Abstract
Objective
The hypothalamus is the brain center that controls the energy balance. Anorexigenic proopiomelanocortin (POMC) neurons and orexigenic AgRP neurons in the arcuate nucleus of the hypothalamus plays critical roles in energy balance regulation. FoxO1 is a transcription factor regulated by insulin signaling that is deacetylated by Sirt1, a nicotinamide adenine dinucleotide- (NAD+-) dependent deacetylase. Overexpression of insulin-resistant constitutively-nuclear FoxO1 (CN-FoxO1) in POMC neurons leads to obesity, whereas Sirt1 overexpression in POMC neurons leads to leanness. Whether overexpression of Sirt1 in POMC neurons could rescue the obesity caused by insulin-resistant CN-FoxO1 was tested here.
Methods
POMC neuron-specific CN-FoxO1/Sirt1 double-KI (DKI) mice were analyzed.
Results
The obese phenotype of CN-FoxO1 KI mice was rescued in male DKI mice. Reduced O2 consumption, increased adiposity, and fewer POMC neurons observed in CN-FoxO1 mice were rescued in male DKI mice without affecting food intake and locomotor activity. Sirt1 overexpression decreased FoxO1 acetylation and protein levels without affecting its nuclear localization in mouse embryonic fibroblasts and hypothalamic N41 cells.
Conclusions
Sirt1 rescues the obesity induced by insulin-resistant CN-FoxO1 in POMC neurons of male mice by decreasing FoxO1 protein through deacetylation. Sirt1 ameliorates obesity caused by a genetic model of central insulin resistance.
Introduction
Since 1980, the prevalence of obesity has nearly doubled worldwide and threefold in the adolescents in the United States 1. Obesity results from an imbalance between energy intake and energy expenditure. The hypothalamus is the key brain region for controlling energy balance, and the central melanocortin system plays a crucial role. Proopiomelanocortin (POMC) neurons secrete α-melanocyte stimulating hormone (α-MSH), which suppresses food intake and increases energy expenditure by activating the melanocortin type-3 receptor (MC3R) and the melanocortin type-4 receptor (MC4R) 2. Agouti-related protein (AgRP) is an inverse agonist for MC3R and MC4R that increases food intake and suppresses energy expenditure.
Insulin signaling in the hypothalamus contributes to the energy balance regulation. The forkhead box-containing protein of the O subfamily (FoxO)1 is a downstream transcription factor for insulin signaling, and it is inactivated by the insulin signaling through phosphorylation by Akt 3. FoxO1 in the hypothalamus increases food intake 4,5. Overexpression of insulin-resistant (phosphorylation mutant) constitutively-nuclear FoxO1 (CN-FoxO1) in POMC neurons results in obesity and hyperphagia 6. Conversely, Foxo1 ablation in POMC neurons results in decreased food intake 7,8.
Sirt1 is a protein deacetylase with numerous substrates and improves insulin sensitivity 9. Sirt1 deacetylates FoxO1, enhances FoxO1 ubiquitination, and decreases FoxO1 protein levels 10. Along with insulin signaling, leptin signaling is another major contributor to the central regulation of energy balance, and POMC neuron-specific Sirt1 overexpression prevents age-associated weight gain by improving leptin sensitivity and by stimulating energy expenditure in male mice 11. Therefore, we asked whether Sirt1 regulates energy balance by improving insulin signaling in POMC neurons as well. Toward this end, we used the genetic overexpression of CN-FoxO1 as a model for insulin resistance and analyzed POMC neuron-specific CN-FoxO1/Sirt1 double knock-in (DKI) mice.
Methods
Animal studies
Pomc-Cre mice, Rosa26CN-Foxo1 mice, and Rosa26Sirt1-WT mice were described previously 6,11. DKI mice were generated by mating Pomc-Cre; Rosa26CN-Foxo1/+ mice with Pomc-Cre; Rosa26Sirt1-WT/+ mice. Mice were housed in individual cages in a temperature-controlled facility with a 12-h light, 12-h dark cycle, and with free access to water and normal chow (60% kcal of carbohydrate, 15% kcal of fat, and 25% kcal of protein) until the experiments. Because high-calorie diet suppresses hypothalamic SIRT1 function, the studies were conducted with normal chow only 11. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at Gunma University. Body weight was measured weekly. Food intake was measured when mice were 25 and 26 weeks old. Oxygen (O2) consumption, carbon dioxide (CO2) production, and locomotor activity were measured in individual 7-month-old mice and locomotor activity was measured as described previously 11.
