Synopsis
Objective
In humans, the process of hair shedding, referred to as exogen, is believed to occur independently of the other hair cycle phases. Although the actual mechanisms involved in hair shedding are not fully known, it has been hypothesized that the processes leading to the final step of hair shedding may be driven by proteases and/or protease inhibitor activity. In this study, we investigated the presence of proteases and protease activity in naturally shed human hairs and assessed enzyme inhibition activity of test materials.
Methods
We measured enzyme activity using a fluorescence-based assay and protein localization by indirect immunohistochemistry (IHC). We also developed an ex vivo skin model for measuring the force required to pull hair fibres from skin.
Results
Our data demonstrate the presence of protease activity in the tissue material surrounding club roots. We also demonstrated the localization of specific serine protease protein expression in human hair follicle by IHC. These data provide evidence demonstrating the presence of proteases around the hair club roots, which may play a role during exogen. We further tested the hypothesis that a novel protease inhibitor system (combination of Trichogen® and climbazole) could inhibit protease activity in hair fibre club root extracts collected from a range of ethnic groups (UK, Brazil, China, first-generation Mexicans in the USA, Thailand and Turkey) in both males and females. Furthermore, we demonstrated that this combination is capable of increasing the force required to remove hair in an ex vivo skin model system.
Conclusion
These studies indicate the presence of proteolytic activity in the tissue surrounding the human hair club root and show that it is possible to inhibit this activity with a combination of Trichogen® and climbazole. This technology may have potential to reduce excessive hair shedding.
Résumé
Objectif
Chez l'homme, le processus de perte de cheveux, désigné comme exogène, est censé se produire indépendamment des autres phases du cycle de cheveux. Bien que les mécanismes réels impliqués dans la perte de cheveux ne soient pas entièrement connus, il a été émis l'hypothèse que les processus conduisant à l'étape finale de la perte de cheveux peuvent être modulés par des protéases et/ou l'activité d'inhibiteurs de protéase. Dans cette étude, nous avons étudié la présence de protéases et de l'activité des protéases dans les cheveux humains perdus naturellement et évalué l'activité inhibitrice d'enzyme de différents matériaux.
Méthodes
Nous avons mesuré l'activité enzymatique en utilisant un dosage basé sur la fluorescence et la localisation des protéines par immunohistochimie indirecte (IHC). Nous avons également développé un modèle de peau ex vivo pour mesurer la force nécessaire pour extraire les fibres capillaires de la peau.
Résultats
Nos données démontrent la présence d'une activité de la protéase dans le matériau de tissu entourant les racines du bulbe. Nous avons également démontré la localisation de l'expression des protéines de la sérine protéase spécifique du follicule pileux humain par IHC. Ces données fournissent des éléments de preuve démontrant la présence de protéases autour des racines du bulbe de cheveux qui peuvent jouer un rôle durant la phase exogène. Nous avons également testé l'hypothèse selon laquelle un nouveau système inhibiteur de protéase (combinaison de Trichogen ® et climbazole) pouvait inhiber l'activité de la protéase dans les extraits des bulbes de la racine des cheveux, recueillies à partir d'un éventail de groupes ethniques (Royaume-Uni, Brésil, Chine, 1ère génération Mexicains aux États-Unis, Thaïlande et Turquie) dans les deux sexes, mâles et les femelles. En outre, nous avons démontré que cette combinaison est capable d'augmenter la force nécessaire pour enlever les poils dans un système de modèle de peau ex vivo.
Conclusion
Ces études indiquent la présence d'une activité protéolytique dans le tissu entourant le bulbe de la racine des cheveux humains et montrent qu'il est possible d'inhiber cette activité avec une combinaison de Trichogen ® et climbazole. Cette technologie peut avoir le potentiel de réduire la perte excessive de cheveux.
Keywords: exogen, follicle, hair growth, hair loss, hair shedding, hair treatment, proteases
Introduction
The process of hair shedding, termed exogen, is believed to occur independently of the hair cycle [1] and is thought to be driven by enzymatic mechanisms [2, 3]. Unwanted hair loss is a common problem and is suffered by much of the world population in both women and men. The condition is characterized by premature hair loss and excessive hair shedding caused by an interruption in the normal hair growth cycle, which can lead to the development of baldness with insufficient replacement of hairs in human scalp. This can have negative effects on the individuals' self-esteem and thus affect their interpersonal relationships 4. Therefore, a better understanding of the factors underlying processes leading to hair shedding may provide insights into how to address the problem of early or excessive hair shedding.
The hair follicle is a regenerating biological system whose primary function is to produce a hair fibre. The hair growth cycle consists of phases of growth (anagen), regression (catagen) and rest (telogen), which occur continuously throughout the follicle lifetime. During catagen, the growing fibre produced in anagen becomes detached from the follicular matrix and is subsequently referred to as the ‘club fibre’. The club fibre eventually sheds from the follicle in a process termed exogen or teloptosis. Although the club fibre is moving up the follicle prior to release, the adjacent follicle continues to cycle and moves back into anagen. In animals, it is not unusual for a new cycle to start producing a growing hair, although the old club fibre is retained alongside in its epithelial silo. In mice, there can be two or three fibres within the same follicle, whereas only one is a growing fibre 5,6, so they always have a full coat of hair. The final timing of club fibre shedding, or exogen, appears to occur independently from the phases of the hair cycle 1,7,8.
