Abstract
Objective
This randomised, double-blind, 12-week study compared efficacy and tolerability of flexible-dose treatment with vortioxetine (10–20 mg/day) versus agomelatine (25–50 mg/day) in major depressive disorder patients with inadequate response to selective serotonin reuptake inhibitor (SSRI)/serotonin–noradrenaline reuptake inhibitor (SNRI) monotherapy.
Methods
Patients were switched directly from SSRI/SNRI to vortioxetine or agomelatine. Primary endpoint was change from baseline to week 8 in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score analysed by mixed model for repeated measurements, using a noninferiority test followed by a superiority test. Secondary endpoints included response and remission rates, anxiety symptoms (Hamilton Anxiety Rating Scale), Clinical Global Impression, overall functioning (Sheehan Disability Scale), health-related quality of life (EuroQol 5 Dimensions), productivity (work limitation questionnaire) and family functioning (Depression and Family Functioning Scale).
Results
Primary endpoint noninferiority was established and vortioxetine (n = 252) was superior to agomelatine (n = 241) by 2.2 MADRS points (p < 0.01). Vortioxetine was also significantly superior in response and remission rates at weeks 8 and 12; MADRS, Hamilton Anxiety Rating Scale, Clinical Global Impression, Sheehan Disability Scale and EuroQol 5 Dimensions scores at week 4 onwards; work limitation questionnaire at week 8 and Depression and Family Functioning Scale at weeks 8 and 12. Fewer patients withdrew because of adverse events with vortioxetine (5.9% vs 9.5%). Adverse events (incidence ≥5%) were nausea, headache, dizziness and somnolence.
Conclusions
Vortioxetine was noninferior and significantly superior to agomelatine in major depressive disorder patients with previous inadequate response to a single course of SSRI/SNRI monotherapy. Vortioxetine was safe and well tolerated.
Keywords: agomelatine, inadequate response, major depressive disorder, switch, vortioxetine
INTRODUCTION
Patients with major depressive disorder (MDD) are usually initially treated with antidepressant monotherapy, often serotonin reuptake inhibitors. Patients who have failed at least one adequate trial of one major class of antidepressant have been characterised as being in stage I of antidepressant resistance (Thase and Rush, 1997). Clinical guidelines recommend switching antidepressant if there has not been clinically meaningful improvement after initial treatment (Bauer et al., 2007; NICE, 2009; APA, 2010). There are very limited data available from double-blind randomised trials (Nolen et al., 1988, 1993; Thase et al., 2002; Lenox-Smith and Jiang, 2008) comparing monotherapy strategies in patients who were unresponsive to first-line treatment with an antidepressant.
Vortioxetine is a novel antidepressant with multimodal activity. It is thought to work through a combination of two pharmacological modes of action: a direct effect on receptor activity and reuptake inhibition. In vitro studies indicate that vortioxetine is a 5-HT3, 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist and an inhibitor of the 5-HT transporter (Bang-Andersen et al., 2011; Westrich et al., 2012). Serotonin (5-HT) transporter occupancy in the raphe nuclei at steady-state conditions in healthy subjects was ≈50% at 5 mg, 65% at 10 mg and >80% at 20 mg of vortioxetine (Areberg et al., 2012; Stenkrona et al., 2013). The antidepressant efficacy of vortioxetine has been demonstrated or supported in the dose range 5–20 mg/day in six placebo-controlled short-term studies of 6–8 weeks duration in adult (Alvarez et al., 2012; Baldwin et al., 2012; Henigsberg et al., 2012; Boulenger et al., 2014; Jacobsen et al., 2013; Mahableshwarkar et al., 2013a, 2013b) or elderly patients with MDD (Katona et al., 2012) and in relapse prevention (Boulenger et al., 2012).
Agomelatine is the only member of a new class of antidepressants with melatonergic agonism (MT1 and MT2 receptors) and 5-HT2C antagonism. Agomelatine is licenced in Europe on the basis of placebo-controlled efficacy in European studies. Subsequent placebo-controlled studies in South America and the United States were less convincing so that meta-analyses of these data have shown more modest efficacy (Koesters et al., 2013; Taylor et al., 2014). In contrast, the individual head to head studies comparing with selective serotonin reuptake inhibitor (SSRI) and serotonin–noradrenaline reuptake inhibitor (SNRI) antidepressants have reported superior efficacy compared with fluoxetine (Hale et al., 2010), sertraline (Kasper et al., 2010) and venlafaxine (Lemoine et al., 2007). The Maudsley Guidelines (Taylor et al., 2012) suggest agomelatine as a possible choice in treatment-resistant depression.
The primary objective of this study was to compare the efficacy of flexible doses of vortioxetine (10–20 mg/day) with agomelatine (25–50 mg/day), after 8 weeks of treatment, on depressive symptoms in patients with MDD who have responded inadequately to antidepressant monotherapy with an SSRI or an SNRI. Agomelatine was chosen as it has a different mechanism of action than SSRIs/SNRIs, which is also the case for vortioxetine. The 12-week duration of treatment was to evaluate the full extent of the improvement of depressive symptoms, overall functioning and health-related quality of life in both treatment groups.
METHOD
Study design
This double-blind, randomised, flexible-dose, active comparator (agomelatine) study included 501 randomised patients recruited from 71 psychiatric inpatient and outpatient settings in 14 countries (Austria, Belgium, Bulgaria, Czech Republic, Estonia, Germany, Italy, Lithuania, Poland, Romania, Russia, Spain, Sweden and the UK) from January 2012 to December 2012. Patients were recruited via advertisements (in Austria, Germany, Estonia, Russia, Sweden and the UK) or referrals from general practitioners. The study was conducted in accordance with the principles of Good Clinical Practice (ICH, 1996) and the Declaration of Helsinki (WMA, 2008). Local research ethics committees approved the study, and eligible patients provided written informed consent before participating.
Patients with an inadequate response to a SSRI/SNRI (SSRIs: citalopram, escitalopram, paroxetine, sertraline, SNRIs: duloxetine and venlafaxine) monotherapy at approved doses for at least 6 weeks prior to the screening visit were eligible. In order to minimise potential interactions, neither fluoxetine (because of its long half-life) nor fluvoxamine (because of its inhibition of agomelatine-sensitive hepatic enzymes) was allowed as previous treatment of the current major depressive episode (MDE). Investigators were asked to gradually decrease the dose of the SSRI or SNRI during the week prior to baseline to reach the minimum therapeutic dose.
