet C, Martin D, Sadzot B, Rikir E, Klitgaard H, Moonen G, Deprez M. Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol Appl Neurobiol. 2014;40:191–204. doi: 10.1111/nan.12054. [DOI] [PubMed] [Google Scholar]
- 63.Stengaard-Pedersen K, Fredens K, Larsson LI. Enkephalin and zinc in the hippocampal mossy fiber system. Brain Res. 1981;212:230–233. doi: 10.1016/0006-8993(81)90058-5. [DOI] [PubMed] [Google Scholar]
- 64.Sutula T. Seizure-induced axonal sprouting: assessing connections between injury, local circuits, and epileptogenesis. Epilepsy Curr. 2002;2:86–91. doi: 10.1046/j.1535-7597.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nissinen J, Lukasiuk K, Pitkanen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- 66.Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, de Graan PN. Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2000;123(Pt 1):19–30. doi: 10.1093/brain/123.1.19. [DOI] [PubMed] [Google Scholar]
- 67.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pirker S, Czech T, Baumgartner C, Maier H, Novak K, Furtinger S, Fischer-Colbrie R, Sperk G. Chromogranins as markers of altered hippocampal circuitry in temporal lobe epilepsy. Ann Neurol. 2001;50:216–226. doi: 10.1002/ana.1079. [DOI] [PubMed] [Google Scholar]
- 69.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Longo BM, Mello LE. Blockade of pilocarpine- or kainate-induced mossy fiber sprouting by cycloheximide does not prevent subsequent epileptogenesis in rats. Neurosci Lett. 1997;226:163–166. doi: 10.1016/s0304-3940(97)00267-x. [DOI] [PubMed] [Google Scholar]
- 72.Longo BM, Mello LE. Effect of long-term spontaneous recurrent seizures or reinduction of status epilepticus on the development of supragranular mossy fiber sprouting. Epilepsy Res. 1999;36:233–241. doi: 10.1016/s0920-1211(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 73.Thom M, Martinian L, Catarino C, Yogarajah M, Koepp MJ, Caboclo L, Sisodiya SM. Bilateral reorganization of the dentate gyrus in hippocampal sclerosis: a postmortem study. Neurology. 2009;73:1033–1040. doi: 10.1212/WNL.0b013e3181b99a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 77.Maccaferri G. Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J Physiol. 2005;562:73–80. doi: 10.1113/jphysiol.2004.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marx M, Haas CA, Haussler U. Differential vulnerability of interneurons in the epileptic hippocampus. Front Cell Neurosci. 2013;7:167. doi: 10.3389/fncel.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toth K, Eross L, Vajda J, Halasz P, Freund TF, Magloczky Z. Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain. 2010;133:2763–2777. doi: 10.1093/brain/awq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- 81.Magloczky Z, Wittner L, Borhegyi Z, Halasz P, Vajda J, Czirjak S, Freund TF. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience. 2000;96:7–25. doi: 10.1016/s0306-4522(99)00474-1. [DOI] [PubMed] [Google Scholar]
- 82.Wittner L, Eross L, Czirjak S, Halasz P, Freund TF, Magloczky Z. Surviving CA1 pyramidal cells receive intact perisomatic inhibitory input in the human epileptic hippocampus. Brain. 2005;128:138–152. doi: 10.1093/brain/awh339. [DOI] [PubMed] [Google Scholar]
- 83.Wittner L, Eross L, Szabo Z, Toth S, Czirjak S, Halasz P, Freund TF, Magloczky ZS. Synaptic reorganization of calbindin-positive neurons in the human hippocampal CA1 region in temporal lobe epilepsy. Neuroscience. 2002;115:961–978. doi: 10.1016/s0306-4522(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 84.Fritschy JM. Epilepsy, E/I balance and GABA(A) receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 86.Huusko N, Romer C, Ndode-Ekane XE, Lukasiuk K, Pitkanen A. Loss of hippocampal interneurons and epileptogenesis: a comparison of two animal models of acquired epilepsy. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0644-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 87.Kovac S, Walker MC. Neuropeptides in epilepsy. Neuropeptides. 2013;47:467–475. doi: 10.1016/j.npep.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 88.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system – II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 89.Vezzani A, Hoyer D. Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis. Eur J Neurosci. 1999;11:3767–3776. doi: 10.1046/j.1460-9568.1999.00838.x. [DOI] [PubMed] [Google Scholar]