The vast majority of hair biology research has focused on the hair growth cycle, and little attention has been devoted to studying the mechanisms involved in exogen. Although a number of mouse and human molecular and genetic studies have uncovered several pathways involved in hair follicle cycling, the molecular signal(s) for exogen remain elusive. The process of hair shedding has been proposed from two viewpoints: one suggesting that the process is a passive event caused by the physical force from the new growing fibre, which dislodges the club fibre 9, and a second suggesting that exogen is an active process controlled by signals, leading to the release of the club fibre 7,10. Club fibres are regarded to be in exogen from the moment of their complete formation (early exogen, with actively retained club fibres) through to their release (late exogen), with club fibre still within the follicle, but ready to be shed 11. In this study, our hair collection method was devised to try and collect human club fibres in late exogen.
Understanding the factors that control exogen may shed light on routes for intervention to reduce hair loss in humans. The club fibre is anchored within an outer root sheath (ORS), and this ORS can be genetically ablated resulting in loss of the club fibre from its silo 12. There is evidence showing a changing gradient of proteins and/or RNA expression of proteolytic enzymes and their respective inhibitors in the transition of early exogen to late exogen 3. In animals, it is thought that an exogen signal results in the release of an enzyme from its inhibitor, which in turn causes the release of the club hair fibre 7. Additionally, in humans, there is an evidence indicating the presence of specific enzymes in or surrounding the club fibre, for example transglutaminases 13, and enzyme inhibitors such as plasminogen activator inhibitor type 2 14 and tissue inhibitor of metalloprotease 3 3, which may have roles in retaining the club fibre. It is therefore reasonable to postulate that proteolytic mechanisms may be involved in the detachment of the club fibre by reducing anchorage to the follicle.
The base of the club fibre is comprised of trichilemmal keratin, which is believed to be important for anchoring the club fibre within the follicle 15. Between the trichilemmal keratin and the surrounding epithelial silo (or ‘trichilemmal sac’), there is an abundance of desmosomal complexes. Milner 7 observed cytoplasmic breakdown in the cells surrounding shed exogen club fibres and subsequently proposed that club fibre release was mediated through proteolytic degradation, indicating that proteolytic activity within the trichilemmal sac was a major activity contributing towards release of the club fibre. Desmosomes in the skin have been shown to play a role in cell cohesion in the stratum corneum and may also be involved in club fibre anchorage. Desmoglein 3 (Dsg3) is a desmosomal transmembrane glycoprotein 16,17 expressed and assembled into desmosomes in the basal and suprabasal keratinocytes 18. However, patients with pemphigus vulgaris (PV) or pemphigus foliaceus develop auto-antibodies against Dsg3 or Dsg1, respectively 19, which results in the loss of cell adhesion. Interestingly, patients with PV and also Dsg3 knockout mice (DSG3-/-) developed hair loss of club fibres, whereas anagen hairs remained firmly anchored 20. Inactivation of Dsg1 in DSG3-/- mice results in loss of anchorage of anagen hair follicles 21. In addition, a heterozygous non-sense mutation of the corneodesmosin gene has been associated with hypertrichosis simplex of the scalp, a form of alopecia 22. The retention mechanism of the club fibre during exogen could prove invaluable in identifying the therapeutic routes for stabilizing excessive hair shedding and lessen suffering and concern in hair loss.
Investigating disease types resulting in symptoms of hair loss can often provide clues on processes involved. Lympho-epithelial Kazal-type-related inhibitor (LEKTI) is a serine protease inhibitor whose defective expression is observed in a skin disease termed ‘Netherton syndrome’. It is encoded by serine protease inhibitor Kazal type 5 (SPINK5) 23 and is produced as a precursor that is cleaved by furin, generating a number of LEKTI fragments 24. Some of the secreted LEKTI fragments have shown specific or differential inhibition of human kallikreins (KLKs) 5, 7 and/or 14. Interestingly, hair shaft defects have been observed in patients with Netherton syndrome 25. In human hair follicles, KLKs are suggested to be important for hair growth and differentiation and trichilemmal keratinization 26,27.
Although the complexities of exogen and the events leading to exogen are little understood, reducing hair loss through targeting of processes involved in exogen may provide a means for alleviating symptoms of excessive or premature hair shedding. Because serine proteases have been shown to play a role in corneodesmosome degradation, a similar role may be played by proteases in degrading the bonds that anchor the club hair during the telogen or exogen phases of the human hair follicle cycle. In this study, we describe protein expression of specific proteases in the human hair follicle and the presence of protease activity in the material surrounding human exogen club fibres in a range of ethnic groups. Furthermore, as proteases have been implicated in exogen, we searched for a cosmetically acceptable active system that would be capable of inhibiting the protease activity found in the hair follicle with the potential to reduce hair shedding. This study reports the findings that a combination of Trichogen® and Climbazole is able to inhibit hair follicle proteases and that this phenomenon can be observed in a range of ethnic groups. Finally, we demonstrate that this combination is capable of increasing the force required to remove hair in an ex vivo skin model system.
