Abstract
The low frequency of T cells specific for given antigens makes the study of antigen-specific T cell responses difficult. The development of MHC class I and II tetramer staining techniques allows precise quantification and tracking of antigen-specific CD8+ and CD4+ T cell responses. Here, we describe a protocol for MHC class I and II tetramer staining of mouse T cells isolated from various tissues of mice infected with lymphocytic choriomeningitis virus (LCMV) or with murine cytomegalovirus (MCMV).
Keywords: MHC Class I tetramer staining, MHC-peptide complexes, CD8+ T cells, CD4+ T cells, Db gp33, Db M45, I-A Ab gp61, I-A Ab M25, Flow cytometry, LCMV, MCMV
1. Introduction
For many years, analysis of antigen-specific T cell responses was hampered by their lack of identifying markers. Many of the methods used by immunologists to measure antigen-specific responses have important limitations. Limiting dilution assays and cytotoxicity assays were some of the first tools used to measure bulk T cell responses (1–4). While limiting dilution assays are fairly quantitative, they depend largely on expansion, survival, and subsequent function of precursors under particular culture conditions. On the other hand, cytotoxicity assays measure function at a population level, and quantitation is difficult and often lacks precision. TCR transgenic (Tg) mice and adoptive transfer approaches were subsequently developed and can be helpful (5), but nonphysiologically high levels of TCR Tg cells can lead to phenotypic abnormalities in these cells (6). Another technique, intracellular cytokine staining, can identify antigen-specific responding T cells; however, this approach is limited by the cytokine-producing ability of the T cell. The development of MHC “tetramers”, recombinant, multimeric, MHC molecules with attached peptides, has revolutionized our ability to track and quantify antigen-specific T cell responses ex vivo (7). The quadrivalent nature of the MHC/peptide tetramers allows for sufficient avidity to interact and stably bind to T cell receptors to visualize specific T cells by flow cytometry. Indeed, this approach uncovered the vastly underestimated response reported by previous nonquantitative methods (8).
To generate tetramers, MHC class I α-chains and β2-microglobulin are first produced as recombinant proteins in bacteria and then refolded in vitro in the presence of the peptide of interest into MHC/peptide monomers (7, 9). Engineering of a BirA recognition site into the class I α-chain allows for site-specific, single biotinylation by incubation of MHC/peptide monomers. Biotinylated monomers can be flash frozen indefinitely in aliquots that can be thawed and coupled to fluorochrome-labeled streptavidin to generate MHC tetrameric staining reagents.
Unlike MHC class I–peptide complexes that fold together in vitro, mouse MHC class II–peptide complexes are generally assembled in insect cells as preassembled monomers (10, 11). This is because mouse MHC class II molecules are difficult to fold in vitro and the covalent, genetic linkage of peptides to MHC class II β-chains allows for proper folding and assembly in insect cells (10, 11). Similar to class I MHC molecules, purified monomeric MHC class II–peptide complexes are singly biotinylated on a BirA recognition site in the MHC class II β-chain. Biotinylated monomers are assembled into tetramers by coupling with fluorochrome-linked streptavidin.
MHC class I and II tetramer staining can be used to quantify antigen-specific T cells in various mouse tissues such as spleen, lymph nodes (12), and peripheral tissues as livers, gut, kidney (13), and brain (14). After lymphocytic choriomeningitis virus (LCMV) infection of C57BL/6 mice, a major immunodominant epitope recognized by CD8+ T cells is derived from the glycoprotein (GP) peptide amino acids 33–41 presented by MHC class I H-2Db molecules, while a major CD4+ T cell response is directed against another GP epitope (amino acids 61–80) presented by I-Ab (15, 16). After murine cytomegalovirus (MCMV) infection of C57BL/6 mice, a major immunodominant epitope recognized by CD8+ T cells is the MCMV E protein M45 epitope (HGIRNASFI) presented by H-2Db; while another M protein epitope M25 (amino acids 409–423) is presented by I-Ab and recognized by CD4+ T cells (17, 18). In this chapter, we will describe a simple protocol for isolation of cells from tissues and their staining and analysis using MHC class I and II tetramers. Further, we will demonstrate the non-cross-reactivity of CD8+ and CD4+ T cells by staining cells from LCMV- versus MCMV-infected mice using LCMV- and MCMV-specific MHC tetramers.
2. Materials
2.1. Infection of Mice
Armstrong-3 strain of LCMV: LCMV are grown in BHK-21 cells, and the number of plaque-forming units (pfu) is assayed on Vero cells (9).