Following a screening period of 4–10 days, eligible patients were directly switched from their previous treatment by randomisation (1 : 1) to vortioxetine (10–20 mg/day) or agomelatine (25–50 mg/day) for 12 weeks of double-blind treatment. On the basis of the investigator's clinical judgement, the dosage for each patient was optimised by using a flexible-dose design for the first 4 weeks. For both treatments, the dose could be increased on the basis of an unsatisfactory response on depressive symptoms, according to the clinical judgement of the investigator. Patients in the vortioxetine group received vortioxetine 10 mg/day in week 1. At the end of weeks 1, 2, 3 or 4, the dose could be increased to 20 mg/day. Patients in the agomelatine group received 25 mg/day for the first 2 weeks of treatment in accordance with the recommendations provided in the Summary of Product Characteristics for agomelatine (SPC, 2009). At the end of weeks 2, 3 or 4, the dose could be increased to 50 mg/day. For both treatments, the investigator could decrease the dose to 10 mg/day (vortioxetine) or 25 mg/day (agomelatine) for patients who did not tolerate the increased dose. After week 4, the dose was fixed. The investigator was blind to the allocated treatment and consequently did not know if the dose was adjusted at week 1. Changes in dosage were handled by the interactive voice response system.
Patients were seen at baseline and weeks 1, 2, 3, 4, 8 and 12. Patients who withdrew were seen as soon as possible after withdrawal. There was no downtaper schedule. A safety follow-up contact was scheduled for 4 weeks after completion of the treatment period or after withdrawal from the study. Study medication was given as encapsulated tablets of identical appearance. From the day after randomisation, patients were instructed to take one capsule per day, orally, preferably at bedtime.
Main entry criteria
Eligible patients were aged ≥18 and ≤75 years, with a primary diagnosis of a single episode or recurrent MDD according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision criteria (APA, 2000) and a current MDE of <12 months' duration [confirmed using the Mini International Neuropsychiatric Interview (MINI) (Lecrubier et al., 1997)]. Patients were required to have a Montgomery–Åsberg Depression Rating Scale (MADRS) (Montgomery and Åsberg, 1979) total score ≥22 and item 1 (apparent sadness) score ≥3 at screening and baseline visits. Only patients with depressive symptoms considered nonresponsive or partially responsive to a single treatment course of an adequate dose (approved) and duration (≥6 weeks) were eligible for the study (stage I (Thase and Rush, 1997) and stage A (Souery et al., 1999) criteria). In addition, patients had to want to change their current treatment because of an inadequate response and to be considered by the investigators to be candidates for a switch.
Treatment resistance was excluded using both stage II (Thase and Rush, 1997) and stage B (Souery et al., 1999) criteria. Patients with a history of lack of response to agomelatine or previous exposure to vortioxetine were excluded. Patients were also excluded if they had any current axis I disorder other than generalised anxiety disorder (GAD) or social anxiety disorder (SAD), as defined in the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision and assessed using the MINI, or if they had a history of a manic or hypomanic episode, schizophrenia or any other psychotic disorder (including major depression with psychotic features), mental retardation, organic mental disorders or mental disorders because of a general medical condition, any substance abuse disorder within the previous 2 years, a history of a clinically significant neurological disorder, any neurodegenerative disorder or any axis II disorder that might compromise their participation in the study.
Patients at serious risk of suicide, on the basis of the investigator's clinical judgement, and those who had a score ≥5 on item 10 of the MADRS scale (suicidal thoughts) or had attempted suicide within <6 months were excluded, as were those receiving formal cognitive or behavioural therapy or systematic psychotherapy and pregnant or breastfeeding women. Patients were also excluded if they were taking disallowed concomitant medication, as described by Alvarez et al. (2012), as well as the antibiotics rifampicin (broad inducer of CYP450 isoforms) and ciprofloxacin (potent CYP1A2 inhibitor contraindicated with agomelatine), although antiarrhythmics, antihypertensives and proton pump inhibitors (except cimetidine) were permitted. Episodic use of zolpidem, zopiclone or zaleplon for severe insomnia was allowed for a maximum of 2 days per week but not the night before a study visit.
Patients were excluded if they had one or more clinical laboratory test values outside the reference range of potential risk to the patient's safety or a serum alanine aminotransferase (ALT) or aspartate aminotransferase value >2 times the upper limit of the reference range [upper limit of normal (ULN)], a serum creatinine value >1.5 times ULN or a serum total bilirubin value >1.5 times ULN. This was due to hepatotoxicity concerns with agomelatine.
Safety reasons for withdrawal from the study were defined using the criteria described in Baldwin et al. (2012). In addition, patients with a QTcF interval >500 ms confirmed by electrocardiogram (ECG) within 2 weeks or ALT/aspartate aminotransferase values >3 times ULN were to be withdrawn. If adverse events (AEs) contributed to withdrawal, they were regarded as the primary reason for withdrawal.
Efficacy rating
The effect of vortioxetine (10–20 mg/day) versus agomelatine (25–50 mg/day) after 8 weeks of treatment was assessed using the MADRS total score. All raters were psychiatrists involved in normal clinical practice and underwent formal training in the MADRS and the scoring conventions for the Clinical Global Impression [severity of illness (CGI-S) and global improvement (CGI-I)] (Guy, 1976), the MINI and the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959) in order to maximise inter-rater reliability. Only raters who passed the qualification test were allowed to rate patients in this study. The effect of vortioxetine versus agomelatine on MADRS was assessed at screening; baseline; weeks 1, 2, 3, 4, 8 and 12; the CGI-S at baseline onwards; CGI-I from week 1 onwards and the HAM-A at baseline onwards (except weeks 1 and 3). Overall functioning, health-related quality of life and productivity were assessed by patient reports at baseline and onwards (except weeks 1, 2 and 3) using the Sheehan Disability Scale (SDS) (Sheehan and Harnett-Sheehan, 1996), the EuroQol 5 Dimensions (EQ-5D) (The EuroQoL Group, 1990), and at baseline, weeks 8 and 12 with the work limitation questionnaire (WLQ) (Lerner et al., 2001) and the Depression and Family Functioning Scale (DFFS) (DiBenedetti et al., 2012).
Allocation to treatment
Eligible patients were assigned to double-blind treatment according to a randomisation list that was computer generated by H. Lundbeck A/S. The details of the randomisation series were contained in a set of sealed opaque envelopes. At each site, sequentially enrolled patients were assigned the lowest randomisation number available in blocks of 4 using an interactive voice/web response system. All investigators, trial personnel, patients and sponsor were blinded to treatment assignment for the duration of the study. The randomisation code was broken for one patient, who was withdrawn because of a serious AE (SAE; peripheral oedema) after 14 days treatment with vortioxetine. This patient was included in the safety and efficacy analyses.
Analysis sets
Safety analyses were based on the all-patients-treated set (APTS), comprising all randomised patients who took at least one dose of study medication. Efficacy analyses were based on a modified intent-to-treat set—the full-analysis set (FAS), comprising all patients in the APTS who had a valid baseline assessment and at least one valid postbaseline assessment of the primary efficacy variable (MADRS total score).
Power and sample size calculations
Power calculations showed that with a power of ≥80% and an expected withdrawal rate of 20% a total of 500 patients should be randomised. This was based on a noninferiority comparison of the treatment groups in MADRS total score using a two-sided 95% confidence interval (CI) against a margin of +2 points, a standard deviation (SD) of 9.5 and an assumed true advantage of 0.7 points for vortioxetine.