Methodology
Hair collection protocol
A non-invasive protocol to collect exogen hairs from female subjects with normal hair growth and no indication of hair loss or scalp disorders was devised, and ethical approval was granted to collect shed hairs.
Study 1
Protease activity in club fibre roots from hair fibres at the point of being shed naturally, that is, those in late exogen. Shed hair fibres were collected from female subjects (n = 26) as described below.
Study 2
Protease inhibition in late exogen club fibre roots collected from subjects spanning a range of ethnic groups. Shed hairs were collected from adult male and female subjects in the UK (n = 30 females and n = 36 males), Brazil (n = 28 females and n = 32 males), China (n = 30 females and n = 29 males), first-generation Mexicans in the USA (Mexico/USA) (n = 21 females and n = 12 males), Thailand (n = 20 females and n = 30 males) and Turkey (n = 31 females and n = 32 males).
Subjects were asked to collect hairs on six occasions over a period of 3 weeks by running their fingers through the length of their hair for 10 min and collecting all those that fell out. They refrained from washing their hair during the 36- to 48-h period leading up to each hair sample collection, thus allowing a build-up of late exogen club fibres. Subjects were permitted to wash their hair after each hair sample collection.
Protein extraction of material surrounding telogen/exogen club fibre roots
Shed hairs from all subjects were pooled, and roots were clipped and collected into glass homogenizers. Negative control samples were collected by clipping respective hair fibres distal to the root end into adjacent homogenizers. Samples were homogenized in batches of approximately 400 in 400 μL extraction buffer (4 g NaCl, 0.1 g KCl, 1% Triton in 500 mL double distilled H2O). Homogenates were transferred into fresh tubes stored on ice (homogenates from root ends and clipped hairs were pooled in separate tubes) and then centrifuged at 10 500 g for 20 min at 4°C. The supernatants were collected, centrifuged at 10 500 g rpm for 5 min, transferred to fresh tubes, assayed for protein yield (Pierce BCA assay, Perbio, UK, as per manufacturer's instructions) and stored at −20°C for subsequent protease activity detection.
Protease activity determination
Protease activity in root extract (RE) samples or hair extract (HE) samples was assessed for the presence of protease (metallo-, serine and sulfhydryl protease) activity using the EnzCheck® Protease Assay kit as per the manufacturer's instructions (Invitrogen, UK). Root extract samples or hair extract samples were diluted 1 : 3 in 0.1 M NaCl prior to the analysis for protease activity. A range of pH concentrations was tested to determine optimum pH for measuring protease activity in the extracts (data not shown).
Protease inhibition assay
Protease inhibition was also determined using the EnzCheck® Protease Assay kit. Briefly, 200 μL reaction mixtures containing 10 μL test material [climbazole (Crinipan® AD, Symrise, France) or Trichogen® (Trichogen® VEG LS 8960, LS Cognis, France)], 40 μL proteolytic extract (RE), 100 μL BODIPY TR-X casein substrate (10 μg mL-1 working concentration) and 50 μL 50 m M Tris–HCl, pH 8.0, were set up in 96-well plates, covered in foil and incubated for 4 h at 37°C. After incubation, the fluorescence was read at excitation (589 nm)/emission (617 nm). A trypsin-only (5 μg mL−1) control reaction was also included.
Ex vivo pig skin hair follicle extraction model for assessing protease inhibitors
We also established an ex vivo model to measure the force required (in the presence or absence of protease inhibitors) to pluck hairs from pig skin. Pig skin contains a large percentage (up to 80%) of club fibres during the summer months 28. We hypothesized that an increase in the extraction force of club fibres following topical treatment with a protease inhibitor would indicate increased anchorage and therefore may delay shedding of early shed hairs in vivo. Waste skin from pigs (scheduled for disposal) was prepared by dipping in skin-sterilizing solution and rinsing in water. The subcutaneous tissue was separated from the connective tissue, while ensuring hair follicle bulbs remained intact without destruction. To determine whether potential protease inhibitors could increase the extraction force required for removing hair fibres from pig skin, sections of skin were topically applied with Trichogen®+climbazole (prepared in an aqueous leave-on formulation) for 8 h at room temperature. After the treatment period, the pig skin was mounted onto a Zwick Z005 displacement controlled tensile testing machine. Individual hair fibres (40–50) were extracted from each piece of pig skin using custom-made self-locking tweezers and the extraction force recorded. Only intact hair fibres extracted from pig skin were counted; hair fibres that snapped or broke during extraction were excluded.
Statistical analysis
All population data are expressed as mean ± standard deviation. The two-tailed t-test was used when appropriate to examine the statistical significance of the differences between groups of data. Significance level was set at P < 0.05.