- 90.Vezzani A, Sperk G. Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides. 2004;38:245–252. doi: 10.1016/j.npep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Kharlamov EA, Kharlamov A, Kelly KM. Changes in neuropeptide Y protein expression following photothrombotic brain infarction and epileptogenesis. Brain Res. 2007;1127:151–162. doi: 10.1016/j.brainres.2006.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 93.Chan-Palay V, Kohler C, Haesler U, Lang W, Yasargil G. Distribution of neurons and axons immunoreactive with antisera against neuropeptide Y in the normal human hippocampus. J Comp Neurol. 1986;248:360–375. doi: 10.1002/cne.902480306. [DOI] [PubMed] [Google Scholar]
- 94.Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, Bogucki J, Schramm J. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54:847–859. doi: 10.1227/01.neu.0000114141.37640.37. discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 95.Nadler JV, Tu B, Timofeeva O, Jiao Y, Herzog H. Neuropeptide Y in the recurrent mossy fiber pathway. Peptides. 2007;28:357–364. doi: 10.1016/j.peptides.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noe FM, Sorensen AT, Kokaia M, Vezzani A. Gene therapy of focal onset epilepsy using adeno-associated virus vector-mediated overexpression of neuropeptide Y. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda, MD: 2012. 4th edn Available at: http://www.ncbi.nlm.nih.gov/books/NBK98184/ [PubMed] [Google Scholar]
- 97.Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47:431–438. doi: 10.1016/j.npep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Lu X, Mazarati A, Sanna P, Shinmei S, Bartfai T. Distribution and differential regulation of galanin receptor subtypes in rat brain: effects of seizure activity. Neuropeptides. 2005;39:147–152. doi: 10.1016/j.npep.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 99.Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. EXS. 2010;102:183–194. doi: 10.1007/978-3-0346-0228-0_13. [DOI] [PubMed] [Google Scholar]
- 100.Toth K, Wittner L, Urban Z, Doyle WK, Buzsaki G, Shigemoto R, Freund TF, Magloczky Z. Morphology and synaptic input of substance P receptor-immunoreactive interneurons in control and epileptic human hippocampus. Neuroscience. 2007;144:495–508. doi: 10.1016/j.neuroscience.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casillas-Espinosa PM, Powell KL, O'Brien TJ. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia. 2012;53(Suppl. 9):41–58. doi: 10.1111/epi.12034. [DOI] [PubMed] [Google Scholar]
- 102.Deng H, Xiu X, Song Z. The molecular biology of genetic-based epilepsies. Mol Neurobiol. 2014;49:352–367. doi: 10.1007/s12035-013-8523-6. [DOI] [PubMed] [Google Scholar]
- 103.Kasperaviciute D, Catarino CB, Heinzen EL, Depondt C, Cavalleri GL, Caboclo LO, Tate SK, Jamnadas-Khoda J, Chinthapalli K, Clayton LM, Shianna KV, Radtke RA, Mikati MA, Gallentine WB, Husain AM, Alhusaini S, Leppert D, Middleton LT, Gibson RA, Johnson MR, Matthews PM, Hosford D, Heuser K, Amos L, Ortega M, Zumsteg D, Wieser HG, Steinhoff BJ, Kramer G, Hansen J, Dorn T, Kantanen AM, Gjerstad L, Peuralinna T, Hernandez DG, Eriksson KJ, Kalviainen RK, Doherty CP, Wood NW, Pandolfo M, Duncan JS, Sander JW, Delanty N, Goldstein DB, Sisodiya SM. Common genetic variation and susceptibility to partial epilepsies: a genome-wide association study. Brain. 