Analysis of the primary efficacy endpoint
The primary efficacy endpoint was the change from baseline in MADRS total score at week 8 based on the FAS using all available data. A noninferiority followed by a superiority comparison of vortioxetine versus agomelatine was based on estimates from a mixed model for repeated measurements (MMRM) with treatment, week and site as fixed factors and the baseline score as a covariate. The model also included treatment-by-week and baseline MADRS total score-by-week interactions. An unstructured covariance structure was used to model the within-patient variance, and the estimation method was a restricted maximum likelihood based approach. Noninferiority was established if the upper bound of the two-sided 95% CI of the difference between treatment groups in MADRS total score at week 8 did not exceed +2 MADRS points for vortioxetine versus agomelatine. The prefixed margin of noninferiority of +2 was specified according to recommendations that the noninferiority margin should be between one-third and one-half of the advantage of the active comparator over placebo. Under a simple closed test procedure, the same primary measure was used to investigate superiority, at a 5% significance level, in accordance with Committee for Proprietary Medicinal Products guidance (CPMP, 2000). Because there was only one primary endpoint and one comparison, no adjustments for multiple testing were made. A sensitivity analysis was performed using analysis of covariance (ANCOVA) including site, treatment and baseline MADRS total score as fixed effects, on the basis of the FAS using last observation carried forward (LOCF).
Analysis of secondary efficacy endpoints
The analyses of the secondary continuous endpoints (MADRS total score, HAM-A total score, CGI-S and CGI-I scores, SDS total score, EQ-5D overall health state score (VAS), WLQ global productivity index and DFFS total score) were performed using the MMRM and ANCOVA and LOCF models similar to the models described for the primary efficacy endpoint. For analyses of the CGI-I, the CGI-S score served as baseline. Response (≥50% improvement from baseline in MADRS total score or CGI-I ≤2) and remission (MADRS total score ≤10 or CGI-S ≤2) were analysed using logistic regression, with treatment as factor and the baseline score as a covariate (FAS and LOCF). All the statistical tests of the efficacy endpoints were two-sided tests performed on a 5% significance level.
Tolerability assessment
At each visit, starting at baseline, patients were asked a nonleading question (such as, how do you feel?). All AEs either observed by the investigator or reported spontaneously by the patient were recorded. Qualified personnel coded AEs using the lowest level term according to MedDRA, version 15.1. Clinical safety laboratory tests (at screening, weeks 4, 8 and 12), vital signs, weight, ECGs and physical examination findings were also evaluated.
RESULTS
Patient baseline characteristics
The APTS comprised 495 patients after the exclusion of six patients who did not take any study medication (Figure 1). The FAS comprised 493 patients after the exclusion of one patient from each treatment group with no valid postbaseline MADRS total score assessment. Both patients withdrew because of an AE. There were no clinically relevant differences at baseline between treatment groups in demographic or clinical characteristics (Tables 1 and 2). Patients had a mean age of about 46 years, approximately three-quarters were women and almost all were Caucasian.
Figure 1.

Flow chart of patient disposition. MADRS, Montgomery–Åsberg Depression Rating Scale; BL, baseline; APTS, all-patients-treated set; FAS, full-analysis set
Table 1.
Baseline patient characteristics
| APTS | Vortioxetine 10–20 mg (n = 253) | Agomelatine 25–50 mg (n = 242) |
|---|---|---|
| Women, n (%) | 195 (77.1) | 175 (72.3) |
| Mean age ± SD (years) | 47 ± 12 | 46 ± 12 |
| Range (years) | 18–75 | 19–74 |
| Caucasian (%) | 99.6 | 100 |
| Mean duration of current MDE (weeks) | 19 ± 10 | 19 ± 11 |
| Previous MDEs ± SD (n) | 1.9 ± 2.2 | 1.7 ± 1.9 |
| Range (n) | 0–13 | 0–12 |
APTS, all-patients-treated set; MDE, major depressive episode; SD, standard deviation.
Table 2.
Baseline assessments (mean ± SD) and change from baseline (mean ± SE) to week 8 (full-analysis set and mixed model for repeated measurements)
| Vortioxetine 10–20 mg (n = 252) | Agomelatine 25–50 mg (n = 241) | |||
|---|---|---|---|---|
| Baseline assessment | Change from baseline | Baseline assessment | Change from baseline | |
| Primary efficacy variable | ||||
| MADRS total score | 29.1 ± 4.4 | −16.5 ± 0.48** | 28.7 ± 4.0 | −14.4 ± 0.51 |
| Secondary efficacy variables | ||||
| Clinician-rated assessments | ||||
| HAM-A total score | 21.6 ± 6.3 | −11.7 ± 0.4*** | 21.4 ± 6.2 | −9.8 ± 0.4 |
| CGI-S score | 4.4 ± 0.6 | −1.84 ± 0.07** | 4.4 ± 0.6 | −1.55 ± 0.07 |
| CGI-I scorea | — | 1.97 ± 0.06** | — | 2.22 ± 0.07 |
| Patient-reported outcomes | ||||
| SDS total score | 19.2 ± 5.3 | −9.28 ± 0.53** | 19.3 ± 5.2 | −7.06 ± 0.55 |
| SDS family life subscale | 6.3 ± 2.0 | −3.09 ± 0.16** | 6.4 ± 2.0 | −2.51 ± 0.16 |
| SDS work subscale | 6.4 ± 2.1 | −2.95 ± 0.18** | 6.4 ± 2.2 | −2.25 ± 0.19 |
| SDS social life subscale | 6.4 ± 2.1 | −3.04 ± 0.16** | 6.5 ± 2.0 | −2.39 ± 0.17 |
| EQ-5D overall health state score (VAS) | 45.5 ± 18.3 | 20.6 ± 1.2** | 46.8 ± 19.4 | 15.6 ± 1.3 |
| WLQ global productivity index | 0.15 ± 0.06 | −0.06 ± 0.00* | 0.16 ± 0.06 | −0.04 ± 0.00 |
| DFFS total score | 210 ± 29 | −10.8 ± 0.7** | 204 ± 29 | −7.9 ± 0.7 |
CGI-I, Clinical Global Impression—Improvement; CGI-S, Clinical Global Impression—Severity; DFFS, Depression and Family Functioning Scale; EQ-5D, EuroQol 5 Dimensions; HAM-A, Hamilton Anxiety Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; SDS, Sheehan Disability Scale; VAS, visual analogue scale, WLQ, work limitation questionnaire.
Absolute value.
p < 0.05 versus agomelatine.
p < 0.01 versus agomelatine.
p < 0.001 versus agomelatine, decreased values = improvement (except for EQ-5D where increased values = improvement).
The mean baseline MADRS total score was 28.9 ± 4.2 (mean ± SD), indicating moderate to severe depression, as also reflected in the mean CGI-S score of 4.4 ± 0.6 (mean ± SD). There was a substantial level of anxiety symptoms, indicated by a mean baseline HAM-A total score of 21.5 ± 6.2 (mean ± SD) (Table 2). The current MDE had typically started about 19 weeks before enrolment with a maximum duration of 51 weeks. It was the first episode for 28.1% of patients, whereas most patients had had two previous depressive episodes (range: 0–13). The antidepressant taken immediately before switching to either vortioxetine or agomelatine were sertraline (24.0%), escitalopram (18.6%), citalopram (18.4%), venlafaxine (17.2%), paroxetine (14.7%) or duloxetine (6.1%).