Immunohistochemistry for detection of serine proteases
Human scalp skin biopsies were obtained from healthy adult volunteers following ethical approval. Formalin-fixed and paraffin-embedded sections (4 μM) were dewaxed and rehydrated through graded EtOH and rinsed in distilled water for 20 min. The Trilogy antigen retrieval system (Cell Marque) was used to expose the antigen epitopes. After rinsing, the sections were placed in the dark in 3% H2O2 for 10 min, rinsed in tap water and dipped twice in Tris-buffered saline (TBS) for 5 min. Sections were incubated with the KLK polyclonal antibodies (kindly provided by Prof Diamandis, University of Toronto, Canada) at the following dilutions: KLK5, 1 : 200; KLK7 1 : 600; and KLK14, 1 : 300 for 30 min after which they were rinsed with TBS for 10 min. The sections were then incubated with the EnVision™ Detection System, peroxidase/diaminobenzidine rabbit/mouse (Dako, Denmark) for 30 min. After rinsing in TBS, sections were incubated in diaminobenzidine solution for 10 min and rinsed with tap water. The sections were counterstained with haematoxylin, dehydrated, cleared in xylene and mounted. Negative controls were performed by omitting the primary antibody and by replacing it using non-immune serum (dilution 1 : 500).
Results
Shed hair collection data
Human scalp hair follicles cycle independently of one another in a mosaic pattern and at any given time approximately 85–90% of scalp hairs are in the anagen (growing phase), whereas 10–15% are in the telogen (resting phase). On average, anagen lasts ∼3 years with a range of 1–7 years, and telogen lasts 2–4 months after which the club hairs are shed from the scalp. The scalp typically has approximately 80 000-150 000 hairs, and an average of ∼100 hairs can be shed per day. The average number of hairs collected by individuals per 10-min period was determined (data not shown). The data suggest that the number of shed hairs from a cohort of normal healthy adult volunteers can be highly variable. The minimum number of hairs collected was 9- per 10-min period, a maximum of 160 and an average of 48. A total of 15 subjects shed up to 50 hairs, 10 subjects shed between 50 and 100 hairs, whereas only one subject shed over 100 hairs per collection. This is lower than the average as described in the literature; however, it is worth noting that these numbers do not include a larger proportion of hairs, which are lost during washing of the scalp or during the natural course of the day.
Subsequent hair collection studies have demonstrated that a significant proportion of hairs collected, using our gentle grooming protocol, are lost from the root, and a smaller proportion are broken hairs 29. This suggests that our protocol is suitable for collecting scalp hairs on the verge of being shed from the scalp where protease activity can be hypothesized to be at its peak.
Protease activity in club root extracts
To ensure sufficient protein yield for subsequent protease activity assessment, clipped roots from subjects were pooled for protein extraction. Respective control clipped hairs were also pooled in a similar manner. Exogen has been postulated to be regulated by a combination of proteases rather than a specific enzyme 3, and the root and hair extracts were likely to contain more than one protease. The data demonstrate the presence of metallo-, serine or sulfhydryl-like protease activity in root extracts (Fig.1). The relative activity observed in club root extracts was approximately 5× greater than that detected in hair fibres distal to the root. This suggests that the material surrounding the root contains enhanced protease activity. Whether these proteases play a role in the detachment of the maturing club root either, preceding, during or at end of exogen remains to be established. Furthermore, the role of protease inhibitors also merits investigation because a balance of proteases and respective protease inhibitors may well be altered as the hair follicle progresses during the hair cycle and then into exogen until it is finally shed from the scalp.
Figure 1.
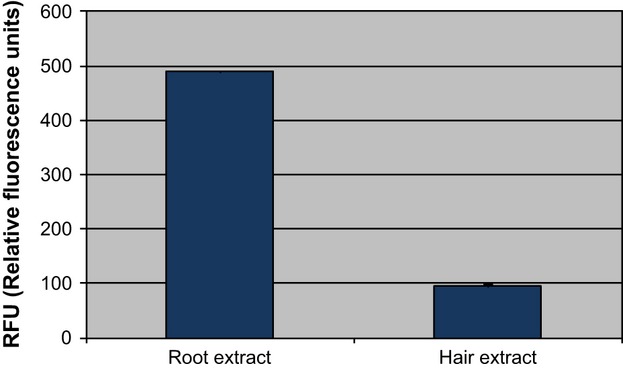
Protease activity detected in material surrounding club root extracts and clipped hairs. Relative fluorescence units (RFU) were determined to be proportional to protease activity. Higher protease activity was found in material surrounding club root extracts compared with hair fibres.
Detection of serine proteases in human hair follicles using immunohistochemistry
The data from the enzyme assays suggested the presence of protease activity in material surrounding the late exogen club fibre roots. Although at this stage it is unclear as to the role of proteases in this region, we wished to determine whether antigens to serine proteases (specifically KLK5, KLK7 and KLK14) were present in/around the telogen hair follicle. KLK5 and KLK7 are serine proteases, which belong to the kallikrein family and play a role of corneodesmosome degradation of corneocytes during skin desquamation 30,31. KLK5, KLK7 and KLK14 have all been postulated to participate in the proteolytic cascade in skin 32. Various studies have demonstrated the presence of protein and mRNA expression of kallikreins in hair follicles 26,33.
