Abstract
Background
While opioids provide effective analgesia, opioid-induced constipation (OIC) can severely impact quality of life and treatment compliance. This pooled analysis evaluated the maintenance of efficacy and safety during long-term treatment with combined oxycodone/naloxone prolonged-release tablets (OXN PR) in adults with moderate-to-severe chronic pain.
Methods
Patients (N = 474) received open-label OXN PR during 52-week extension phases of two studies, having completed 12-week, double-blind, randomized treatment with oxycodone prolonged-release tablets (Oxy PR) or OXN PR. Analgesia and bowel function were assessed at each study visit using ‘Average pain over last 24 h scale and Bowel Function Index (BFI), respectively. Treatment Satisfaction Questionnaire for Medication was assessed at study end only.
Key Results
Improvement in bowel function was particularly marked in patients who switched from Oxy PR in the double-blind phase to OXN PR during the extension phase, resulting in a clinically meaningful reduction (≥12 points) in BFI score: at the start of the extension phases, mean (SD) BFI score was 44.3 (28.13), and was 29.8 (26.36) for patients who had received OXN PR in the double-blind phase. One week later, BFI scores were similar for the two groups (26.5 [24.40] and 27.5 [25.60], respectively), as was observed throughout the following months. Fewer than 10% of patients received laxatives regularly. Mean 24-h pain scores were low and stable throughout the extension phases. No unexpected adverse events were observed.
Conclusions & Inferences
Pooled data demonstrate OXN PR is an effective long-term therapy for patients with chronic non-cancer pain, and can address symptoms of OIC. No new safety issues were observed which were attributable to the long-term administration of OXN PR.
Keywords: chronic pain, constipation, naloxone, opioid, oxycodone
Key Messages.
Pooled data from the 12-month extension phases of two Phase III trials demonstrate combined oxycodone/naloxone prolonged-release tablets (OXN PR) are an effective long-term therapy for patients with chronic non-cancer pain, and can address symptoms of opioid-induced constipation. Comparable pain control but with improved bowel function was observed with OXN PR vs oxycodone PR and was maintained during the open-label, long-term treatment. No new safety issues were observed which were attributable to the long-term administration of OXN PR.
Introduction
Chronic pain affects approximately 20% of the population worldwide,1–5 and is highly debilitating, typically resulting in depression, anxiety, and loss of independence.6 Opioids are effective treatments for moderate-to-severe chronic cancer pain and non-cancer pain.7,8 However, their use can be complicated by side effects including opioid-induced bowel dysfunction (OIBD). A key symptom of OIBD is opioid-induced constipation (OIC), which can severely impact patients’ quality of life to the point where treatment compliance and subsequent pain relief are compromised.9–12
Laxatives are frequently used to address the symptoms of OIC. Most laxatives aid defecation by stimulating colonic motility and/or softening stools.13 Laxatives can be effective in some circumstances, including for constipation arising from delayed colonic transit.13 However, OIC has a unique etiology, arising from interaction between opioids and μ-opioid receptors present throughout the entire gut.14 Stimulation of peripheral μ-opioid receptors affects numerous gastrointestinal functions, including neural activity, motility, secretion, resorption of fluid, and blood flow.14,15 Consequently, opioids delay gastric emptying and prolong transit time throughout the small and large intestines.14–18 As opioids affect the entire gastrointestinal tract, it is unsurprising that laxatives, which predominantly act on the colon, frequently do not address the symptoms of OIC.18 Indeed, many patients report that despite taking laxatives they miss or decrease doses of opioids to make it easier to have a bowel movement.10 No single laxative is considered optimal for OIC.19 Furthermore, laxatives are associated with side effects including bloating, gas, and gastroesophageal reflux.18 Therefore, aggressive laxative regimens may be associated with tolerability issues.