Withdrawals from the study
The overall withdrawal rate during the entire study was slightly lower for vortioxetine (20.9%) than agomelatine (26.0%) (Figure 1). The most frequent reasons for withdrawal were AEs [5.9% (vortioxetine) and 9.5% (agomelatine)], lack of efficacy [4.3% (vortioxetine) and 7.0% (agomelatine)] and withdrawal of consent [5.5% (vortioxetine) and 5.0% (agomelatine)].
Dosage
Patients received vortioxetine 10 mg/day for the first week and agomelatine 25 mg/day for the first 2 weeks. During the first 4 weeks, 64 patients (25.5%) in the vortioxetine group and 48 patients (20.8%) in the agomelatine group did not change their initial dose and stayed on the low dose during the study. The dose was changed for 179 vortioxetine patients (71.0%) and 166 agomelatine patients (68.9%). Of these, four vortioxetine patients (2.2%) and two agomelatine patients (1.2%) had an AE shortly after the dose increase that led to their withdrawal from the study. At the start of week 5, 64.7% of the patients in the vortioxetine group received the higher dose (20 mg/day) whilst 71.7% did so in the agomelatine group (50 mg/day). The total exposure accrued was 50 (vortioxetine) and 45 (agomelatine) patient years.
Efficacy
Primary endpoint
In the primary efficacy analysis, the mean change from baseline in MADRS total score at week 8 (FAS and MMRM) was −16.5 (vortioxetine) and −14.4 points (agomelatine) (Table 2). The mean difference for vortioxetine to agomelatine was −2.2 (95% CI: −3.5 to −0.8; p = 0.0018). Noninferiority was established, as the upper bound of the 95% CI for the vortioxetine, and agomelatine comparison was −0.81 MADRS points and therefore clearly less than the noninferiority margin of +2 MADRS points. In the present study, the upper bound of the 95% confidence limit was not only less than this margin but also less than 0, establishing significantly superior efficacy.
To analyse the robustness of the results of the primary efficacy analysis, sensitivity analyses were performed using ANCOVA (FAS, LOCF). Vortioxetine was significantly superior to agomelatine by −3.1 (95% CI: −4.6 to −1.7; p < 0.0001) MADRS points at week 8 (Table 3).
Table 3.
Mean change from baseline to weeks 8 and 12 (full-analysis set): difference between vortioxetine and agomelatine (±SE)
| MMRM | ANCOVA, LOCF | |||
|---|---|---|---|---|
| Efficacy variable | Week 8 | Week 12 | Week 8 | Week 12 |
| Primary efficacy variable | ||||
| MADRS total score | −2.2 ± 0.7**a | −2.0 ± 0.7** | −3.1 ± 0.8*** | −3.5 ± 0.8*** |
| Secondary efficacy variables | ||||
| Clinician-rated assessments | ||||
| HAM-A total score | −1.9 ± 0.6*** | −1.9 ± 0.6*** | −2.4 ± 0.6*** | −2.8 ± 0.7*** |
| CGI-S score | −0.3 ± 0.1** | −0.3 ± 0.1** | −0.4 ± 0.1*** | −0.4 ± 0.1*** |
| CGI-I score | −0.3 ± 0.1** | −0.3 ± 0.1** | −0.4 ± 0.1*** | −0.5 ± 0.1*** |
| Patient-reported outcomes | ||||
| SDS total score | −2.2 ± 0.7** | −1.8 ± 0.8* | −2.7 ± 0.7*** | −2.8 ± 0.8*** |
| SDS family life subscale | −0.58 ± 0.22** | −0.43 ± 0.22 | −0.76 ± 0.22*** | −0.70 ± 0.23** |
| SDS work subscale | −0.70 ± 0.25** | −0.55 ± 0.26* | −0.88 ± 0.25*** | −0.86 ± 0.26** |
| SDS social life subscale | −0.66 ± 0.23** | −0.55 ± 0.22* | −0.77 ± 0.23*** | −0.76 ± 0.24** |
| EQ-5D overall health state score (VAS) | 5.0 ± 1.7** | 4.7 ± 1.9* | 6.2 ± 1.7*** | 6.6 ± 1.9*** |
| WLQ global productivity index | −0.01 ± 0.01* | −0.01 ± 0.01 | −0.01 ± 0.01* | −0.01 ± 0.01 |
| DFFS total score | −2.9 ± 0.9** | −2.5 ± 1.0* | −2.9 ± 0.9** | −3.0 ± 1.0** |
CGI-I, Clinical Global Impression—Improvement; CGI-S, Clinical Global Impression—Severity; DFFS, Depression and Family Functioning Scale; EQ-5D, EuroQol 5 Dimensions; HAM-A, Hamilton Anxiety Rating Scale; LOCF, last observation carried forward; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed model for repeated measurements; SDS, Sheehan Disability Scale; VAS, visual analogue scale; WLQ, work limitation questionnaire; ANCOVA, analysis of covariance.
Primary efficacy analysis.
Absolute value.
*p < 0.05 versus agomelatine.
**p < 0.01 versus agomelatine.
***p < 0.001 versus agomelatine, decreased values = improvement (except for EQ-5D where increased values = improvement).
Secondary efficacy analysis
Clinician-rated assessments
Vortioxetine was significantly superior to agomelatine in predefined secondary efficacy analyses (MADRS total score, HAM-A total score, CGI-S score and CGI-I score) (Tables 2 and 3), including response and remission based on the MADRS, the CGI-S and the CGI-I (Figure 2).
Figure 2.

Montgomery–Åsberg Depression Rating Scale (MADRS) response (≥50% improvement from baseline) and MADRS remission (MADRS total score ≤10) rates (logistic regression, full-analysis set and last observation carried forward). **p < 0.01; ***p < 0.001 versus agomelatine
Montgomery–Åsberg Depression Rating Scale
At week 12, the MADRS total mean score decreased (improved) from 29.1 at baseline to 9.9 (vortioxetine) and from 28.7 to 11.9 (agomelatine) (FAS and MMRM). Vortioxetine was significantly superior to agomelatine (p < 0.05) in reducing the MADRS total score from week 4 onwards (MMRM) (Figure 3) and from week 3 onwards (ANCOVA and LOCF). Vortioxetine was significantly superior to agomelatine in response and remission at week 8 and onwards. At week 12, 69.8% of the patients in the vortioxetine group were MADRS responders compared with 56.0% of the patients in the agomelatine group, and 55.2% of the patients in the vortioxetine group were MADRS remitters compared with 39.4% of the patients in the agomelatine group (LOCF; p < 0.01) (Figure 2). In addition, vortioxetine separated from agomelatine during the 12-week study for each of the MADRS single items at weeks 8, 12 or earlier with the exception of ‘inability to feel’ and ‘reduced sleep’ (FAS and MMRM).
Figure 3.