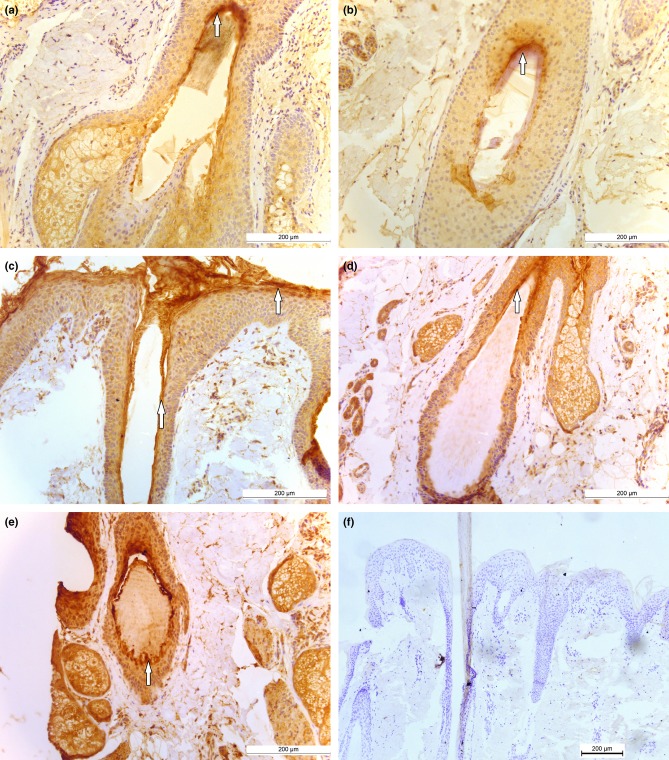
Using immunohistochemistry, we found expression of KLK5, KLK7 and KLK14 in human scalp telogen club hairs (Fig.2). Telogen club hairs can be discriminated when the lower section of the hair follicle is examined for the presence of trichilemmal club (data not shown). Figure2a shows staining for KLK5 in telogen club at the level of the isthmus. In Fig.2b, punctate staining for KLK5 is seen to be most prominent in the innermost layer of the outer root sheath, which has been defined as the companion layer. The staining for KLK7 could be detected in the cornified layers of the infundibulum, and staining was also observed at the skin surface (Fig.2c), in line with that shown by Ekhholm and Egelrud (33). Interestingly, intense staining for KLK7 was also seen in cells adjacent to the trichilemma (Fig.2d). Staining for KLK14 appeared to be most prominent in a layer of cells adjacent to the trichilemma of the telogen club (Fig.2e). These early findings on the expression patterns of three KLKs in telogen clubs suggest that they may play a role in hair follicle biology during telogen. Various proteases including serine, cysteine and aspartic proteases have been identified in the stratum corneum, and it has been suggested that they play a role in desquamation 34,35. It is possible that serine proteases play a role similar to desquamation in skin in releasing the club hair from the follicle, but this remains to be established.
Figure 2.
(a) KLK5 staining at the level of the isthmus. (b) KLK5 staining around the mid-upper level of the hair follicle. Arrows show staining in the innermost layer of the outer root sheath. (c) KLK7 was observed in the cornified layers of the infundibulum and extended to the skin surface at which point the staining appeared more intense. (d) KLK7 staining at the level of the isthmus. Here, the staining appeared highest in the cells adjacent to the trichilemma. KLK14 staining around the telogen club (e) and in the hair follicle. Negative control section counterstained with haematoxylin (f).
Effect of potential protease inhibitors on protease activity in vitro
The effect of Trichogen®, climbazole and a combination of Trichogen®+climbazole was investigated for their ability to inhibit serine-like protease activity in root extracts using trypsin as the model serine protease. Climbazole (0.05%) alone had no effect on trypsin activity (Fig.3). Trichogen® alone at all concentrations tested and in combination with climbazole (0.05%) was capable of inhibiting trypsin activity. Both Trichogen® and Trichogen®+climbazole demonstrated a statistically significant inhibition in trypsin activity. The effect of combining climbazole with Trichogen® resulted in a greater inhibition of protease activity compared with Trichogen® alone and greater still compared with climbazole alone, which showed no inhibitory activity. These data suggest that combining Trichogen® with climbazole results in a synergistic protease inhibition effect.
Figure 3.
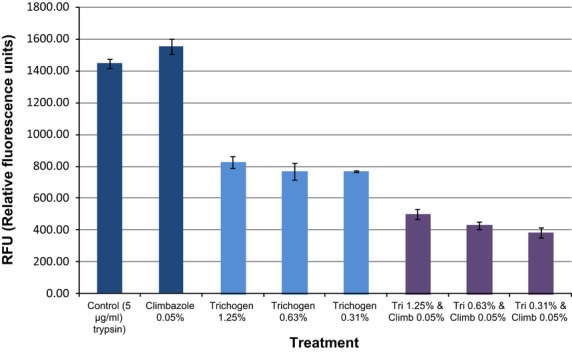
Inhibition of trypsin activity by climbazole, Trichogen® and a combination of Trichogen® + climbazole.