Oxycodone (Oxy) is a semi-synthetic, opioid analgesic demonstrated to be efficacious for cancer-related pain, postoperative pain, osteoarthritis-related pain, and neuropathic non-malignant pain syndromes.20 To address OIC symptoms, which are associated with all opioid agonists, a novel analgesic was developed combining Oxy with naloxone, an opioid-receptor antagonist.21 When administered orally, naloxone has ≤2% systemic bioavailability due to extensive first-pass hepatic metabolism.22 Consequently, oral naloxone acts on opioid receptors in the gastrointestinal tract, where it has greater affinity than Oxy.21 Combined oxycodone/naloxone prolonged-release tablets (OXN PR) provide effective analgesia for patients with moderate-to-severe chronic pain in studies of 12 weeks’ duration. Meaningful improvements were also observed in the symptoms of OIC.23–26
Given the nature of chronic pain, effective management often necessitates prolonged therapy. Consequently, the long-term effects of treatments must be established. Here, we present a pooled analysis of efficacy and safety data based on data from two 52-week extension phases which followed completion of two double-blind, randomized studies. These studies were conducted in patients with moderate-to-severe non-cancer pain and OIC between January 2006 and July 2007 to compare the efficacy and safety of OXN PR vs Oxy PR.23–25 The aim of this pooled analysis was to investigate whether the analgesia, safety profile, and improvements in bowel function and quality of life associated with OXN PR are maintained long term in a large number of patients.
Materials and Methods
Study design
This pooled analysis comprised two open-label, 52-week extension phases (OXN3001S and OXN3006S) investigating OXN PR in patients who had completed one of two Phase III, double-blind, multicenter, randomized, 12-week studies (OXN3001; EudraCT: 2005-002398-5725 and OXN3006; EudraCT: 2005-003510-15).23,24
Details of the designs of the two Phase III, double-blind studies have been reported.23–25 Patients were male or female, aged ≥18 years, with a documented history of moderate-to-severe non-cancer pain that required round-the-clock opioid therapy. All patients suffered from OIC on entry to the double-blind studies, defined as <3 complete spontaneous bowel movements in the prior 7 days. Patients were randomized to OXN PR (n = 162) or Oxy PR (n = 160) at doses equivalent to 20–50 mg/day of Oxy (OXN3001), or to OXN PR (n = 130) or Oxy PR (n = 135) at doses equivalent to 60–120 mg/day Oxy (OXN3006). Oral bisacodyl (10 mg/day) was permitted 72 h after a bowel movement, but could be taken sooner if patients exhibited discomfort. Patients completing the 12-week double-blind treatment could participate in the 52-week extension phase if they were considered likely to benefit from opioid therapy during this period (Fig.1).
Figure 1.
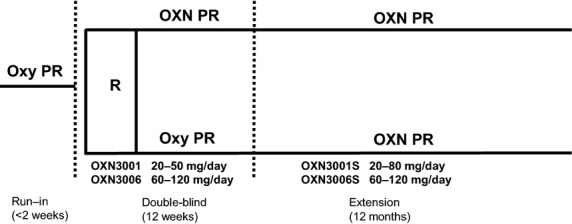
Study schema. Dose titration was permitted at the discretion of the investigator to a maximum of 80 mg/day (OXN3001S) or 120 mg/day (OXN3006S). OXN PR, combined oxycodone / naloxone prolonged-release tablets; Oxy PR, oxycodone prolonged-release; R, randomization.
All patients received open-label OXN PR during the extension phase but were unaware of the treatment group they had been assigned to during the double-blind phase of the study. The starting dose of OXN PR was the effective analgesic dose of Oxy or OXN that the patient received at the end of the double-blind phase. Dose titration was permitted to a maximum of 80 mg/day (OXN3001S) or 120 mg/day (OXN3006S) at the discretion of the investigator.
Use of concomitant medication including laxatives and analgesic rescue therapy was recorded in patient diaries. Oxy immediate-release (IR) and bisacodyl were provided for the first 7 days of the extension phase; thereafter analgesic rescue medications and laxatives were prescribed according to standard protocols of the investigational sites.
There were seven mandated office visits: Visit 9 at Day 1 of study treatment in the extension phases, which was likely to be the same day as Visit 8, the end of double-blind assessment; Visit 10 at 7 ± 3 days, Visit 11 at 30 ± 7 days, Visit 12 at 90 ± 7 days, Visit 13 at 180 ± 7 days, Visit 14 at 270 ± 7 days, and Visit 15 at 360 ± 7 days after Visit 8.