PCR genotyping
We extracted genomic DNA from tail samples using phenol/chloroform and genotyped them using Rosa26 genotyping PCR with ExTaq polymerase (TAKARA, Otsu, Japan) as described previously 11. The primer sequences are listed in Supporting Information Table 1. For PCR identification of the Rosa26Sirt1-WT locus and the Rosa26CN-Foxo1 locus, the Sirt1–1918F and M13F primer set and the Flag S1 and SE 1 primer set were used, respectively.
Immunohistochemistry
For immunofluorescence analysis of samples from the hypothalamus, 4% paraformaldehyde-fixed frozen sections were stained with anti-POMC antibody. Pictures were taken, and the number of neurons that were positive for POMC immunostaining was counted and marked digitally using Adobe Photoshop to prevent re-counting. The results are expressed as the number of POMC-positive, nucleus-containing cell bodies per hemisection (the average of the left and the right) or the sum of these cells in all five counted sections of the ARC.
Cell culture, transfection, and adenovirus infection
Mouse embryonic fibroblasts (MEFs) were prepared as described previously 12. Hypothalamic N41 cells (Cosmo Bio, Tokyo, Japan) and MEFs were maintained in high-glucose Dulbecco's modified Eagle's mediaum (DMEM) supplemented with 10% fetal bovine serum (FBS). Transient transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Adenovirus infection was performed using a standard protocol at an MOI (multiplicity of infection) of 50. The cells were harvested 24 h after transfection or infection.
Western blot analyses
Proteins from cell lysates or MEFs were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes (Pall Life Sciences, Dreieich, Germany), and blotted with antibodies. The antibodies used in this study are listed in Supporting Information Table 2. Immunoreactive proteins were assessed using the LAS-4000 Image analyzer (Fuji Film, Tokyo, Japan) and densitometry using Image-J software.
Preparation of cell lysates from cultured cells
Total cell lysates and nuclear lysates were prepared using lysis buffer supplemented with a Complete Mini Protease Inhibitor Cocktail tablet (Roche), 40 µM MG132 (Sigma-Aldrich, St. Louis, MO), and 0.25 µg/ml ubiquitin aldehyde (Peptide Institute, Minoh, Japan). To prepare cytoplasmic and nuclear lysates, ice-cold cytoplasmic lysis buffer (5 mM PIPES (KOH) pH 8.0, 65 mM KCl, and 0.5% NP 40) was added and the supernatants after centrifugation recovered as cytoplasmic lysates. Remaining nuclear pellets were mixed with the lysis buffer to make nuclear lysates.
Statistical analyses
Data are expressed as the mean value ± SEM. Statistical significance was assessed using one-way analysis of variance (ANOVA) with post hoc Fisher's Least Significant Difference (LSD) Test. A P value of <0.05 was considered statistically significant.
Results
Sirt1 overexpression in POMC neurons rescues the obese phenotype caused by CN-FoxO1 in male mice by improving energy expenditure
Male CN-FoxO1 KI mice were obese whereas male Sirt1 KI mice were lean as reported previously 6,11,13. The DKI mice were leaner than CN-FoxO1 mice starting at 12 weeks of age (Figure 1a). The increase in body weight in CN-FoxO1 KI male mice was accompanied by increases in body length and adiposity, and these phenotypes were rescued in DKI mice (Figure 1b-d). There were no significant differences in body weight and adiposity in female mice among four groups (data not shown). Daily food intake was not different in the examined groups (Figure 1e). Oxygen consumption was significantly decreased in CN-FoxO1 KI mice and it was rescued in DKI mice (Figure 1f,g), without significant difference in respiratory exchange ratio (Figure 1h). The locomotor activity did not differ in the groups studied (Figure 1i). These results suggest that the rescue of CN-FoxO1-induced obesity by Sirt1 overexpression in POMC neurons could be explained by an effect on energy expenditure.