Smith strain of MCMV: MCMV are prepared by in vivo propagation in Balb/C mice; salivary gland homogenates are prepared 14 days postinfection, and viral titer in plaque-forming units (pfu) is measured by plaque assays on mouse embryo fibroblasts (19, 20).
2.2. Preparation of Cell Suspensions from Tissues
20× Balanced salt solution (BSS): 5.6 mM glucose, 0.4 mM KH2PO4, 1.3 mM Na2HPO4, 1.3 mM CaCl2·2H2O, 5.4 mM KCl, 137 mM NaCl, 0.8 mM MgSO4, 1 mM MgCl2·6H2O, and 0.001% phenol red. Mix and filter sterilize (see Note 1).
ACK lysis buffer: Prepare 155 mM NH4Cl buffer, adjust the pH to 7.5, and then add KHCO3 at a final concentration of 10 mM and phenol red 0.0005%. Filter sterilize.
CTM: Minimum essential medium (S-MEM) complemented with 2 mM L-glutamine, 0.1 mM nonessential amino acids, 0.5× essential amino acid mixture, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, 50 µg/ml gentamicin, 50 µM β-mercaptoethanol, 3 mM dextrose, and 10% Fetal Bovine Serum (FBS) (see Note 2).
Click’s medium (EHAA) (available from multiple vendors).
5.1 mg/ml Liberase +0.8 mg/ml DNAse I solution in EHAA medium.
0.1 M EDTA.
37.5% isotonic Percoll.
70 µm mesh cell strainers, 50 ml tubes, 96-well plates.
Tabletop centrifuge.
2.3. Staining for Flow Cytometry
Flow buffer: BSS supplemented with 2% FBS and 0.1% w/v sodium azide (see Note 3).
Paraformaldehyde (PFA) solution (2% w/v) in Phosphate Buffered Saline (PBS) (see Note 4).
MHC class I tetramers: Db–gp33 or Db–M45 coupled with SA-PE (see Note 5).
MHC class II tetramer: I-Ab–gp61 or I-Ab–M25 coupled with SA-PE.
Antibodies against mouse CD4 and CD8 and/or other surface markers, coupled to desired fluorochromes other than PE (multiple vendors).
Flow tubes, flow cytometry analyzer.
3. Methods
3.1. Infection with LCMV
Inject mice with 2 × 105 pfu of LCMV or with 1 × 105 pfu of MCMV intraperitoneally. Allow for expansion of T cells for at least 5–6 days (see Note 6). Specific T cells can be stained with MHC tetramers for the lifetime of the infected animal.
3.2. Preparation of Single Cell Suspensions from Mouse Spleen
Harvest the spleen of the infected mouse, and crush through a 70 µm mesh cell strainer into 50 ml tube to generate a single cell suspension.
Add 35 ml of BSS through the strainer to allow passage of the cells into the tube.
Spin the cells down at 300 × g for 5 min at 4 °C. Discard the supernatant.
To lyse red blood cells, add 1.5 ml of ACK lysis buffer, and incubate for 2.5 min at room temperature (RT).
Increase volume to 50 ml by adding BSS and centrifuge at 300 × g for 5 min, at 4 °C.
Discard the supernatant.
Repeat steps 5 and 6 to wash one more time (see Note 7).
Resuspend the cells in 3 ml of CTM.
Count the cells and adjust concentration to add 2 × 106 cells per well of a 96-well plate.
3.3. Preparation of Single Cell Suspensions from Mouse Liver
Harvest liver tissue from the mouse into 5 ml of serum-free EHAA medium and mince into small pieces.
Add 50 µl of Liberase + DNAse I solution on the liver pieces in a 50 ml conical tube, and incubate at 37 °C for 30 min, swirling every 5 min.
Add 2 ml of 0.1 M EDTA to a final concentration of 0.03 M.
Filter liver tissue through cell strainers, and add BSS up to 35 ml (see Note 8).
Centrifuge cells at 300 × g for 5 min at 4 °C. Discard the supernatant.
Resuspend cells with 25 ml of 37.5% isotonic Percoll.
Centrifuge cells at 690 × g for 12 min. Pipet the supernatants off the cell pellet carefully.
Add 50 ml BSS and wash the cells by repeating step 5.
Lyse the red cells by following the steps 4–9 of Subheading 3.2.
3.4. MHC Class I Tetramer Staining
Add 100 µl of flow buffer onto 2 × 106 cells seeded in wells.