The studies in this pooled analysis were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Guidelines for Good Clinical Practice, and the European Union Clinical Trials Directive. The procedures were approved by local ethics committees, and all patients gave informed, written consent prior to enrollment.27–29
Outcomes and assessments
Bowel function was assessed using the validated Bowel Function Index (BFIa). BFI score comprised the arithmetic mean score of three distinct items (0–100 scale): ease of defecation, feeling of incomplete bowel evacuation, and personal judgment of constipation. A change in BFI score of ≥12 points is considered to be clinically meaningful30 and BFI score of 0–28.8 is the reference range for non-constipated patients with chronic pain.31
Analgesic efficacy was assessed using ‘Average pain over the last 24 h' using a numeric analog scale (NAS; 0–10, single question). Frequency of analgesic rescue medication (Oxy IR) and laxative use (bisacodyl) was documented (intake of laxatives and opioid analgesic medications was recorded as concomitant medication after the first 7 days of the extension phases).
Quality of life was assessed at the end of the extension phases only, to ascertain patients’ assessment of overall treatment, using the general questionnaire ‘Treatment Satisfaction Questionnaire for Medication (TSQM)’ which comprised 14 items. Subscale scores were calculated for effectiveness, side effects, convenience, and global satisfaction (0–100 for each score).
Safety was monitored via the documentation of adverse events (classified by system organ class and Medical Dictionary for Regulatory Activities [MedDRA] preferred terms) and serious adverse events (SAEs); monitoring of vital signs, hematology, blood chemistry, and electrocardiograms (ECG); and Subjective Opiate Withdrawal Scale (SOWS) scores (excluding the item number 16 ‘I feel like shooting up today’, which is intended for opiate abusers and therefore did not apply to the target population of these studies32).
Statistical methods
Given the prospectively planned, similar designs of the extension phases, pooled analyses of data were considered appropriate to provide further insight into the long-term efficacy and safety of OXN PR in a large number of patients. The extension-phase population comprised all patients who had received at least one dose of OXN PR in the extension phases and had at least one safety assessment. Data on safety, bowel function, pain, and use of rescue analgesic medication, and laxatives were collected at each study visit in the extension phases (Days 1, 7 ± 3 days, 30, 90, and 180 [end of study] ± 7 days). Modified SOWS scores were collected on Day 7 and at the end of the study.
Oxy IR and bisacodyl intake during Week 1 of the extension phases is described using summary statistics. Concomitant intake of analgesic therapy and laxatives during the subsequent weeks is presented for patients with available descriptions of doses and frequencies. Summary statistics (n, mean and standard deviation [SD]) are described for continuous variables. BFI scores are presented using the last observation carried forward (LOCF) method. A change in BFI score of ≥12 points was considered to be clinically meaningful.30
Results
Of the 859 patients who were enrolled in the two double-blind studies, 587 were randomized to treatment; 581 patients received ≥1 dose of study medication, and 499 patients completed the studies.24 The pooled analysis population comprised the 474 patients who received open label OXN PR; 399 of these patients completed the extension phase (Fig.2).
Figure 2.
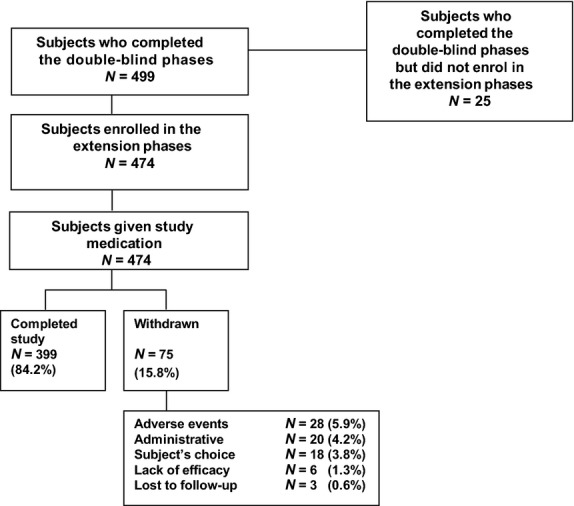
Patient disposition in the pooled analysis.
The mean age of the analysis population was 57.3 years and most patients were female (Table1). The overall mean (SD) daily dose of OXN PR was 57.4 mg (26.86). The median exposure was 360 days, indicating that at least 50% of the patients received exactly 12 months of treatment, per the study protocols.