Figure 1.
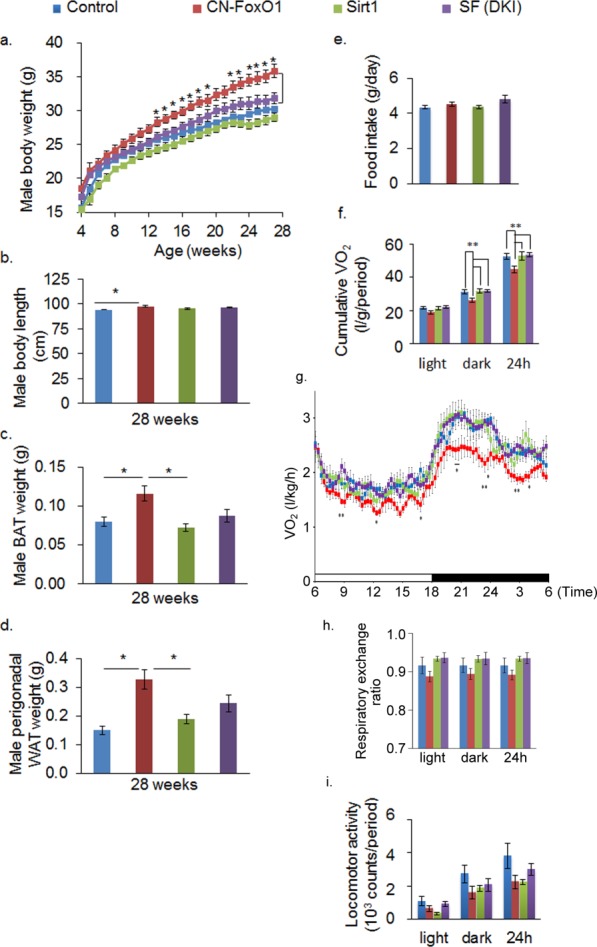
Sirt1 overexpression in POMC neurons rescues the obese phenotype caused by CN-FoxO1 in male mice by improving energy expenditure. (a) Changes in the body weight of control mice, CN-FoxO1 KI mice, Sirt1 KI mice, and double-KI male (n = 8–12) mice. (b) The body length of mice in the four groups (n = 8–12). (c, d) The weight of the brown adipose tissue (BAT) (c) and perigonadal white adipose tissue (WAT) (d) of the studied mice (n = 8–12). (e) Daily food intake at 25–26 weeks of age. (f-i) O2 consumption (VO2) (f-g), respiratory exchange ratio (h), and locomotor activity (i) in control mice, CN-FoxO1 KI mice, Sirt1 KI mice, and DKI mice. Data are reported as mean ± SEM. *P < 0.05; **P < 0.01 by ANOVA with post hoc LSD procedure. For Figure 1g, only the significant differences between CN-FoxO1 KI mice and DKI mice were marked.
POMC neurons are known to exert stronger effects on energy expenditure than on food intake, partly because POMC neurons have direct innervation on the sympathetic preganglionic neurons of the intermediolateral nucleus of the thoracic spinal cord, whereas AgRP neurons do not 14. Postganglionic neurons that innervate the interscapular brown adipose tissue receive projections from the intermediolateral nucleus 15. Re-expression of MC4R in cholinergic neurons normalizes energy expenditure without affecting food intake 16, whereas deletion of MC4R in sympathetic preganglionic neurons reduces energy expenditure and causes obesity 17, providing a pathway by which POMC neurons can regulate energy expenditure.
Sirt1 overexpression ameliorates the effect of CN-FoxO1 on the number of POMC neurons
FoxO1 has been shown to regulate the number of POMC neurons 18. Therefore, we tested whether Sirt1 overexpression affected the number of POMC neurons in DKI mice. Contrary to the previous study, we found that the number of POMC neurons (both the average number and the total number) tended to be decreased in CN-FoxO1 KI mice; it was rescued in DKI mice (Figure 2a-c). Sirt1 overexpression by itself did not affect the number of POMC neurons as described previously 11. Therefore, Sirt1 overexpression in POMC neurons rescued the obese phenotype caused by CN-FoxO1, possibly by normalizing the effect of CN-FoxO1 on the number of POMC neurons. One possible explanation for the differing results is that the genetic background of the mice differs in the two studies. Another possibility is that the loss of function (FoxO1 knock-out) and the gain of function (CN-FoxO1 KI) differentially affect the FoxO1 target genes. Because FoxO1 regulates both cell proliferation and survival 10,19, the lack of FoxO1 may promote POMC neuronal cell death, whereas overexpression of CN-FoxO1 may suppress the proliferation of POMC neurons and/or their progenitors.