Centrifuge the cells down at 300 × g for 5 min at 4 °C. Discard the supernatant.
Add appropriate amount of Db–gp33 or Db–M45 coupled with SA-PE in 70 µl of flow buffer/well (see Note 9).
Incubate the cells with the tetramer for 45 min at 4 °C.
Prepare antibody mixtures against CD8, CD16/32, and other surface molecules of interest in 20 µl of BSS/well. Add on top of each well, without washing away the tetramer stain, and incubate for an additional 45 min at 4 °C (see Note 10).
Add 150 µl of flow buffer/well and centrifuge the cells down at 300 × g for 5 min at 4 °C. Discard the supernatant.
Add 200 µl of flow buffer/well and centrifuge the cells down at 300 × g for 5 min at 4 °C. Discard the supernatant.
Fix the cells by adding 100 µl of 2% PFA for 20 min at RT.
Add 150 µl of flow buffer/well and centrifuge the cells down at 300 × g for 5 min at 4 °C. Discard the supernatant.
Resuspend the cells with 200 µl of flow buffer/well, and analyze on a flow cytometer.
3.5. MHC Class II Tetramer Staining
Add 100 µl of CTM onto 2 × 106 cells seeded in wells.
Centrifuge the cells at 300 × g for 5 min at 4 °C. Discard the supernatant.
Add appropriate amount of I-Ab–gp61 coupled with SA-PE in 70 µl of CTM/well.
Incubate the cells with the tetramer for 75 min at 37 °C.
Prepare antibody mixtures against CD4, CD16/32, and other surface molecules of interest in 20 µl of CTM/well. Add on top of each well, without washing away the tetramer stain, and incubate for an additional 45 min at 37 °C (see Note 11).
Add 150 µl of CTM/well and centrifuge the cells at 300 × g for 5 min at 4 °C. Discard the supernatant.
Fix the cells by adding 100 µl of 2% PFA for 20 min at RT.
Add 150 µl of flow buffer/well and centrifuge the cells at 300 × g for 5 min at 4 °C. Discard the supernatant.
Resuspend the cells with 200 µl of flow buffer/well, and analyze on a flow cytometer.
Fig. 1.
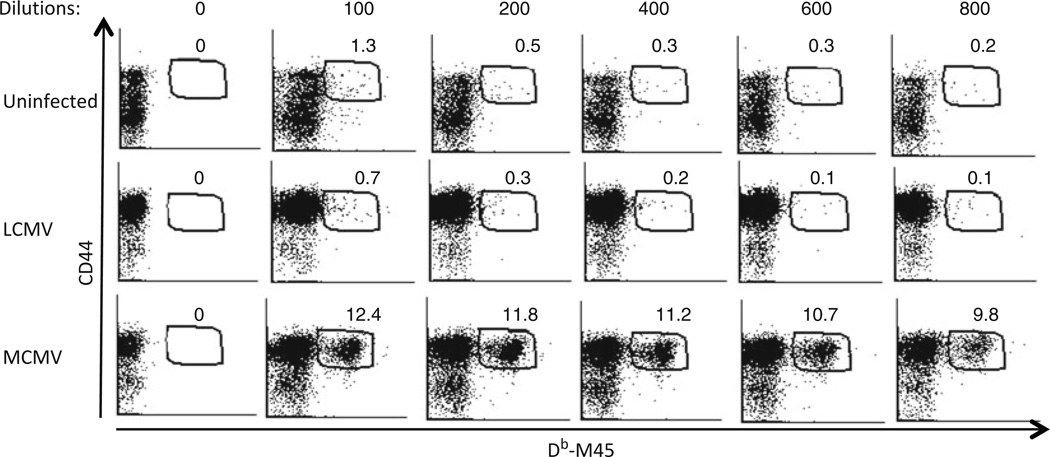
Determination of appropriate MHC tetramer concentration to use for staining. Representative dot plots show staining with various dilutions of Db–M45 tetramer and antibody against CD44 after gating on CD8+ T cells. Splenocytes from uninfected naïve C57/BL6 mice and mice infected with LCMV or with MCMV are stained with Db–M45 tetramer at the dilutions indicated. Specific Db–M45+ CD8+ CD44+ T cells are only detected in MCMV-infected mice, and their percentages within total CD8+ T cells are indicated on plots. The appropriate dilution to use the tetramer is 1:400 dilution because the percentage of Db–M45+ CD44+ cells within CD8+ T cells is high, the mean fluorescence intensity (MFI) of the Db–M45 stain within the tetramer + population remains high, and the nonspecific staining in naïve and LCMV-infected mice are low at this concentration.