Table 1.
Patient characteristics at baseline
| Variable | OXN PR (N = 474) |
|---|---|
| Age (years) | |
| Mean (SD) | 57.3 (10.97) |
| Median | 58 |
| Min, Max | 26, 88 |
| Age group, n (%) | |
| ≤65 years | 362 (76.4) |
| >65 years | 112 (23.6) |
| Sex, n (%) | |
| Male | 175 (36.9) |
| Female | 299 (63.1) |
| Race*, n (%) | |
| Caucasian | 473 (99.8) |
| Other | 1 (0.2) |
| Weight (kg) | |
| Mean (SD) | 84.5 (19.02) |
| Median | 83 |
| Min, Max | 42, 150 |
SD, standard deviation.
Self-assigned ethnicity, using nationally agreed guidelines.
Bowel function
Similar BFI scores (approximately 62) were observed during the Screening and Run-in phases for patients subsequently randomized to double-blind treatment with Oxy PR or OXN PR (Fig.3). Statistically significant improvements in bowel function were observed with OXN PR compared with Oxy PR from Week 1 of treatment and mean BFI scores were approximately 15 points lower at Week 12 (p < 0.0001).24
Figure 3.
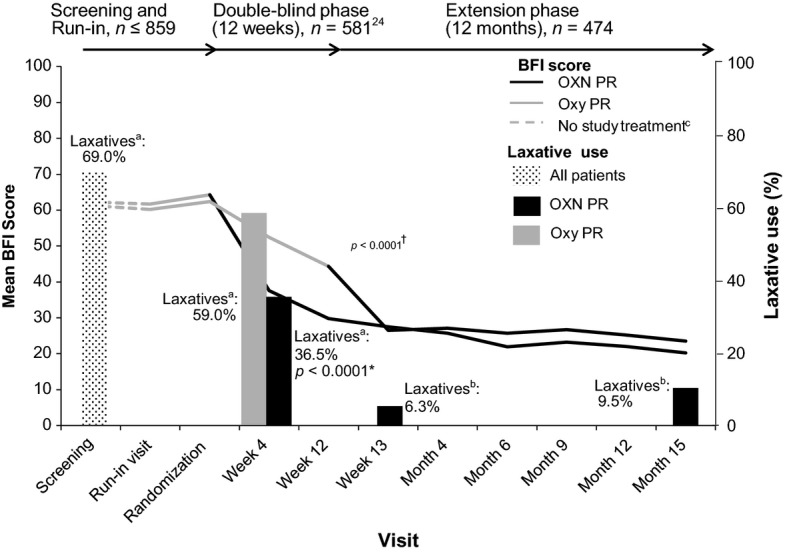
Mean Bowel Function Index scores (LOCF) and laxative use throughout 15 months of treatment. LOCF, last observation carried forward. 587 patients were randomized to treatment in the double-blind phase, 581 patients received ≥1 dose of study medication and were included in the full analysis population. Laxative use was captured differently during the study: aScreening and double-blind phases: patients who required laxatives (patients provided with bisacodyl by the study investigator, according to the study protocol). bExtension phases: patients who used laxatives regularly (according to specific dosing and treatment instructions provided by the investigator). cNo study treatment was received during Screening. At Run-in, patients had prestudy opioid converted to open-label Oxy PR, titrated to an effective analgesic dose. *Laxative intake Weeks 1–4 (Fisher's Exact Test) OXN PR vs Oxy PR, p < 0.0001. †BFI score Weeks 1–12 (mixed effects linear model with repeated measurements by subject) OXN PR vs Oxy PR, p < 0.0001.
Improvement in bowel function, indicated by a decrease in BFI scores, throughout the extension phases was particularly marked in patients who switched from receiving Oxy PR in the double-blind studies, to OXN PR at the start of the extension phases (Fig.3). At the start of the extension phases (Visit 9), patients who were taking Oxy PR in the double-blind studies and who had only just switched to OXN PR, had a mean (SD) BFI score of 44.3 (28.13) while patients who had received OXN PR during the double-blind studies had a lower mean (SD) BFI score of 29.8 (26.36). After only 1 week of extension-phase treatment (Visit 10), the BFI score of patients originally receiving Oxy PR had dropped to a mean (SD) of 26.5 (24.40), which was similar to the scores reported in patients who had also been taking OXN PR in the double-blind studies (mean [SD] 27.5 [25.60]). From Visit 10 onwards, the scores dropped at similar rates in both groups, culminating at Month 12 of the extension phases (Visit 15) with mean (SD) BFI scores of 23.5 (24.86) in patients who had originally received Oxy PR and 20.2 (22.84) in individuals who had originally received OXN PR in the double-blind trials (Fig.3).