Estimated change in Montgomery–Åsberg Depression Rating Scale (MADRS) total scores from baseline to week 12 (FAS and MMRM by visit) and LOCF (FAS and ANCOVA) at week 12. FAS, full-analysis set; LOCF, last observation carried forward; MMRM, mixed model repeated measures. Patient numbers at each visit are shown below the x-axis for each treatment group. **p < 0.01; ***p < 0.001 versus agomelatine. The primary endpoint is at week 8 (FAS and MMRM)
Hamilton Anxiety Rating Scale
In line with the MADRS total score, the mean HAM-A total score improved from baseline to week 12 in both treatment groups (FAS and MMRM) (Figure 4). There was a significant difference (p < 0.05) in favour of vortioxetine from week 4 onwards (Figure 4). This was supported by the ANCOVA (FAS and LOCF) results.
Figure 4.
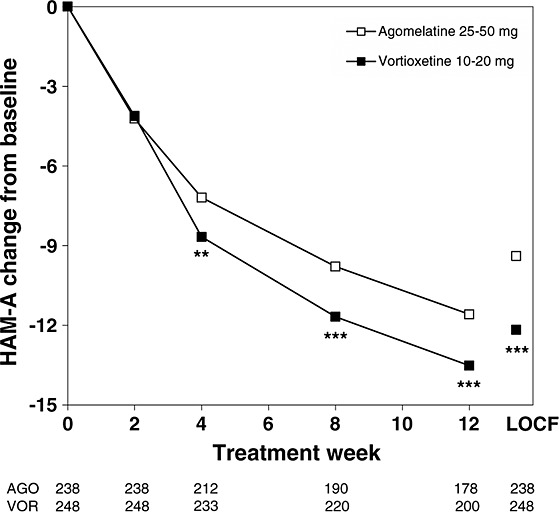
Estimated change in Hamilton Anxiety Rating Scale (HAM-A) total scores from baseline to week 12 (FAS and MMRM by visit) and LOCF (FAS and ANCOVA) at week 12. FAS, full-analysis set; LOCF, last observation carried forward; MMRM, mixed model repeated measures. Patient numbers at each visit are shown below the x-axis for each treatment group. **p < 0.01, ***p < 0.001 versus agomelatine
Clinical Global Impression
The mean CGI-I and CGI-S scores improved throughout the 12-week treatment period in both treatment groups (FAS and MMRM) (Table 2). Significant differences in favour of vortioxetine (p < 0.05) were seen from week 4 onwards for both. This was supported by the ANCOVA (FAS, LOCF) results. Vortioxetine was also significantly superior to agomelatine in response and remission based on the CGI-I and the CGI-S. Separation from agomelatine in favour of vortioxetine was seen at weeks 8 and 12 for CGI-I response (p < 0.05) and at week 4 onwards for CGI-S remission (p < 0.05).
Patient-reported outcomes
The scores improved in both treatment groups for all patient-reported outcomes relating to overall functioning [SDS total score and all three subscales (family, work and social life)], health-related quality of life (EQ-5D), productivity (WLQ) and family functioning (DFFS) (Table 2). Vortioxetine was significantly superior to agomelatine from week 4 onwards on the SDS total score and all three subscales (except for family at week 12) and EQ-5D, for the family functioning (DFFS) at weeks 8 and 12 and for productivity (WLQ) at week 8 (FAS and MMRM; p < 0.05). This was supported by the ANCOVA (FAS and LOCF) results.
Tolerability and safety
Treatment-emergent adverse events
During the 12-week treatment period, approximately half of the patients in each treatment group had one or more treatment-emergent adverse event (TEAEs) (Table 4). During this period, 34 patients withdrew because of TEAEs, 5.5% in the vortioxetine group and 8.3% in the agomelatine group. The only TEAEs leading to withdrawal of ≥2 patients in either treatment group were vomiting (1.2%) and nausea (0.8%) in the vortioxetine group and dizziness (2.1%) and headache (0.8%) in the agomelatine group.
Table 4.
Treatment-emergent adverse events (TEAEs) with an incidence of ≥5% in either treatment group in the 12-week treatment period (all-patients-treated set)
| Preferred term | Vortioxetine 10–20 mg, n (%) (n = 253) | Agomelatine 25–50 mg, n (%) (n = 242) |
|---|---|---|
| Patients with TEAEs | 137 (54.2) | 127 (52.5) |
| Nausea | 41 (16.2) | 22 (9.1) |
| Headache | 26 (10.3) | 32 (13.2) |
| Dizziness | 18 (7.1) | 28 (11.6) |
| Somnolence | 10 (4.0) | 19 (7.9) |
The most common TEAEs reported by at least 5% of patients in either treatment group were nausea, headache, dizziness and somnolence. Of these, nausea was the only TEAE with a higher incidence in the vortioxetine group (16.2%) than in the agomelatine group (9.1%). For the remaining TEAEs, the incidence was lower in the vortioxetine group compared with the agomelatine group; headache (10.3% vs 13.2%), dizziness (7.1% vs 11.6%) and somnolence (4.0% vs 7.9%). The incidence of sleep-related TEAEs (insomnia, somnolence, initial insomnia, middle insomnia, terminal insomnia and sleep disorder) was 11.1% (n = 28) in the vortioxetine group and 10.7% (n = 26) in the agomelatine group. The incidence of TEAEs related to sexual dysfunction was 0.4% (n = 1) (vortioxetine) and 0% (agomelatine).
Serious AEs were reported by seven patients, three in the vortioxetine group and four in the agomelatine group. No SAE was reported by more than one patient. Three patients in the vortioxetine group and one patient in the agomelatine group withdrew because of an SAE. For the patient treated with agomelatine, the SAE leading to withdrawal was related to the liver with an ALT >3 times ULN. No patients had SAEs related to suicidal behaviour or self-harm. No deaths occurred during this study.
During the study, none of the patients had suicidal behaviour, and self-injurious ideation was reported by no vortioxetine patients and one agomelatine patient. An improvement from baseline in the scores for MADRS item 10 (suicidal thoughts) was seen in both treatment groups, with a significantly greater effect for vortioxetine at weeks 4 onwards (p < 0.05).
Except for one agomelatine patient with an abnormal liver function test, no clinically relevant changes over time or differences between treatment groups were seen in clinical laboratory test results, vital signs, weight or ECG parameters.
DISCUSSION
Almost all clinical guidelines recommend that following an unsuccessful adequate length single course of treatment with one or other SSRI or SNRI, treatment with an antidepressant class with a different mechanism of action should be tried (Bauer et al., 2007; NICE, 2009; APA, 2010). This advice to switch to an antidepressant class with a different mechanism of action following an inadequate response to treatment is based on theoretical pharmacological considerations and the assumption that a different mechanism of action would have a better chance of success. However, the question of which different mechanism of action has the better chance of achieving response or remission has not been adequately tested. The present study of patients with an unsatisfactory response to a representative range of SSRIs and SNRIs who were randomly switched to treatment with one of two antidepressants with different mechanisms of action directly tests these assumptions.