2010;133:2136–2147. doi: 10.1093/brain/awq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hwang SK, Hirose S. Genetics of temporal lobe epilepsy. Brain Dev. 2012;34:609–616. doi: 10.1016/j.braindev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Salzmann A, Malafosse A. Genetics of temporal lobe epilepsy: a review. Epilepsy Res Treat. 2012;2012:863702. doi: 10.1155/2012/863702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kasperaviciute D, Catarino CB, Matarin M, Leu C, Novy J, Tostevin A, Leal B, Hessel EV, Hallmann K, Hildebrand MS, Dahl HH, Ryten M, Trabzuni D, Ramasamy A, Alhusaini S, Doherty CP, Dorn T, Hansen J, Kramer G, Steinhoff BJ, Zumsteg D, Duncan S, Kalviainen RK, Eriksson KJ, Kantanen AM, Pandolfo M, Gruber-Sedlmayr U, Schlachter K, Reinthaler EM, Stogmann E, Zimprich F, Theatre E, Smith C, O'Brien TJ, Meng Tan K, Petrovski S, Robbiano A, Paravidino R, Zara F, Striano P, Sperling MR, Buono RJ, Hakonarson H, Chaves J, Costa PP, Silva BM, da Silva AM, de Graan PN, Koeleman BP, Becker A, Schoch S, von Lehe M, Reif PS, Rosenow F, Becker F, Weber Y, Lerche H, Rossler K, Buchfelder M, Hamer HM, Kobow K, Coras R, Blumcke I, Scheffer IE, Berkovic SF, Weale ME, Delanty N, Depondt C, Cavalleri GL, Kunz WS, Sisodiya SM. Epilepsy, hippocampal sclerosis and febrile seizures linked by common genetic variation around SCN1A. Brain. 2013;136:3140–3150. doi: 10.1093/brain/awt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aronica E, Boer K, Doorn KJ, Zurolo E, Spliet WG, van Rijen PC, Baayen JC, Gorter JA, Jeromin A. Expression and localization of voltage dependent potassium channel Kv4.2 in epilepsy associated focal lesions. Neurobiol Dis. 2009;36:81–95. doi: 10.1016/j.nbd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl. 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gonzalez MI, Brooks-Kayal A. Altered GABA(A) receptor expression during epileptogenesis. Neurosci Lett. 2011;497:218–222. doi: 10.1016/j.neulet.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- 112.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 113.Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134:1033–1040. doi: 10.1093/brain/awq385. [DOI] [PubMed] [Google Scholar]
- 114.Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, Palkovits M, Magloczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Magloczky Z, Toth K, Karlocai R, Nagy S, Eross L, Czirjak S, Vajda J, Rasonyi G, Kelemen A, Juhos V, Halasz P, Mackie K, Freund TF. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia. 2010;51(Suppl. 3):115–120. doi: 10.1111/j.1528-1167.2010.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ben-Ari Y, Holmes GL. The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol. 2005;18:141–145. doi: 10.1097/01.wco.0000162855.75391.6a. [DOI] [PubMed] [Google Scholar]
- 117.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 118.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 120.Sen A, Martinian L, Nikolic M, Walker MC, Thom M, Sisodiya SM. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res. 2007;74:220–227. doi: 10.1016/j.eplepsyres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 121.Thom M, Martinian L, Caboclo LO, McEvoy AW, Sisodiya SM. Balloon cells associated with granule cell dispersion in the dentate gyrus in hippocampal sclerosis. Acta Neuropathol. 2008;115:697–700. doi: 10.1007/s00401-008-0341-y. [DOI] [PubMed] [Google Scholar]
- 122.Miyahara H, Ryufuku M, Fu YJ, Kitaura H, Murakami H, Masuda H, Kameyama S, Takahashi H, Kakita A. Balloon cells in the dentate gyrus in hippocampal sclerosis associated with non-herpetic acute limbic encephalitis. Seizure. 2011;20:87–89. doi: 10.1016/j.seizure.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Martinian L, Boer K, Middeldorp J, Hol EM, Sisodiya SM, Squier W, Aronica E, Thom M. Expression patterns of glial fibrillary acidic protein (GFAP)-delta in epilepsy-associated lesional pathologies. Neuropathol Appl Neurobiol. 2009;35:394–405. doi: 10.1111/j.1365-2990.2009.00996.x. [DOI] [PubMed] [Google Scholar]