Fig. 2.
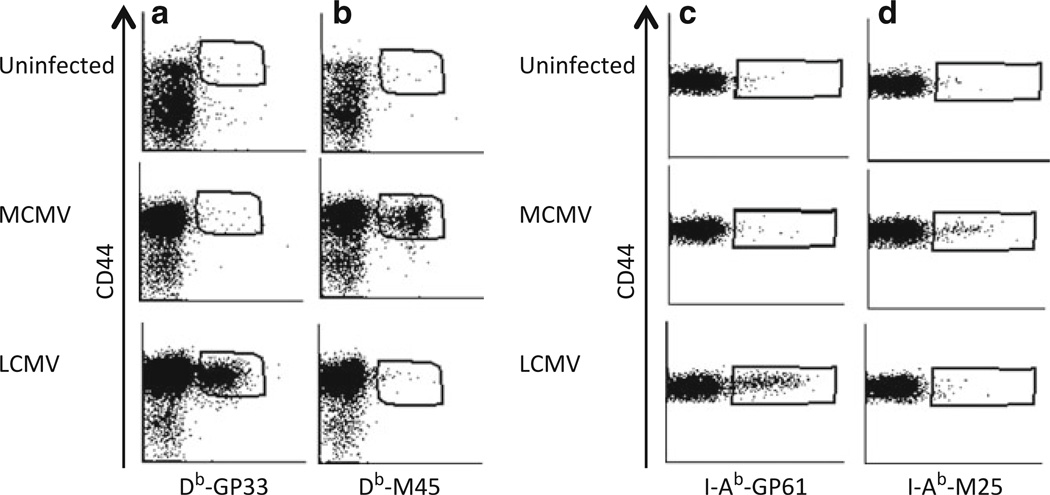
Specificity of MHC class I and class II tetramers. Single cell suspensions from spleens of C57BL/6 mice uninfected or infected with either LCMV or MCMV were stained with antibodies against CD8 and CD44 and with Db–GP33 (a) or Db–M45 (b) tetramers. (a, b) Representative dot plots show splenocytes stained with antibody against CD44 and with Db–GP33 (a) or Db–M45 (b) tetramer after gating on CD8+ T cells in naïve, LCMV- or MCMV-infected mice. (c, d) Splenocytes from each mouse are stained with antibodies against CD4 and CD16/32 and with I-Ab–GP61 (c) or I-Ab–M25 class II tetramers. Dot plots show CD4 and tetramer staining after gating on CD4+ CD16/32− cells.
Fig. 3.
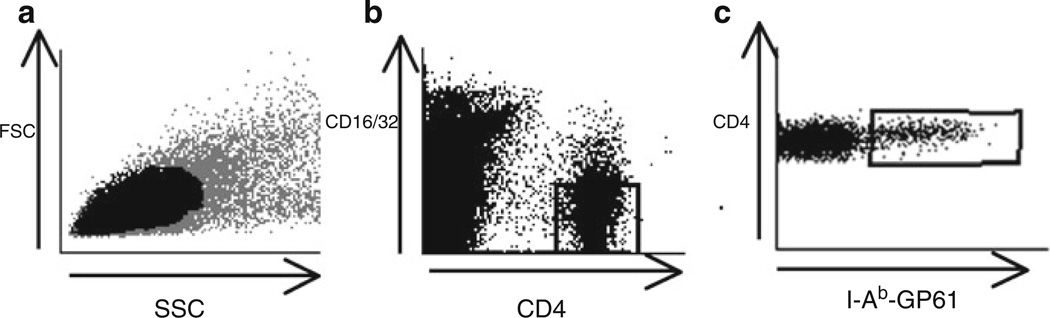
Gating strategy for MHC class II tetramer staining. Representative dot plots showing the flow cytometry analysis of staining of splenocytes from LCMV-infected mice with I-Ab–GP61 tetramer and antibodies against CD4 and CD16/32. (a) Dot plot showing Forward Scatter (FSC) and Side Scatter (SSC) of stained cells. Plot area shown in black indicates the live gate. (b) Dot plot shows CD4 by CD16/32 staining on gated live cells. (c) Dot plot shows CD4 staining by I-Ab–GP61 tetramer staining on CD4+ CD16/32-gated events in (b).
Acknowledgments
This work was supported by NIH-Public Health Service Grants AI057753, DK081175 (D.A.H.). The authors also thank Drs. Kasper Hoebe and Rhonda Cardin for their assistance with MCMV infection.