Figure 2.
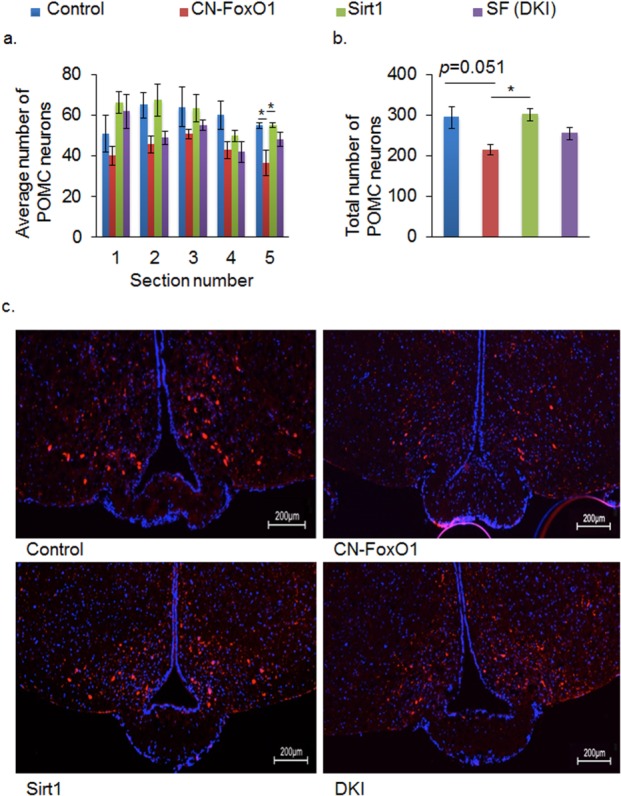
Sirt1 and FoxO1 regulate the number of POMC neurons. (a,b) The average number (a) and the total number (b) of POMC neurons in the arcuate nucleus of the indicated mice. (c) Representative arcuate nucleus sections with POMC neurons from control, CN-FoxO1, Sirt1, and DKI mice. Sections were immunostained with anti-POMC (red) and stained with DAPI (blue). 400-fold magnification. Data are reported as mean ± SEM. *P < 0.05 by ANOVA.
Sirt1 overexpression decreases FoxO1 acetylation and protein levels without affecting the nuclear localization of CN-FoxO1
Deacetylation of the FoxO1 protein affects its stability by promoting poly-ubiquitination of FoxO1 10, while deacetylation of FoxO1 overrides the cytoplasmic localization signal conferred by phosphorylation via Akt 20. Therefore, Sirt1 overexpression could promote CN-FoxO1 deacetylation and affect either protein stability and/or the nuclear localization. To address these questions, we assessed the effect of Sirt1 overexpression on FoxO1 acetylation, protein level, and subcellular localization in hypothalamic N41 cells and MEFs. Sirt1 overexpression in hypothalamic N41 cells promoted FoxO1 deacetylation and decreased the CN-FoxO1 protein level without affecting its nuclear localization (Figure 3a-c). The degree of Sirt1 overexpression achieved in MEFs via infection with adenovirus encoding the Cre recombinase was sufficient to decrease the level of FoxO1 protein in the nucleus without affecting the FoxO1 protein level in the cytoplasm of DKI MEFs (Figure 3d).
Figure 3.