During the first 7 days of the extension phases, 30 subjects (6.3%) received laxatives on a regular basis. After the first 7 days, 45 subjects (9.5%) were given laxatives on a regular basis (Fig.3).
Analgesic efficacy
Mean ‘average pain over the last 24 h’ scores of the analysis population were very similar at each visit during the extension phases and when patients were subgrouped according to the treatment they received during the double-blind phases (Table2). This indicates that stable pain control was maintained with OXN PR throughout the 12-month treatment period.
Table 2.
Mean 24-h average pain scores
| Visit | Treatment received during the double-blind phase* | Total (N = 474) | |
|---|---|---|---|
| OXN PR (N = 239) | Oxy PR (N = 235) | ||
| Visit 9† (Day 1) | |||
| n | 238 | 234 | 472 |
| Mean (SD) | 3.5 (1.65) | 3.6 (1.64) | 3.6 (1.65) |
| Visit 10 (Day 7 ± 3 days) | |||
| n | 233 | 224 | 457 |
| Mean (SD) | 3.5 (1.77) | 3.6 (1.60) | 3.5 (1.69) |
| Visit 11 (Day 30 ± 7 days) | |||
| n | 229 | 223 | 452 |
| Mean (SD) | 3.5 (1.80) | 3.4 (1.69) | 3.4 (1.74) |
| Visit 12 (Day 90 ± 7 days) | |||
| n | 219 | 207 | 426 |
| Mean (SD) | 3.6 (1.91) | 3.4 (1.65) | 3.5 (1.79) |
| Visit 13 (Day 180 ± 7 days) | |||
| n | 211 | 205 | 416 |
| Mean (SD) | 3.6 (1.86) | 3.6 (1.66) | 3.6 (1.76) |
| Visit 14 (Day 270 ± 7 days) | |||
| n | 205 | 201 | 406 |
| Mean (SD) | 3.5 (1.78) | 3.5 (1.78) | 3.5 (1.78) |
| Visit 15‡ (Day 360 ± 7 days) | |||
| n | 234 | 228 | 462 |
| Mean (SD) | 3.5 (1.96) | 3.6 (1.96) | 3.6 (1.96) |
Days refer to number of days of OXN PR treatment during the extension phases only (excluding double-blind treatment). SD, standard deviation. *Scores for the total population and scores subgrouped according to treatment patients received during the double-blind phases of the studies.
Pain scores were not reported by two patients at Visit 9. Visit 9 was likely to be the same day as Visit 8, the end of double-blind assessment.
LOCF.
Analgesic rescue medication was provided for the first week of the extension phases, after which, rescue medication use was recorded as concomitant medication. During the first week, 68.5% of the total subject days were recorded as days where no analgesic rescue intake was required. Mean daily (SD) use of Oxy IR during the first week of the extension phases was low at 4.47 mg (7.97) as was the use of Oxy IR during the last week of the double-blind trials (3.42 mg [6.64]). Concomitant medication records show that opioid analgesics were used by 34% of subjects and other analgesics/antipyretics were used by 27.4% of subjects, at the discretion of the investigator. The concomitant opioid analgesics used were largely IR products at low doses, for example, co-codamol, tramadol, and Oxy IR, and were used in accordance with guidelines for pain management. Due to the prolonged-release formulation, OXN PR is not intended for the treatment of breakthrough pain.
Quality of Life
TSQM scores (possible score: 0–100) at the end of the extension phases were relatively high, indicating that subjects were satisfied with the study medication they were receiving following the 52-week treatment duration. Mean (SD) TSQM subscale scores were: ‘effectiveness’ 68.6 (20.17); ‘side-effects’ 86.3 (24.62); ‘convenience’ 84.9 (14.90); and ‘global satisfaction’ 73.5 (21.60). Data were available from at least 451 patients for each TSQM subscale.