- 124.Steinhauser C, Seifert G, Bedner P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia. 2012;60:1192–1202. doi: 10.1002/glia.22313. [DOI] [PubMed] [Google Scholar]
- 125.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- 127.Bedner P, Steinhauser C. Altered Kir and gap junction channels in temporal lobe epilepsy. Neurochem Int. 2013;63:682–687. doi: 10.1016/j.neuint.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 128.Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929:105–116. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- 129.Garbelli R, Frassoni C, Condorelli DF, Trovato Salinaro A, Musso N, Medici V, Tassi L, Bentivoglio M, Spreafico R. Expression of connexin 43 in the human epileptic and drug-resistant cerebral cortex. Neurology. 2011;76:895–902. doi: 10.1212/WNL.0b013e31820f2da6. [DOI] [PubMed] [Google Scholar]
- 130.de Lanerolle NC, Lee TS. New facets of the neuropathology and molecular profile of human temporal lobe epilepsy. Epilepsy Behav. 2005;7:190–203. doi: 10.1016/j.yebeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 132.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 133.Michalak Z, Lebrun A, Di Miceli M, Rousset MC, Crespel A, Coubes P, Henshall DC, Lerner-Natoli M, Rigau V. IgG leakage may contribute to neuronal dysfunction in drug-refractory epilepsies with blood-brain barrier disruption. J Neuropathol Exp Neurol. 2012;71:826–838. doi: 10.1097/NEN.0b013e31826809a6. [DOI] [PubMed] [Google Scholar]
- 134.Kovacs R, Heinemann U, Steinhauser C. Mechanisms underlying blood-brain barrier dysfunction in brain pathology and epileptogenesis: role of astroglia. Epilepsia. 2012;53(Suppl. 6):53–59. doi: 10.1111/j.1528-1167.2012.03703.x. [DOI] [PubMed] [Google Scholar]
- 135.Kastanauskaite A, Alonso-Nanclares L, Blazquez-Llorca L, Pastor J, Sola RG, DeFelipe J. Alterations of the microvascular network in sclerotic hippocampi from patients with epilepsy. J Neuropathol Exp Neurol. 2009;68:939–950. doi: 10.1097/NEN.0b013e3181b08622. [DOI] [PubMed] [Google Scholar]
- 136.Mott RT, Thore CR, Moody DM, Glazier SS, Ellis TL, Brown WR. Reduced ratio of afferent to total vascular density in mesial temporal sclerosis. J Neuropathol Exp Neurol. 2009;68:1147–1154. doi: 10.1097/NEN.0b013e3181b9d75f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aronica E, Sisodiya SM, Gorter JA. Cerebral expression of drug transporters in epilepsy. Adv Drug Deliv Rev. 2012;64:919–929. doi: 10.1016/j.addr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 138.Feldmann M, Asselin MC, Liu J, Wang S, McMahon A, Anton-Rodriguez J, Walker M, Symms M, Brown G, Hinz R, Matthews J, Bauer M, Langer O, Thom M, Jones T, Vollmar C, Duncan JS, Sisodiya SM, Koepp MJ. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013;12:777–785. doi: 10.1016/S1474-4422(13)70109-1. [DOI] [PubMed] [Google Scholar]
- 139.Meyer A, Beck E. The hippocampal formation in temporal lobe epilepsy. Proc R Soc Med. 1955;48:457–462. doi: 10.1177/003591575504800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meyer A, Falconer MA, Beck E. Pathological findings in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1954;17:276–285. doi: 10.1136/jnnp.17.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, Connelly A. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951–1959. doi: 10.1093/brain/awf202. [DOI] [PubMed] [Google Scholar]
- 142.Men S, Lee DH, Barron JR, Munoz DG. Selective neuronal necrosis associated with status epilepticus: MR findings. AJNR Am J Neuroradiol. 2000;21:1837–1840. [PMC free article] [PubMed] [Google Scholar]