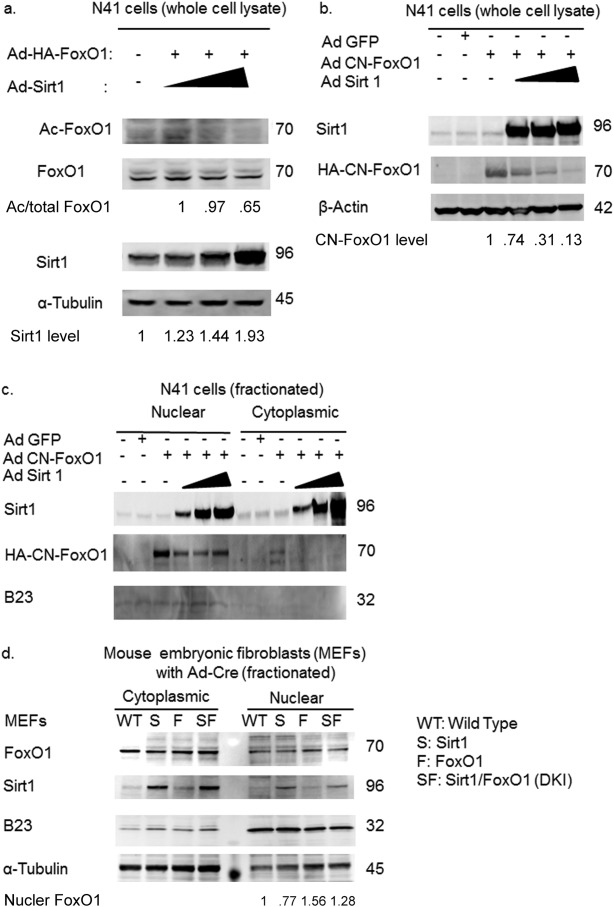
Sirt1 affects the acetylation and amount of CN-FoxO1 protein but not its nuclear localization. (a) Sirt1 overexpression decreases Foxo1 acetylation in hypothalamic N41 cells in a dose-dependent manner. (b) Sirt1 overexpression decreases CN-FoxO1 protein in hypothalamic N41 cells. (c) Sirt1 overexpression does not affect CN-FoxO1 localization in hypothalamic N41 cells, while it decreases nuclear CN-FoxO1 level. (d) The amount of nuclear FoxO1 protein is decreased in Ad-Cre-infected DKI MEFs (SF) compared to CN-FoxO1 MEF (F) mice. Nuclear FoxO1 levels were determined by ratio between FoxO1 signals and B23 signals.
Based on these results, we conclude that Sirt1 overexpression in POMC neurons rescues the obesity caused by CN-Foxo1 by decreasing FoxO1 acetylation and FoxO1 protein levels.
Acknowledgments
Authors thank Ms. C. Osawa for excellent technical assistance, Ms. Kanako Ohtani for secretarial assistance, and the members of the Kitamura lab for constructive discussions of the data. They also thank the Biosignal Genome Resource Center (Gunma University) for the use of the facility. Authors thank Dr. Jun Nakae at Keio University for providing us with Rosa 26CN-FoxO1 mice.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Coll AP, Farooqi IS, Challis BG, Yeo GS, O'Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 3.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim MS, Pak YK, Jang PG, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura T, Feng Y, Kitamura YI, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 6.Iskandar K, Cao Y, Hayashi Y, et al. PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. Am J Physiol Endocrinol Metab. 2010;298:E787–E798. doi: 10.1152/ajpendo.00512.2009. [DOI] [PubMed] [Google Scholar]
- 7.Ropelle ER, Pauli JR, Prada P, et al. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol. 2009;587:2341–2351. doi: 10.1113/jphysiol.2009.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plum L, Lin HV, Dutia R, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 2010;57:939–946. doi: 10.1507/endocrj.k10e-320. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki T, Kikuchi O, Shimpuku M, et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia. 2014;57:819–831. doi: 10.1007/s00125-013-3140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413–422. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki T, Kim HJ, Kobayashi M, et al. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- 14.Elias CF, Lee C, Kelly J, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 15.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 16.Rossi J, Balthasar N, Olson D, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglund ED, Liu T, Kong X, et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plum L, Lin HV, Aizawa KS, Liu Y, Accili D. InsR/FoxO1 signaling curtails hypothalamic POMC neuron number. PLoS One. 2012;7:e31487. doi: 10.1371/journal.pone.0031487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiang L, Banks AS, Accili D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J Biol Chem. 2010;285:27396–27401. doi: 10.1074/jbc.M110.140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