Safety
Adverse events (all causality, any grade) were experienced by 370 patients (78.1%). The most common adverse events were musculoskeletal or connective tissue disorders (n = 174, 36.7%). This was anticipated as musculoskeletal and connective tissue disorders accounted for 86% of the underlying pain conditions which led to patients being included in the studies.24
In total, 218 patients (46.0%) experienced treatment-related adverse events (defined as ‘unlikely’, ‘possibly’, ‘probably’, or ‘definitely’ related to study treatment; Table3). Of the 59 patients who experienced constipation (12.4%), 39 (8.2%) experienced constipation that was classed as possibly, probably, or definitely related to study drug. In seven patients (1.5%), constipation was considered unlikely to be related to study medication. Diarrhea was considered possibly, probably, or definitely related to study drug in only 13 patients (2.7%), and was considered unlikely related to study medication in five patients (1.1%).
Table 3.
Treatment-related adverse events (considered by study investigator to be definitely, probably, possibly, or unlikely related to study medication) by organ class (≥5%) and preferred term (adverse events occurring in ≥1.0%)
| System organ class and MedDRA preferred term | OXN PR (N = 474) n (%) |
|---|---|
| Gastrointestinal disorders | 96 (20.3) |
| Abdominal pain | 6 (1.3) |
| Abdominal pain upper | 9 (1.9) |
| Constipation | 46 (9.7) |
| Diarrhea | 18 (3.8) |
| Dyspepsia | 5 (1.1) |
| Nausea | 17 (3.6) |
| Vomiting | 7 (1.5) |
| General disorders and administrative site conditions | 45 (9.5) |
| Drug withdrawal syndrome | 7 (1.5) |
| Fatigue | 11 (2.3) |
| Oedema peripheral | 7 (1.5) |
| Pain | 9 (1.9) |
| Infections and infestations | 32 (6.8) |
| Sinusitis* | 5 (1.1) |
| Musculoskeletal and connective tissue disorders | 47 (9.9) |
| Arthralgia* | 8 (1.7) |
| Back pain | 19 (4.1) |
| Osteoarthritis | 7 (1.5) |
| Pain in extremity | 6 (1.3) |
| Nervous system disorders | 41 (8.6) |
| Dizziness | 6 (1.3) |
| Headache | 9 (1.9) |
| Psychiatric disorders | 26 (5.5) |
| Depression | 7 (1.5) |
| Insomnia | 7 (1.5) |
| Skin and subcutaneous tissue disorders | 38 (8.0) |
| Hyperhidrosis | 21 (4.4) |
| Rash | 5 (1.1) |
MedDRA, Medical Dictionary for Regulatory Activities.
All treatment-related events were considered unlikely to be related to study medication.
Twenty subjects (4.2%) experienced SAEs which the investigator suspected had a causal relationship with study drug. The Sponsor considered additional SAEs in two subjects to have a positive causality to study drug (gastrointestinal disorder: possibly related; fall with femoral neck fracture: unlikely to be related).
Most adverse events resulting in treatment discontinuation, dose interruptions or dose reductions occurred only once during the extension phases. Such adverse events occurring in 3 or more subjects included seven incidences of hyperhidrosis, five incidences of diarrhea, four incidences of nausea and three incidences of fatigue. There were two deaths during the extension phases, one due to sepsis and one due to necrotizing fasciitis; neither was considered related to study treatment. In addition, one patient with a malignant tumor of the abdomen and metastases died from pulmonary embolism more than 1 month after study medication had been discontinued; the investigator considered this event unlikely to be related to study medication.
Modified SOWS scores were stable and low throughout the extension phases. Mean (SD) SOWS scores were 6.5 (6.45) and 7.6 (7.51) at Visit 10 and Visit 15, respectively. Only seven subjects (1.5%) had adverse events associated with opioid withdrawal considered by the Investigator to be related to study medication (four incidences were possibly related, one incidence was probably related and two incidences were thought to be definitely related to study medication). Three further events were considered not related to study medication.
No signal was detected that pointed to a specific effect of OXN PR on any of the investigated laboratory parameters. No clinically important changes in vital signs were observed, and ECG changes were infrequent and isolated.