The data presented in Fig.3 represent a demonstration of in vitro inhibition of a model serine protease. Our next aim was to determine whether Trichogen®+climbazole was capable of inhibiting root protease activity isolated from the material surrounding the telogen/exogen club fibre roots from subjects with a range of ethnic backgrounds/geographical regions (see Table1). Firstly, the data verify the presence of proteolytic activity in human telogen/exogen club fibre roots in all of the different ethnic groups examined. Additionally, the combination of Trichogen®+Climbazole was able to inhibit proteolytic activity in the telogen/exogen club fibre root extracts in all groups. The magnitude of the inhibition ranged from 32.2% (UK females) to 57.3% (Mexico/USA females). These studies clearly demonstrate the inhibitory properties of the Trichogen®+Climbazole combination ex vivo.
Table 1.
Inhibition of protease activity using 10 : 1 ratio of Trichogen® (0.3%)/climbazole (0.03%) in scalp telogen/exogen club hair fibre root extracts collected from subjects of differing ethnic background/geographical regions
| Country | Gender | Protease activity (RFU) | % Inhibition | |
|---|---|---|---|---|
| Root extract only | Root extract + Trichogen® + climbazole | |||
| UK | Male | 226 | 124 | 45.1 |
| Female | 205 | 139 | 32.2 | |
| Brazil | Male | 233 | 109 | 53.2 |
| Female | 231 | 105 | 54.5 | |
| China | Male | 183 | 103 | 43.7 |
| Female | 187 | 103 | 44.9 | |
| Mexico/USA | Male | 226 | 100 | 55.8 |
| Female | 253 | 108 | 57.3 | |
| Thailand | Male | 238 | 118 | 50.4 |
| Female | 228 | 128 | 43.9 | |
| Turkey | Male | 291 | 170 | 41.6 |
| Female | 243 | 140 | 42.4 | |
Effect of a cosmetic system on hair fibre extraction force in a pig skin model
To ascertain the potential benefit of protease inhibition in vivo, we developed an ex vivo hair fibre extraction force model using pig skin. This model was used to determine the correlation between the force required to remove/pluck hair from skin and protease activity. Using this model, we investigated whether the force required to remove hairs from pig skin was increased following treatment with a protease inhibitor. Crucially, we observed that after treatment with the Trichogen®+Climbazole, club fibres were actively retained (Table2). We found that the force required to remove hair club fibres was significantly (P = 0.02) enhanced using Trichogen®+Climbazole compared with vehicle. Although, morphology of the plucked hair fibres was not assessed in this study, previous studies have demonstrated that up to 80% of the hairs in pig skin pig are in telogen 28, and we have therefore assumed that the hairs we plucked were club fibres.
Table 2.
Average plucking force (40 HFs per treatment; n = 3 experiments) required to pluck hairs from pig skin following treatment with a 10 : 1 ratio of Trichogen® (5%)/climbazole (0.5%) or vehicle (50% EtOH). Pairwise comparisons of the treatments averaged over the 4 days gave a significant difference between the test material and vehicle, P = 0.02
| Time | 24 h | 48 h | 72 h | 96 h | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Vehicle | Test* | Vehicle | Test* | Vehicle | Test* | Vehicle | Test* |
| Mean pucking force (newtons) | 1.28 | 1.31 | 1.07 | 1.32 | 1.04 | 1.24 | 0.94 | 1.20 |
| SD | 0.61 | 0.29 | 0.26 | 0.35 | 0.21 | 0.31 | 0.25 | 0.30 |
5% Trichogen + 0.5% climbazole.
P = 0.02 (pairwise comparisons of the treatments (test vs. vehicle) averaged over 4 days).
Discussion
Evidence from animal studies has indicated a potential role for proteases in hair shaft anchorage. Further studies have demonstrated the presence of proteases and protease inhibitors in human anagen and telogen hair follicles. We postulated that serine proteases may have a role in mediating exogen in humans and therefore investigated the activity and expression of specific serine proteases surrounding human club fibres. Our data indicate that protease activity can be detected in the material surrounding club hairs, and protein expression can be observed around club hairs. Because serine proteases have been shown to play a role in corneodesmosome degradation, a similar role may be played by proteases in degrading the bonds that anchor the club hair during the exogen phase of human hair follicle.
We hypothesized that materials with anti-protease activity may inhibit the proteases, present in the club root of the human hair, that have been implicated in hair shedding (exogen). Trichogen® is a mixture of ingredients including zinc gluconate, and zinc salts are known to inhibit protease activity 36. Interestingly, we found that Trichogen® could not only inhibit trypsin activity. Climbazole is an imidazole anti-fungal agent commonly used to treat dandruff, but has no known protease inhibition activity 37. However, we sought to combine a protease inhibition agent with an anti-dandruff agent and surprisingly found that trypsin activity was further reduced with the addition of climbazole compared with Trichogen® alone. In addition, the combination of Trichogen®+climbazole was capable of inhibiting the proteases present in club root extracts from ethnic and geographical groups across the world. This finding indicates that this combination of ingredients may be able to provide a global solution to reduce excessive hair fall.