- 143.Pitkanen A, Nissinen J, Nairismagi J, Lukasiuk K, Grohn OH, Miettinen R, Kauppinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- 144.Salmenpera T, Kalviainen R, Partanen K, Mervaala E, Pitkanen A. MRI volumetry of the hippocampus, amygdala, entorhinal cortex, and perirhinal cortex after status epilepticus. Epilepsy Res. 2000;40:155–170. doi: 10.1016/s0920-1211(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 145.Kumar G, Mittal S, Moudgil SS, Kupsky WJ, Shah AK. Histopathological evidence that hippocampal atrophy following status epilepticus is a result of neuronal necrosis. J Neurol Sci. 2013;334:186–191. doi: 10.1016/j.jns.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 146.Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, Xu Y, Macfall J, Gomes WA, Moshe SL, Mathern GW, Pellock JM, Nordli DR, Jr, Frank LM, Provenzale J, Shinnar RC, Epstein LG, Masur D, Litherland C, Sun S. Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol. 2014;75:178–185. doi: 10.1002/ana.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol. 2004;17:161–164. doi: 10.1097/00019052-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 149.Baulac S, Gourfinkel-An I, Nabbout R, Huberfeld G, Serratosa J, Leguern E, Baulac M. Fever, genes, and epilepsy. Lancet Neurol. 2004;3:421–430. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi E, D'Agostino MD, Lopes-Cendes I, Berkovic SF, Li ML, Andermann E, Andermann F, Cendes F. Hippocampal atrophy and T2-weighted signal changes in familial mesial temporal lobe epilepsy. Neurology. 2003;60:405–409. doi: 10.1212/wnl.60.3.405. [DOI] [PubMed] [Google Scholar]
- 151.Cavalleri GL, Lynch JM, Depondt C, Burley MW, Wood NW, Sisodiya SM, Goldstein DB. Failure to replicate previously reported genetic associations with sporadic temporal lobe epilepsy: where to from here? Brain. 2005;128:1832–1840. doi: 10.1093/brain/awh524. [DOI] [PubMed] [Google Scholar]
- 152.Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571–574. [PubMed] [Google Scholar]
- 153.Stogmann E, Zimprich A, Baumgartner C, Aull-Watschinger S, Hollt V, Zimprich F. A functional polymorphism in the prodynorphin gene promotor is associated with temporal lobe epilepsy. Ann Neurol. 2002;51:260–263. doi: 10.1002/ana.10108. [DOI] [PubMed] [Google Scholar]
- 154.Busch RM, Floden D, Lineweaver TT, Chapin JS, Unnwongse K, Wehner T, Diaz-Arrastia R, Najm IM. Effect of apolipoprotein epsilon4 allele on hippocampal and brain volume in intractable temporal lobe epilepsy. Epilepsy Behav. 2011;21:88–90. doi: 10.1016/j.yebeh.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 155.Henshall DC. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans. 2007;35:421–423. doi: 10.1042/BST0350421. [DOI] [PubMed] [Google Scholar]
- 156.Dericioglu N, Soylemezoglu F, Gursoy-Ozdemir Y, Akalan N, Saygi S, Dalkara T. Cell death and survival mechanisms are concomitantly active in the hippocampus of patients with mesial temporal sclerosis. Neuroscience. 2013;237:56–65. doi: 10.1016/j.neuroscience.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 157.Sano T, Reynolds JP, Jimenez-Mateos EM, Matsushima S, Taki W, Henshall DC. MicroRNA-34a upregulation during seizure-induced neuronal death. Cell Death Dis. 2012;3:e287. doi: 10.1038/cddis.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feast A, Martinian L, Liu J, Catarino CB, Thom M, Sisodiya SM. Investigation of hypoxia-inducible factor-1alpha in hippocampal sclerosis: a postmortem study. Epilepsia. 2012;53:1349–1359. doi: 10.1111/j.1528-1167.2012.03591.x. [DOI] [PubMed] [Google Scholar]
- 159.Mathern GW, Babb TL, Mischel PS, Vinters HV, Pretorius JK, Leite JP, Peacock WJ. Childhood generalized and mesial temporal epilepsies demonstrate different amounts and patterns of hippocampal neuron loss and mossy fibre synaptic reorganization. Brain. 1996;119:965–987. doi: 10.1093/brain/119.3.965. [DOI] [PubMed] [Google Scholar]
- 160.Paine SML, Bell DNR, Melchionda V, Tan J, Weereratne C, Harrison S, Vargha-Khadem F, Harkness W, Cross JH, Harding BN, Pizarro L, Jacques TS. Patterns of hippocampal sclerosis in children. Neuropathol Appl Neurobiol. 2013;39:42–43. [Google Scholar]
- 161.Azakli H, Gurses C, Arikan M, Aydoseli A, Aras Y, Sencer A, Gokyigit A, Bilgic B, Ustek D. Whole mitochondrial DNA variations in hippocampal surgical specimens and blood samples with high-throughput sequencing: a case of mesial temporal lobe epilepsy with hippocampal sclerosis. Gene. 2013;529:190–194. doi: 10.1016/j.gene.2013.06.077. [DOI] [PubMed] [Google Scholar]