Discussion
Comparable pain control but with improved bowel function was observed with OXN PR compared with Oxy PR in two double-blind 12-week, Phase III studies in patients with chronic, moderate-to-severe, non-malignant pain.23–25 This pooled analysis of data from two subsequent extension-phases indicates that the improvements were maintained when patients continued to receive OXN treatment for up to 52 additional weeks.
As anticipated, the improvement in bowel function, indicated by BFI scores, was most pronounced in those patients who switched from receiving Oxy PR in the double-blind studies to OXN PR when they entered the extension phase. In these patients, a substantial fall in mean BFI score was observed within the first week of OXN PR treatment. Furthermore, improvement in bowel function was maintained long term, with a clinically meaningful (≥12 points) mean reduction of 20.8 points on the BFI score observed over the 52-week extension phases for patients who received Oxy PR in the double-blind trials and then OXN PR in the extension phases. For patients who received OXN PR in both the double-blind and extension phases the reduction in BFI score (9.6 points) was less owing to the already significantly improved bowel function experienced during the double-blind studies.24 For all patients receiving OXN PR in the extension phases, mean BFI scores were within the normal range for non-constipated patients with chronic pain (≤28.8 points) from Week 1 to Month 12 of treatment.31
At screening, prior to randomization in the 12-week double-blind studies, most patients (69.0%) were documented to ‘require laxatives’ (Fig.3). Marked differences in laxative intake between patients randomized to Oxy PR and OXN PR had already been observed during the first 4 weeks of the double-blind trials. This was demonstrated by a significant reduction in the incidence of laxative intake for patients randomized to OXN PR (36.5%) compared with those randomized to Oxy PR (59.0%; p < 0.0001).24 Compared with during double-blind treatment, laxative intake was captured differently in the extension phases, to reflect clinical practice, and consequently direct comparison of laxative use during these time periods cannot be made. However, during the long-term therapy fewer than 10% of patients from the pooled analysis population were documented to have ‘received laxatives on a regular basis’. The infrequent use of laxatives is particularly noteworthy given many patients were transitioning from Oxy PR in the double-blind studies to OXN PR in the extension phases.
Opioids induce OIBD, including OIC, via interaction with μ-opioid receptors present throughout the entire gastrointestinal tract.14 The effects of OXN PR on the small intestine and the colon likely play a significant part in addressing symptoms of OIC. In a study of healthy volunteers, OXN PR normalized the delayed arrival of 99mTechnetium-labelled tablets into the colon observed with Oxy alone.17 Mean colonic arrival times (transit time from the stomach through the small intestine) were significantly longer following treatment with Oxy compared with placebo (7.19 vs 5.15 h) and numerically longer compared with OXN (5.16 h).17 This indicates that the small intestine plays an important role in Oxy-related prolongation of gastrointestinal transit time, and this can be normalized by OXN PR. Furthermore, data from healthy volunteers indicate a substantial proportion of Oxy is absorbed in the small intestine.33 These observations go some way to explain why laxatives which act predominantly in the colon often do not satisfactorily relieve the symptoms of OIC.10,18,34
Pain control was maintained with OXN PR throughout 52 weeks of treatment. Average ‘pain over the previous 24 h scores were low and stable over the 12-month period and were similar when patients were subgrouped according to the treatment they received during the double-blind phase of the studies, indicating OXN PR provides effective long-term analgesia. The mean pain scores in this pooled analysis of the extension phases were similar to those reported in the Oxy PR and the OXN PR groups during the double-blind studies (Week 12 of the double-blind phases: mean Oxy PR patients' score was 3.5; mean OXN PR patients' score was 3.6).24 Mean daily use of Oxy IR during the first week of the extension phases was low and patient satisfaction with OXN PR (as shown by the TSQM scores) was high following long-term treatment, in concordance with the analgesic efficacy and improvements in bowel function associated with OXN PR.