In the present study, vortioxetine is significantly superior to agomelatine in the treatment of inadequate responders to a single adequate course of treatment with SSRIs or SNRIs. The robustness of this finding is shown by the consistent demonstration of superiority of vortioxetine on the secondary efficacy analyses and by the significant findings on the overall functioning and health-related quality of life measures. This is the first prospective randomised double-blind study to find an advantage of one class of antidepressants over another in inadequate responders and provides for the first time sound evidence that informs about the management of inadequate response.
The clinical relevance of the significant advantage of vortioxetine is demonstrated by a number of measures. The difference in treatment effect on the MADRS total score of −2.2 MADRS points (MMRM) compared with agomelatine is as large as the difference from placebo reported with a range of different antidepressants in the pivotal placebo-controlled studies (Kirsch et al., 2002; Melander et al., 2008). A difference of 2 points or more therefore meets criteria used to judge clinical relevance (Montgomery and Möller, 2009). The difference was reflected in the high number of individual items on the MADRS that showed a significant difference. The advantage of vortioxetine is also observed in the response and remission analyses where both outcome measures showed a significant and clinically relevant advantage compared with agomelatine. In the responder analysis, a difference between the two antidepressant in response rates at week 8 of 14.2 (MADRS) and 15.7 (CGI-I) percentage points was seen. This is similar to the level often used as a criterion of clinical relevance of antidepressant effect compared with placebo (Melander et al., 2008; Broich, on behalf of the CHMP, 2009; Montgomery and Möller, 2009). Remission is not common in short-term studies, because it often occurs beyond the study period. The 12-week period of treatment allowed 69.8% of patients to respond and 55.2% to remit (on the basis fo the MADRS) following a change of treatment to vortioxetine.
The significant advantage of vortioxetine over agomelatine seen on all of the many functional measures in the study reaffirms the clinical importance of the finding. The SDS self-report and its three subscales (family, work and social life) all demonstrated a clear and significant advantage. The 2.2-point difference observed on the SDS total score is greater than the 2-point difference to placebo reported by Sheehan and Sheehan (2008) to be clinically relevant. The consistent significant advantage in this study of vortioxetine over agomelatine on the EuroQol Qualify of Life Scale (EQ-5D), the WLQ and the DFFS all attest to the functional advantage of using vortioxetine as treatment following unsatisfactory response to SSRIs or SNRIs.
The present study was long enough (12 weeks) to allow patients with a slow response to respond to treatment. However, the analysis showed that the significant difference in favour of vortioxetine was seen early and that the magnitude of the significant difference seen at 3 or 4 weeks was similar at 8 and 12 weeks, suggesting that the early advantage of vortioxetine was sustained. The data show that depressive symptomatology improves at least until week 12 and also suggest that useful information concerning treatment may be obtained in studies as short as 4 weeks.
The patients represented a population of inadequate responders with moderate to severe MDD with a substantial level of anxiety symptoms. The significant advantage of vortioxetine on the HAM-A suggests a potential advantage in those with comorbid anxiety. This is also supported by the finding that vortioxetine was statistically significantly (p < 0.05) better than agomelatine in reducing the MADRS single-item score of the symptom inner tension.
Stopping antidepressants abruptly has been shown in many cases to be associated with the development of discontinuation symptoms. An attempt was made to reduce these possible effects in the present study by suggesting to the investigators that they downtitrate the doses of the previous antidepressants to the lowest dose in the last week to reduce potential discontinuation effects in patients entering the study. Discontinuation symptoms are normally maximal in the first week and return to close to the placebo level by the second week (Montgomery et al., 2004; Baldwin et al., 2007). Any potential differences in the amelioration of the effects of discontinuation symptoms in depression or anxiety by vortioxetine and agomelatine should therefore be reflected in the scores on the MADRS and HAM-A in the first 2 weeks. An examination of the data in this study shows that there is no perceptible difference in the scores of MADRS or HAM-A between the two antidepressants in the first 2 weeks and that the advantage in differential efficacy is only observed in the third or fourth week. This suggests that discontinuation symptoms on stopping the previous antidepressant, which may be mistaken for depressive or anxiety symptoms, did not appear to contribute to the clinical advantage of vortioxetine over agomelatine seen in the present study.
Vortioxetine separated significantly from agomelatine on most measured parameters in this population. It may well be that in addition to the serotonin system, the diverse downstream effects of vortioxetine on neurotransmitter systems [norepinephrine, dopamine, histamine, acetylcholine, γ-aminobutyric acid and glutamate (Mørk et al., 2013)] provide a particular benefit in a patient population with inadequate response to SSRIs and SNRIs. In favour of this, interpretation are data from an animal model of low serotonin levels, in which vortioxetine is able to reverse depression-like phenotypes, whereas escitalopram and duloxetine are inactive (du Jardin et al., 2014).
In the light of the present finding, the advice in the clinical guidelines of using any different class of antidepressant with a different mechanism of action as switch strategy could be considered to be updated, because it appears that some mechanisms with different classes of antidepressants are more effective than others. It would be better to use the evidence base to specify which antidepressants have been shown in large well-conducted studies to be more effective as treatment of patients with an inadequate response. The present result from a substantial study is in contrast to the limited data available from published randomised double-blind studies (Nolen et al., 1988, 1993; Thase et al., 2002; Lenox-Smith and Jiang, 2008) in which a comparison of monotherapy strategies in MDD patients who were unresponsive to first-line treatment with an antidepressant either showed no significant difference in efficacy between treatments (Nolen et al., 1988, 1993; Thase et al., 2002) or a significant difference only in a subset of more severely depressed patients (Lenox-Smith and Jiang, 2008).
The efficacy of vortioxetine compared with agomelatine was established here under conditions of fair comparison. The selected population of patients with moderate to severe MDD does not compromise agomelatine, which is more efficacious in severe depression (Montgomery and Kasper, 2007).
Furthermore the relevant population of inadequate responders was ensured by including patients nonpartially or partially responsive to a single treatment course of one of six commonly prescribed SSRIs/SNRIs optimised with respect to duration and dose. The patients also wished to change their current treatment and were considered by the investigators to be candidates for a switch. A direct switch was chosen to reflect normal clinical practice.
Agomelatine has a good tolerability profile with a low risk of sexual dysfunction and sleep disturbances (Lemoine et al., 2007; Kennedy et al., 2008), which is often observed during antidepressant treatment (Nierenberg et al., 1994; Yang et al., 2010). In the present study, the incidences of both treatment-emergent sexual dysfunction and sleep-related symptoms were equally low for vortioxetine and agomelatine. The withdrawal rate because of TEAEs associated with dose increases before week 5, indicating possible tolerability issues, was low in both treatment groups.
Limitations
The present study is a direct comparison of two effective antidepressants in inadequate responders to SSRI or SNRI treatment. The absence of placebo in the comparison makes it difficult to know whether the less effective treatment, agomelatine, was efficacious. The efficacy of vortioxetine by contrast is established because it was more effective than agomelatine under conditions of fair comparison, which is generally accepted as least as good if not better than placebo-controlled efficacy data.