Footnotes
4. Notes
When preparing BSS, two separate solutions should be prepared to prevent precipitation. Glucose, KH2PO4, and Na2HPO4 should be dissolved together. CaCl2·2H2O, KCl, 137 mM NaCl, MgSO4, and MgCl2·6H2O should be prepared in a separate solution and should be mixed afterwards.
Supplements for the CTM media except for FBS can be prepared as a 100× stock solution and stored at −20 °C.
Sodium azide is very toxic if ingested or inhaled. Avoid contact with skin, eyes, or clothing.
Prepare a stock 4% w/v PFA solution. First heat deionized distilled H2O (ddH2O) until bubbles form, and then decrease the heat to 60 °C. Dissolve PFA in the water which takes around 10 min. If PFA does not dissolve, add 1–2 drops of NaOH into the mixture. After PFA dissolves, cool the solution down, and add 10× PBS to a final concentration of 1×. Adjust the pH to 7.6. Complete to the appropriate volume and store at −20 °C. If desired, a 10× stock PBS solution can be prepared (137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, and 2 mM KH2PO4), with pH adjusted to 7.2. Dilute 4% PFA to 2% PFA with ddH2O.
Db–gp33 or Db–M45 monomers can be flash frozen in a dry ice/ethanol bath and stored at −80 °C, but once they are conjugated to fluorochromes, they are kept at +4 °C in the dark like other fluorochrome-conjugated antibodies.
Typically, endogenous T cell responses to acute viral infections can be quantified as early as 5 days after infection. However, peak CD8+ T cell numbers occur between days 8 and 10 after infection. In the next 10–15 days after this point, most of the T cells undergo apoptotic cell death (12); however, sufficient numbers of detectable cells persist for the lifetime of the animal. Depending upon the markers to be interrogated on antigen-specific T cells, it might be useful to collect 1–2 million events on the flow cytometer. This may mean scaling up the staining of 4–6 million cells per sample.
Do not skip the second wash as this could affect cell viability.
We find it useful to crush the liver pieces over the cell strainer to get the cells through the strainer.
The appropriate amount of the tetramer is determined by comparing several dilutions of the tetramer staining in infected cells to uninfected cells or to a stain with an irrelevant tetramer. The dilutions that have substantial staining on infected cells but having the lowest background in uninfected cells are chosen (Fig. 1). As an independent test to determine whether or not MHC tetramers stain activated cells, we infected mice with either MCMV or LCMV and assessed staining of splenocytes from each mouse with both MCMV and LCMV tetramers. Results show fine specificity of both class I and class II MHC tetramers (Fig. 2).
CD16/32 refers to the murine Fcγ receptors. In flow staining, these receptors are generally blocked with antibodies to avoid nonspecific staining. Anti-CD16/32 antibody is produced by the 2.4G2 hybridoma (available from ATCC) and can be purified from 2.4G2 cell culture supernatant using a protein G column.
For class II staining, we include anti-CD16/32 antibody that is conjugated to a fluorochrome. CD16/32+ cells will be excluded from CD4+ gate to eliminate CD4+ myeloid cells from the analysis. We have found that CD16/32 is not expressed on CD4+ T cells and that cells bearing Fcγ receptors nonspecifically stain with MHC class II tetramers produced in baculoviral systems. Therefore, when analyzing the stains, a strategy that gates out CD16/32+ events helps to decrease the background (Fig. 3).
References
- 1.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 2.Razvi ES, Welsh RM, McFarland HI. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 3.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripp RA, Hou S, McMickle A, Houston J, Doherty PC. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 5.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 8.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 9.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 10.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 11.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127 (lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 14.Lin AA, Tripathi PK, Sholl A, Jordan MB, Hildeman DA. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol. 2009;83:8604–8615. doi: 10.1128/JVI.02477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gairin JE, Mazarguil H, Hudrisier D, Oldstone MB. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J Virol. 1995;69:2297–2305. doi: 10.1128/jvi.69.4.2297-2305.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 17.Gold MC, Munks MW, Wagner M, Koszinowski UH, Hill AB, Fling SP. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J Immunol. 2002;169:359–365. doi: 10.4049/jimmunol.169.1.359. [DOI] [PubMed] [Google Scholar]
- 18.Arens R, Wang P, Sidney J, Loewendorf A, Sette A, Schoenberger SP, Peters B, Benedict CA. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol. 2008;180:6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]