Furthermore, we developed in ex vivo model (using pig skin) for measuring force required to remove club hairs from skin. Consequently, we were able to demonstrate that a combination of Trichogen®+climbazole can increase the extraction force required to remove club hairs from the pig skin. This advance demonstrates that we were successfully able to translate our in vitro studies to an ex vivo skin model. Utilizing a Trichogen®+climbazole in cosmetic applications may serve to increase the retention strength of club hairs and therefore reduce unwanted club hair shedding to promote a fuller appearance of hair on the scalp.
Summary
Although little focus has been placed on investigating the pathways and signals involved in exogen compared with other phases of the follicle, further understanding of the human exogen phase may provide novel routes for attenuating excessive hair loss due to pathways involved in incorrect anchorage. Alternatively, it may also allude to novel therapeutic interventions to boost the mechanisms of exogen and club fibre shedding for alleviating unwanted hair. Finally, we also developed an ex vivo model for measuring the force required to remove hairs, which can be exploited for measuring the anchorage strength of hair fibres.
Acknowledgments
We would like to thank the following experts for their respective contributions to the above research: Prof E. P. Diamandis (University of Toronto, Canada), Dr C. Petraki (Evangelismos Hospital, Greece) and Dr G. E. Westgate (Westgate Consultancy, UK). We would also like to thank Ning Chang (Unilever R&D Shanghai) and Ravine Gungabissoon (Unilever R&D Colworth), Dr F. Baines (Unilever R&D Port Sunlight), S. Paterson and Dr J. Matheson (Unilever R&D Port Sunlight). The work described in this manuscript was funded by Unilever plc.
References
- 1.Stenn KS, Parimoo S, Prouty SM. Growth of the hair follicle: a cycling and regenerating biological system. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis (Molecular Biology Intelligence Unit 1) Austin, TX: RG Landes Company; 1998. pp. 111–130. [Google Scholar]
- 2.Tobin DJ, Foitzik K, Reinheckel T, Mecklenburg L, Botchkarev VA, Peters C, Paus R. The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am. J. Pathol. 2002;160:1807–1821. doi: 10.1016/S0002-9440(10)61127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins CA, Westgate GE, Jahoda CA. Modulation in proteolytic activity is identified as a hallmark of exogen by transcriptional profiling of hair follicles. J. Invest. Dermatol. 2011;131:2349–2357. doi: 10.1038/jid.2011.227. [DOI] [PubMed] [Google Scholar]
- 4.Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J. Am. Acad. Dermatol. 1993;29:568–575. doi: 10.1016/0190-9622(93)70223-g. [DOI] [PubMed] [Google Scholar]
- 5.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol. Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 6.Dry F. The coat of a mouse (Mus musculus. J. Genet. 1926;16:281–340. [Google Scholar]
- 7.Milner Y, Sudnik J, Filippi M, Kizoulis M, Kashgarian M, Stenn K. Exogen, shedding phase of the hair growth cycle: characterization of a mouse model. J. Invest. Dermatol. 2002;119:639–644. doi: 10.1046/j.1523-1747.2002.01842.x. [DOI] [PubMed] [Google Scholar]
- 8.Stenn K. Exogen is an active, separately controlled phase of the hair growth cycle. J. Am. Acad. Dermatol. 2005;52:374–375. doi: 10.1016/j.jaad.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Kligman AM. Pathologic dynamics of human hair loss I. Telogen effluvium. Arch. Dermatol. 1961;83:175–198. doi: 10.1001/archderm.1961.01580080005001. [DOI] [PubMed] [Google Scholar]
- 10.Higgins CA, Westgate GE, Jahoda CA. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J. Invest. Dermatol. 2009;129:2100–2108. doi: 10.1038/jid.2009.66. [DOI] [PubMed] [Google Scholar]
- 11.Higgins CA, Richardson GD, Westgate GE, Jahoda CA. Exogen involves gradual release of the hair club fibre in the vibrissa follicle model. Exp. Dermatol. 2009;18:793–795. doi: 10.1111/j.1600-0625.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoneda K, Akiyama M, Morita K, Shimizu H, Imamura S, Kim SY. Expression of transglutaminase 1 in human hair follicles, sebaceous glands and sweat glands. Br. J. Dermatol. 1998;138:37–44. doi: 10.1046/j.1365-2133.1998.02024.x. [DOI] [PubMed] [Google Scholar]