- 162.Nakahara H, Konishi Y, Beach TG, Yamada N, Makino S, Tooyama I. Infiltration of T lymphocytes and expression of icam-1 in the hippocampus of patients with hippocampal sclerosis. Acta Histochem Cytochem. 2010;43:157–162. doi: 10.1267/ahc.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simoes PS, Perosa SR, Arganaraz GA, Yacubian EM, Carrete H, Jr, Centeno RS, Varella PP, Santiago JF, Canzian M, Silva JA, Jr, Mortara RA, Amado D, Cavalheiro EA, Mazzacoratti Mda G. Kallikrein 1 is overexpressed by astrocytes in the hippocampus of patients with refractory temporal lobe epilepsy, associated with hippocampal sclerosis. Neurochem Int. 2011;58:477–482. doi: 10.1016/j.neuint.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 164.Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 165.Zattoni M, Mura ML, Deprez F, Schwendener RA, Engelhardt B, Frei K, Fritschy JM. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31:4037–4050. doi: 10.1523/JNEUROSCI.6210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aronica E, Gorter JA. Gene expression profile in temporal lobe epilepsy. Neuroscientist. 2007;13:100–108. doi: 10.1177/1073858406295832. [DOI] [PubMed] [Google Scholar]
- 167.Yang T, Zhou D, Stefan H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: uncontrolled inflammation drives disease progression? J Neurol Sci. 2010;296:1–6. doi: 10.1016/j.jns.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 168.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl. 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 169.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, Vincent A, Elger CE. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- 171.Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Niehusmann P, Mittelstaedt T, Bien CG, Drexler JF, Grote A, Schoch S, Becker AJ. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia. 2010;51:2478–2483. doi: 10.1111/j.1528-1167.2010.02741.x. [DOI] [PubMed] [Google Scholar]
- 173.Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, Becker A, Cepeda C, Cendes F, Colombo N, Crino P, Cross JH, Delalande O, Dubeau F, Duncan J, Guerrini R, Kahane P, Mathern G, Najm I, Ozkara C, Raybaud C, Represa A, Roper SN, Salamon N, Schulze-Bonhage A, Tassi L, Vezzani A, Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thom M, Eriksson S, Martinian L, Caboclo LO, McEvoy AW, Duncan JS, Sisodiya SM. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol. 2009;68:928–938. doi: 10.1097/NEN.0b013e3181b05d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baulac M, De Grissac N, Hasboun D, Oppenheim C, Adam C, Arzimanoglou A, Semah F, Lehericy S, Clemenceau S, Berger B. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol. 1998;44:223–233. doi: 10.1002/ana.410440213. [DOI] [PubMed] [Google Scholar]
- 176.Fernandez G, Effenberger O, Vinz B, Steinlein O, Elger CE, Dohring W, Heinze HJ. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis. Neurology. 1998;50:909–917. doi: 10.1212/wnl.50.4.909. [DOI] [PubMed] [Google Scholar]
- 177.Grunewald RA, Farrow T, Vaughan P, Rittey CD, Mundy J. A magnetic resonance study of complicated early childhood convulsion. J Neurol Neurosurg Psychiatry. 2001;71:638–642. doi: 10.1136/jnnp.71.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blumcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002;12:199–211. doi: 10.1111/j.1750-3639.2002.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsai MH, Pardoe HR, Perchyonok Y, Fitt GJ, Scheffer IE, Jackson GD, Berkovic SF. Etiology of hippocampal sclerosis: evidence for a predisposing familial morphologic anomaly. Neurology. 