The influence of possible discontinuation symptoms on efficacy is another possible limitation. The attempt by investigators to minimise this by downtitrating the dose of the previous antidepressant might well have minimised this problem. The failure to detect any difference in efficacy between the two treatments in the first 1 or 2 weeks when discontinuation symptoms are likely to be highest suggests that these symptoms did not influence the result.
The study population of unsatisfactory response to one of a range of commonly used SSRIs or SNRIs, representing their approximate proportion of use in the general population (Wu et al., 2009), allows the results to be generalised to both SSRIs and SNRIs. However, the failure to include other classes of antidepressants means that the results may not be generalised confidently to other classes of antidepressants apart from the tricyclic antidepressants, which have a similar mechanism of action.
The exclusion of comorbidity with other disorders (with the exception of GAD and SAD), which was necessary to allow confidence in addressing the specific question of the treatment of MDD, has had the effect of limiting the population studied to those with relatively pure depression, as in the placebo-controlled studies. Consequently, the results cannot necessarily be generalised to a population of MDD with high comorbidity. The significant advantage of vortioxetine on the HAM-A, however, suggests a potential advantage in those with comorbid anxiety, especially GAD and SAD. The limitation of excluding those at risk of suicide, those younger than 18 years, pregnant women and those who are excluded by regulatory restrictions, means that results cannot be confidently generalised to these groups.
CONCLUSION
This study appears to be the first large double-blind randomised study to show a statistically and clinically relevant difference in efficacy, as shown in response, remission and function, between two antidepressants in patients with MDD who had previously failed to show an adequate response of their current depressive episode after treatment with a representative range of SSRIs and SNRIs. Vortioxetine was significantly more effective than agomelatine on the primary measure at 4 weeks onwards, an advantage that is supported by significant differences on all the secondary efficacy measures and on all the functional and quality of life measures.
CONFLICT OF INTEREST
S. A. M. has received honoraria and/or has participated in advisory boards on behalf of Alkermes, AstraZeneca, Grunenthal, Johnson & Johnson, Lundbeck, Merck, Merz, M's Science Corporation, Otsuka Pharmaceuticals, Pierre Fabre Pharmaceuticals, Pfizer, PharmaNeuroBoost, Richter, Roche, Servier, Synosis, Takeda, Theracos, Targacept, Transcept and Xytis. L. H. has received grant funding, as well as honoraria and consultancy fees from Lundbeck, and has lectured at meetings sponsored by several companies that manufacture antidepressants and mood stabilisers. R. Z. N. and L. H. P. are employees of Lundbeck.
Acknowledgments
H. Lundbeck A/S sponsored the study and was involved in the study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication. The authors would like to thank all patients for their participation in the study. The authors gratefully acknowledge the participation of the patients and the following investigators in the psychiatric sites in the trial: Austria: Margot Schmitz, Belgium: Stefaan Geerts and Lieven F. E. de Weirdt, Bulgaria: Loris Sayan, Dora Atanasova, Luchezar Hranov, Rinaldo Shishkov and Diana Shkodrova, Czech Republic: Erik Herman, Michaela Klabusayova, Jaroslav Lestina, Silvia Musilova, Tibor Miklos, Zdenek Solle, Bronislav Kobeda, Simona Papezova and Jan Holan, Estonia: Anu Arold, Peeter Laane, Ants Puusild, Innar Töru and Ellen Grüntal-Oja, Germany: Cornelia Drubig, Dagmar Koethe, Klaus Sallach and Jana Thomsen, Italy: Carlo Altamura and Filippo Bogetto, Lithuania: Virginija Adomaitiene, Gintautas Daubaras, Daiva Deltuviene, Algirdas Narinkevicius, Rima Samusyte and Robertas Bunevicius, Poland: Hanna Badzio-Jagiello, Leszek Bidzan, Przemyslaw Bogacki, Mieczyslaw Janiszewski, Hanna Karakula, Dariusz Malicki, Tomasz Mysliwiec and Jaroslaw Strzelec, Romania: George Mihai Badescu, Irina Dan, Maria Ladea, Mirela Manea, Delia Marina Podea and Elena Tocari, Russia: Alyona Sidenkova, Elena Zagoruyko, Alexey Agarkov, Julia Barylnik, Mikhail Burdukovskiy, Natalia Dobrovolskaya, Liubov Gluskina, Andrey Gribanov, Alexandr Kotsubinkyi, Vitaly Tadtaev, Sergey Zolotarev and Anna Vasileva, Spain: Ricard Gandia, Diego Palao, Francisco Quintero-Gutierrez and Rosa Villanueva, Sweden: Peter Bosson, Maj-Liz Persson and Angela Marré-Lippitz, UK: Mark Dale and Chris McWilliam. The authors thank D. J. Simpson (H. Lundbeck A/S) for providing support in the preparation, revision and editing of the manuscript.
REFERENCES
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active-reference study of Lu AA21004 in patients with major depressive disorder (MDD) Int J Neuropsychopharmacol. 2012;15:589–600. doi: 10.1017/S1461145711001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 2000. 4th edn Text Revision (DSM-IV-TR). [Google Scholar]
- American Psychiatric Association (APA) Practice guidelines for the treatment of patients with MDD. 2010. 3rd edn.
- Areberg J, Luntang-Jensen M, Søgaard B, Nilausen DO. Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol. 2012;110:401–404. doi: 10.1111/j.1742-7843.2011.00810.x. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10:73–84. doi: 10.1017/S1461145705006358. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD) Eur Neuropsychopharmacol. 2012;22:482–491. doi: 10.1016/j.euroneuro.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Bang-Andersen B, Ruhland T, Jørgensen M, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54:3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bschor T, Pfennig A, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World J Biol Psychiatry. 2007;8:67–104. doi: 10.1080/15622970701227829. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26:1408–1416. doi: 10.1177/0269881112441866. [DOI] [PubMed] [Google Scholar]
- Boulenger J-P, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29:138–149. doi: 10.1097/YIC.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broich K Committee for Medicinal Products for Human Use. Committee for Medicinal Products for Human Use (CHMP) assessment on efficacy of antidepressants. Eur Neuropsychopharmacol. 2009;19:305–308. doi: 10.1016/j.euroneuro.2009.01.012. [DOI] [PubMed] [Google Scholar]
- CPMP (Committee for Proprietary Medicinal Products) Points to consider on switching between superiority and non-inferiority. 2000. Jul, Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003658.pdf. Accessed December 12, 2013. [DOI] [PMC free article] [PubMed]
- DiBenedetti DB, Danchenko N, François C, Lewis S, Davis KH, Fehnel SE. Development of a family functioning scale for major depressive disorder. Curr Med Res Opin. 2012;28:303–313. doi: 10.1185/03007995.2012.658910. [DOI] [PubMed] [Google Scholar]
- Guy W. Clinical Global Impressions. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. pp. 217–222. Revised ed. [Google Scholar]
- Hale A, Corral RM, Mencacci C, Ruiz JS, Severo CA, Gentil V. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double-blind study. Int Clin Psychopharmacol. 2010;25:305–314. doi: 10.1097/YIC.0b013e32833a86aa. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Henigsberg N, Mahableshwarkar A, Jacobsen P, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73:953–959. doi: 10.4088/JCP.11m07470. [DOI] [PubMed] [Google Scholar]
- ICH Harmonised Tripartite Guideline E6. Guideline for good clinical practice. 1996. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073122.pdf. Accessed December 12, 2013.