- 14.Lavker RM, Risse B, Brown H, Ginsburg D, Pearson J, Baker MS, Jensen PJ. Localization of plasminogen activator inhibitor type 2 (PAI-2) in hair and nail: implications for terminal differentiation. J. Invest. Dermatol. 1998;110:917–922. doi: 10.1046/j.1523-1747.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 15.Roth W, Deussing J, Botchkarev VA, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 2000;14:2075–2086. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- 16.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 17.Koch PJ, Franke WW. Desmosomal cadherins - another growing multigene family of adhesion molecules. Curr. Opin. Cell Biol. 1994;6:682–687. doi: 10.1016/0955-0674(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 18.Amagai M, Koch PJ, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (Desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J. Invest. Dermatol. 1996;106:351–355. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- 19.Stanley J. Cell-Adhesion Molecules As Targets of Autoantibodies in Pemphigus and Pemphigoid, Bullous Diseases Due to Defective Epidermal-Cell Adhesion. In: Dixon FJ, editor. Advances in Immunology. Vol. 53. Cambridge, Massachusetts: Academic Press; 1993. pp. 291–325. [DOI] [PubMed] [Google Scholar]
- 20.Koch PJ, Mahoney MG, Cotsarelis G, Rothenberger K, Lavker RM, Stanley JR. Desmoglein 3 anchors telogen hair in the follicle. J. Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]
- 21.Hanakawa Y, Li H, Lin CY, Stanley JR, Cotsarelis G. Desmogleins 1 and 3 in the companion layer anchor mouse anagen hair to the follicle. J. Invest. Dermatol. 2004;123:817–822. doi: 10.1111/j.0022-202X.2004.23479.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy-Nissenbaum E, Betz RC, Frydman M, et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat. Genet. 2003;34:151–153. doi: 10.1038/ng1163. [DOI] [PubMed] [Google Scholar]
- 23.Magert HJ, Standker L, Kreutzmann P, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J. Biol. Chem. 1999;274:21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 24.Deraison C, Bonnart C, Lopez F, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol. Biol. Cell. 2007;18:3607–3619. doi: 10.1091/mbc.E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JD, Linden KG. Netherton syndrome: a case report and review of the literature. Int. J. Dermatol. 2006;45:693–697. doi: 10.1111/j.1365-4632.2005.02637.x. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu N, Takata M, Otsuki N, Toyama T, Ohka R, Takehara K, Saijoh K. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J. Invest. Dermatol. 2003;121:542–549. doi: 10.1046/j.1523-1747.2003.12363.x. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu N, Saijoh K, Toyama T, et al. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br. J. Dermatol. 2005;153:274–281. doi: 10.1111/j.1365-2133.2005.06754.x. [DOI] [PubMed] [Google Scholar]
- 28.Mowafy M, Cassens RG. Hair growth in the domestic pig - Quantitative aspects. J. Am. Leather Chem. Assoc. 1976;71:71–81. [Google Scholar]
- 29.Chang N, Gungabissoon R, Bhogal RK, Turner GA, Paterson SE. The inhibition of proteases involved in the exogen process in a range of ethnic groups. 2011. p. PE3 021.
- 30.Egelrud T, Lundstrom A. A chymotrypsin-like proteinase that may be involved in desquamation in plantar stratum-corneum. Arch. Dermatol. Res. 1991;283:108–112. doi: 10.1007/BF00371618. [DOI] [PubMed] [Google Scholar]
- 31.Hansson L, Stromqvist M, Backman A, Wallbrandt P, Carlstein A, Egelrud T. Cloning, expression, and characterization of stratum-corneum chymotryptic enzyme - a skin-specific human serine proteinase. J. Biol. Chem. 1994;269:19420–19426. [PubMed] [Google Scholar]
- 32.Borgono CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 33.Ekholm E, Egelrud T. The expression of stratum corneum chymotryptic enzyme in human anagen hair follicles: further evidence for its involvement in desquamation-like processes. Br. J. Dermatol. 1998;139:585–590. doi: 10.1046/j.1365-2133.1998.02452.x. [DOI] [PubMed] [Google Scholar]
- 34.Horikoshi T, Igarashi S, Uchiwa H, Brysk H, Brysk MM. Role of endogenous cathepsin D-like and chymotrypsin-like proteolysis in human epidermal desquamation. Br. J. Dermatol. 1999;141:453–459. doi: 10.1046/j.1365-2133.1999.03038.x. [DOI] [PubMed] [Google Scholar]
- 35.Watkinson A. Stratum corneum thiol protease (SCTP): a novel cysteine protease of late epidermal differentiation. Arch. Dermatol. Res. 1999;291:260–268. doi: 10.1007/s004030050406. [DOI] [PubMed] [Google Scholar]
- 36.Debela M, Debela M, Goettig P, Magdolen V, Huber R, Schechter NM, Bode W. Structural basis of the zinc inhibition of human tissue kallikrein 5. J. Mol. Biol. 2007;373:1017–1031. doi: 10.1016/j.jmb.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt-Rose T, Braren S, Folster H, et al. Efficacy of a piroctone olamine/climbazol shampoo in comparison with a zinc pyrithione shampoo in subjects with moderate to severe dandruff. Int. J. Cosmet. Sci. 2011;33:276–282. doi: 10.1111/j.1468-2494.2010.00623.x. [DOI] [PubMed] [Google Scholar]