2013;81:144–149. doi: 10.1212/WNL.0b013e31829a33ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bis JC, DeCarli C, Smith AV, van der Lijn F, Crivello F, Fornage M, Debette S, Shulman JM, Schmidt H, Srikanth V, Schuur M, Yu L, Choi SH, Sigurdsson S, Verhaaren BF, DeStefano AL, Lambert JC, Jack CR, Jr, Struchalin M, Stankovich J, Ibrahim-Verbaas CA, Fleischman D, Zijdenbos A, den Heijer T, Mazoyer B, Coker LH, Enzinger C, Danoy P, Amin N, Arfanakis K, van Buchem MA, de Bruijn RF, Beiser A, Dufouil C, Huang J, Cavalieri M, Thomson R, Niessen WJ, Chibnik LB, Gislason GK, Hofman A, Pikula A, Amouyel P, Freeman KB, Phan TG, Oostra BA, Stein JL, Medland SE, Vasquez AA, Hibar DP, Wright MJ, Franke B, Martin NG, Thompson PM, Nalls MA, Uitterlinden AG, Au R, Elbaz A, Beare RJ, van Swieten JC, Lopez OL, Harris TB, Chouraki V, Breteler MM, De Jager PL, Becker JT, Vernooij MW, Knopman D, Fazekas F, Wolf PA, van der Lugt A, Gudnason V, Longstreth WT, Jr, Brown MA, Bennett DA, van Duijn CM, Mosley TH, Schmidt R, Tzourio C, Launer LJ, Ikram MA, Seshadri S. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44:545–551. doi: 10.1038/ng.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, Toro R, Appel K, Bartecek R, Bergmann O, Bernard M, Brown AA, Cannon DM, Chakravarty MM, Christoforou A, Domin M, Grimm O, Hollinshead M, Holmes AJ, Homuth G, Hottenga JJ, Langan C, Lopez LM, Hansell NK, Hwang KS, Kim S, Laje G, Lee PH, Liu X, Loth E, Lourdusamy A, Mattingsdal M, Mohnke S, Maniega SM, Nho K, Nugent AC, O'Brien C, Papmeyer M, Putz B, Ramasamy A, Rasmussen J, Rijpkema M, Risacher SL, Roddey JC, Rose EJ, Ryten M, Shen L, Sprooten E, Strengman E, Teumer A, Trabzuni D, Turner J, van Eijk K, van Erp TG, van Tol MJ, Wittfeld K, Wolf C, Woudstra S, Aleman A, Alhusaini S, Almasy L, Binder EB, Brohawn DG, Cantor RM, Carless MA, Corvin A, Czisch M, Curran JE, Davies G, de Almeida MA, Delanty N, Depondt C, Duggirala R, Dyer TD, Erk S, Fagerness J, Fox PT, Freimer NB, Gill M, Goring HH, Hagler DJ, Hoehn D, Holsboer F, Hoogman M, Hosten N, Jahanshad N, Johnson MP, Kasperaviciute D, Kent JW, Jr, Kochunov P, Lancaster JL, Lawrie SM, Liewald DC, Mandl R, Matarin M, Mattheisen M, Meisenzahl E, Melle I, Moses EK, Muhleisen TW, Nauck M, Nothen MM, Olvera RL, Pandolfo M, Pike GB, Puls R, Reinvang I, Renteria ME, Rietschel M, Roffman JL, Royle NA, Rujescu D, Savitz J, Schnack HG, Schnell K, Seiferth N, Smith C, Steen VM, Valdes Hernandez MC, Van den Heuvel M, van der Wee NJ, Van Haren NE, Veltman JA, Volzke H, Walker R, Westlye LT, Whelan CD, Agartz I, Boomsma DI, Cavalleri GL, Dale AM, Djurovic S, Drevets WC, Hagoort P, Hall J, Heinz A, Jack CR, Jr, Foroud TM, Le Hellard S, Macciardi F, Montgomery GW, Poline JB, Porteous DJ, Sisodiya SM, Starr JM, Sussmann J, Toga AW, Veltman DJ, Walter H, Weiner MW, Bis JC, Ikram MA, Smith AV, Gudnason V, Tzourio C, Vernooij MW, Launer LJ, DeCarli C, Seshadri S, Andreassen OA, Apostolova LG, Bastin ME, Blangero J, Brunner HG, Buckner RL, Cichon S, Coppola G, de Zubicaray GI, Deary IJ, Donohoe G, de Geus EJ, Espeseth T, Fernandez G, Glahn DC, Grabe HJ, Hardy J, Hulshoff Pol HE, Jenkinson M, Kahn RS, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meyer-Lindenberg A, Morris DW, Muller-Myhsok B, Nichols TE, Ophoff RA, Paus T, Pausova Z, Penninx BW, Potkin SG, Samann PG, Saykin AJ, Schumann G, Smoller JW, Wardlaw JM, Weale ME, Martin NG, Franke B, Wright MJ, Thompson PM. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Connolly MB. Hippocampal malrotation and temporal lobe epilepsy: what is the relationship? Can J Neurol Sci. 2013;40:626–627. doi: 10.1017/s0317167100014839. [DOI] [PubMed] [Google Scholar]
- 183.Sen A, Thom M, Martinian L, Dawodu S, Sisodiya SM. Hippocampal malformations do not necessarily evolve into hippocampal sclerosis. Epilepsia. 2005;46:939–943. doi: 10.1111/j.1528-1167.2005.61104.x. [DOI] [PubMed] [Google Scholar]