- Jacobsen PL, Mahableshwarkar AR, Serenko M, Chan S, Trivedi M. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. San Francisco, CA USA: Poster NR9-06 presented at the 166th Annual Meeting of the APA; 2013. Abstract available at http://www.psychiatry.org/learn/library--archives. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Jensen JB, Sanchez C, Pehrson AL. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur Neuropsychopharmacol. 2014;24:160–171. doi: 10.1016/j.euroneuro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Kasper S, Hajak G, Wulff K, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71:109–120. doi: 10.4088/JCP.09m05347blu. [DOI] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–223. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi S, Fulton K, Rasmussen J. A double-blind comparison of sexual functioning, antidepressant efficacy, and tolerability between agomelatine and venlafaxine XR. J Clin Psychopharmacol. 2008;28:329–333. doi: 10.1097/JCP.0b013e318172b48c. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor's new drugs. An analysis of antidepressant medication data submitted to the US Food and Drug Administration. Prevention Treatment 2002;5. 2002. art. 23. Available online at: http://psycnet.apa.org/?&fa=main.doiLanding&doi=10.1037/1522-3736.5.1.523a.
- Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials. Br J Psychiatry. 2013;203(3):179–187. doi: 10.1192/bjp.bp.112.120196. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224–231. [Google Scholar]
- Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. 2007;68:1723–1732. doi: 10.4088/jcp.v68n1112. [DOI] [PubMed] [Google Scholar]
- Lenox-Smith AJ, Jiang Q. Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor. Int Clin Psychopharmacol. 2008;23:113–119. doi: 10.1097/YIC.0b013e3282f424c2. [DOI] [PubMed] [Google Scholar]
- Lerner D, Amick BC, 3rd, Rogers WH, Malspeis S, Bungay K, Cynn D. The work limitations questionnaire. Med Care. 2001;39:72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar A, Jacobsen PL, Ml S, Chen Y, Trivedi M. A duloxetine-referenced fixed dose study comparing efficacy and safety of 2 vortioxetine doses in the acute treatment of adult patients with MDD. San Francisco, CA USA: Poster NR9-01 presented at the 166th Annual Meeting of the APA; 2013a. Abstract available at http://www.psychiatry.org/learn/library--archives. [Google Scholar]
- Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013b;29:217–226. doi: 10.1185/03007995.2012.761600. [DOI] [PubMed] [Google Scholar]
- Melander H, Salmonson T, Abadie E, van Zwieten-Boot B. A regulatory apologia—a review of placebo-controlled studies in regulatory submissions of new-generation antidepressants. Eur Neuropsychopharmacol. 2008;18:623–627. doi: 10.1016/j.euroneuro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Kasper S. Severe depression and antidepressants: focus on a pooled analysis of placebo-controlled studies on agomelatine. Int Clin Psychopharmacol. 2007;22:283–291. doi: 10.1097/YIC.0b013e3280c56b13. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24:111–118. doi: 10.1097/YIC.0b013e32832a8eb2. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int Clin Psychopharmacol. 2004;19:271–280. doi: 10.1097/01.yic.0000137184.64610.c8. [DOI] [PubMed] [Google Scholar]
- Mørk A, Montezinho LP, Miller S, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav. 2013;105:1–5. doi: 10.1016/j.pbb.2013.01.019. [DOI] [PubMed] [Google Scholar]
- NICE. (National Institute for Health and Clinical Excellence) Clinical Guideline 90 (CG90)—the treatment and management of depression in adults. 2009. Available at: http://guidance.nice.org.uk/CG90. Accessed December 12, 2013. [PubMed]
- Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M. Trazodone for antidepressant associated insomnia. Am J Psychiatry. 1994;151:1069–1072. doi: 10.1176/ajp.151.7.1069. [DOI] [PubMed] [Google Scholar]
- Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. I. Non-tricyclic and selective reuptake inhibitors in resistant depression: a double-blind partial crossover study on the effects of oxaprotiline and fluvoxamine. Acta Psychiatr Scand. 1988;78:668–675. doi: 10.1111/j.1600-0447.1988.tb06402.x. [DOI] [PubMed] [Google Scholar]
- Nolen WA, Haffmans PM, Bouvy PF, Duivenvoorden HJ. Monoamine oxidase inhibitors in resistant major depression. A double-blind comparison of brofaromine and tranylcypromine in patients resistant to tricyclic antidepressants. J Affect Disord. 1993;28:189–197. doi: 10.1016/0165-0327(93)90104-r. [DOI] [PubMed] [Google Scholar]
- Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the Discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23:70–83. doi: 10.1097/YIC.0b013e3282f2b4d6. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- Souery D, Amsterdam J, Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9:83–91. doi: 10.1016/s0924-977x(98)00004-2. [DOI] [PubMed] [Google Scholar]
- SPC (Summary of Product Characteristics) for agomelatine (Valdoxan) 2009. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000915/WC500046227.pdf. Accessed 12 December 2013.
- Stenkrona P, Halldin C, Lundberg J 5-HTT and 5-HT(1A) receptor occupancy of the novel substance vortioxetine (Lu AA21004) A PET study in control subjects. Eur Neuropsychopharmacol. 2013;23:1190–1198. doi: 10.1016/j.euroneuro.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. London: Wiley-Blackwell; 2012. [Google Scholar]
- Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888. doi: 10.1136/bmj.g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(Suppl 13):23–29. [PubMed] [Google Scholar]
- Thase ME, Rush AJ, Howland RH, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry. 2002;59:233–239. doi: 10.1001/archpsyc.59.3.233. [DOI] [PubMed] [Google Scholar]
- The EuroQoL Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Westrich L, Pehrson A, Zhong H, et al. In vitro and in vivo effects of the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets. Int J Psychiatry Clin Pract. 2012;16(Suppl 1):47. [Google Scholar]
- World Medical Association (WMA) [Homepage on the Internet] Declaration of Helsinki: Ethical principles for medical research involving human subjects; 2008. [Google Scholar]
- Wu EQ, Greenberg PE, Yang E, Yu AP, Ben-Hamadi R, Erder MH. Treatment persistence, healthcare utilization and costs in adult patients with major depressive disorder: a comparison between escitalopram and other SSRI/SNRIs. J Med Econ. 2009;12:124–135. doi: 10.3111/13696990903093537. [DOI] [PubMed] [Google Scholar]
- Yang H, Sinicropi-Yao L, Chuzi S, et al. Residual sleep disturbance and risk of relapse during the continuation/maintenance phase treatment of major depressive disorder with the selective serotonin reuptake inhibitor fluoxetine. Ann Gen Psychiatry. 2010;9:10. doi: 10.1186/1744-859X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