- 184.Sloviter RS, Kudrimoti HS, Laxer KD, Barbaro NM, Chan S, Hirsch LJ, Goodman RR, Pedley TA. Tectonic’ hippocampal malformations in patients with temporal lobe epilepsy. Epilepsy Res. 2004;59:123–153. doi: 10.1016/j.eplepsyres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 185.Thom M, Sisodiya SM, Lin WR, Mitchell T, Free SL, Stevens J, Scaravilli F. Bilateral isolated hippocampal malformation in temporal lobe epilepsy. Neurology. 2002;58:1683–1686. doi: 10.1212/wnl.58.11.1683. [DOI] [PubMed] [Google Scholar]
- 186.Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005;128:1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- 187.Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res. 2012;98:97–103. doi: 10.1016/j.eplepsyres.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 188.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 189.Blanc F, Martinian L, Liagkouras I, Catarino C, Sisodiya SM, Thom M. Investigation of widespread neocortical pathology associated with hippocampal sclerosis in epilepsy: a postmortem study. Epilepsia. 2011;52:10–21. doi: 10.1111/j.1528-1167.2010.02773.x. [DOI] [PubMed] [Google Scholar]
- 190.Sinjab B, Martinian L, Sisodiya SM, Thom M. Regional thalamic neuropathology in patients with hippocampal sclerosis and epilepsy: a postmortem study. Epilepsia. 2013;54:2125–2133. doi: 10.1111/epi.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res. 2000;41:63–73. doi: 10.1016/s0920-1211(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 192.Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello A, Costantino L, Biagini G. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem. 2014;21:663–688. doi: 10.2174/0929867320666131119152201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bertram EH. Temporal lobe epilepsy: where do the seizures really begin? Epilepsy Behav. 2009;14(Suppl. 1):32–37. doi: 10.1016/j.yebeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bertram EH. Neuronal circuits in epilepsy: do they matter? Exp Neurol. 2013;244:67–74. doi: 10.1016/j.expneurol.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Desalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered structural connectome in temporal lobe epilepsy. Radiology. 2014;270:842–848. doi: 10.1148/radiol.13131044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Jr, Stern JM. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Engel J, Jr, Thompson PM. Going beyond hippocampocentricity in the concept of mesial temporal lobe epilepsy. Epilepsia. 2012;53:220–223. doi: 10.1111/j.1528-1167.2011.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thom M, Mathern GW, Cross JH, Bertram EH. Mesial temporal lobe epilepsy: how do we improve surgical outcome? Ann Neurol. 2010;68:424–434. doi: 10.1002/ana.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laufs H, Richardson MP, Salek-Haddadi A, Vollmar C, Duncan JS, Gale K, Lemieux L, Loscher W, Koepp MJ. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011;77:904–910. doi: 10.1212/WNL.0b013e31822c90f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kahane P, Bartolomei F. Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia. 2010;51(Suppl. 1):59–62. doi: 10.1111/j.1528-1167.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 201.Dawodu S, Thom M. Quantitative neuropathology of the entorhinal cortex region in patients with hippocampal sclerosis and temporal lobe epilepsy. Epilepsia. 2005;46:23–30. doi: 10.1111/j.0013-9580.2005.21804.x. [DOI] [PubMed] [Google Scholar]
- 202.Thom M, Liu JY, Thompson P, Phadke R, Narkiewicz M, Martinian L, Marsdon D, Koepp M, Caboclo L, Catarino CB, Sisodiya SM. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134:2969–2981. doi: 10.1093/brain/awr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Labate A, Gambardella A, Andermann E, Aguglia U, Cendes F, Berkovic SF, Andermann F. Benign mesial temporal lobe epilepsy. Nat Rev Neurol. 2011;7:237–240. doi: 10.1038/nrneurol.2010.212. [DOI] [PubMed] [Google Scholar]
- 204.Malmgren K, Thom M. Hippocampal sclerosis – origins and imaging. Epilepsia. 2012;53(Suppl. 4):19–33. doi: 10.1111/j.1528-1167.2012.03610.x. [DOI] [PubMed] [Google Scholar]