Based on the observed adverse events, vital signs, hematology, blood chemistry, and ECG profiles, long-term therapy with OXN PR was well-tolerated. Most adverse events did not have a major impact on long-term treatment with OXN PR as few subjects experienced events which resulted in dose reduction or discontinuation. Adverse events in the pooled analysis were slightly more frequent than observed previously during the double-blind studies (61% of OXN PR-treated patients had adverse events, with 36% experiencing treatment-related adverse events24). This difference was anticipated due to the considerably longer duration of the extension phases (12 months) compared with the double-blind studies (12 weeks). During the pooled analysis, diarrhea and constipation considered by the study investigator to be definitely, probably, or possibly related to study medication were infrequent. This is particularly noteworthy, given the high incidence of constipation reported in studies of patients receiving opioid therapy despite the use of laxatives.10
No unexpected safety signals were detected in the pooled analysis and the safety profile of OXN PR associated with long-term administration appears consistent with previous observations and the expected profile of the opioid analgesic class of drugs.20 SOWS scores were stable during the extension phase and were similar to the scores seen during the double-blind studies, indicating that drug withdrawal was not a problem associated with long-term administration of OXN PR.
In summary, this pooled analysis of data from a large number of patients indicates that OXN PR is an effective long-term therapy for patients with chronic non-cancer pain, and can address symptoms of OIC. While all patients received OXN PR for 12 months, approximately 50% of patients had also received OXN PR for an additional 12 weeks during the preceding randomized, double-blind studies. Data from this subgroup indicate that OXN PR is effective throughout 15 months of therapy. This pooled analysis demonstrated that average pain scores remained low and stable throughout the extension phases and use of analgesic rescue medication was infrequent. The improvement in bowel function seen with OXN PR during the double-blind studies was continued throughout the 52 weeks of extended treatment in this pooled analysis. No new or unexpected safety issues were observed which were attributable to the long-term administration of OXN PR. Patient satisfaction with OXN PR was also high and maintained throughout the 52 weeks. Findings from this pooled analysis are consistent with a prior analysis of long-term OXN PR in patients with non-cancer chronic pain.26
Acknowledgments
The authors wish to thank all participating patients, and the network of investigators, study coordinators, and operations staff. Medical writing services were provided by Siân Marshall PhD of SIANTIFIX Inc., Cambridgeshire, UK and funded by Mundipharma Research GmbH & Co. KG.
Glossary
- BFI
Bowel Function Index
- ECG
electrocardiogram
- IR
immediate-release
- LOCF
last observation carried forward
- MedDRA
Medical Dictionary for Regulatory Activities
- NAS
numerical analog scale
- OIBD
opioid-induced bowel dysfunction
- OIC
opioid-induced constipation
- OXN
combined oxycodone/naloxone
- Oxy
oxycodone
- PR
prolonged-release
- SAE
serious adverse event
- SD
standard deviation
- SOWS
Subjective Opioid Withdrawal Scale
- TSQM
Treatment Satisfaction Questionnaire for Medication
Footnotes
Copyright for the BFI is owned by Mundipharma Laboratories GmbH, Switzerland 2002; the BFI is subject of European Patent Application Publication No. EP 1 860 988 and corresponding patents and applications in other countries.
Funding
This research was financially supported by Mundipharma Research GmbH & Co. KG, Limburg, Germany.
Disclosure
Johannes Hafer and Mark Blagden declare no competing financial and other interests. Heike Duerr, Michael Hopp, and Björn Bosse are employees of Mundipharma Research GmbH & Co. KG.
Author Contribution
All authors had access to the data, critically reviewed and revised the article for intellectual content, and approved the final version for publication. Johannes Hafer and Mark Blagden were investigators on the study (JH was principal investigator) and involved in acquisition of data; and Heike Duerr, Michael Hopp, and Björn Bosse played key roles in the development of the study design, analysis, and interpretation of the data.
References
- 1.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–9. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Ng KF, Tsui SL, Chan WS. Prevalence of common chronic pain in Hong Kong adults. Clin J Pain. 2002;18:275–81. doi: 10.1097/00002508-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bekkering GE, Kleijnen J. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449–62. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- 6.Strine TW, Hootman JM, Chapman DP, Okoro CA, Balluz L. Health-related quality of life, health risk behaviors, and disability among adults with pain-related activity difficulty. Am J Public Health. 2005;95:2042–8. doi: 10.2105/AJPH.2005.066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl. 7):vii139–54. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 9.Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137–44. doi: 10.5055/jom.2009.0014. [DOI] [PubMed] [Google Scholar]
- 10.Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1) Pain Med. 2009;10:35–42. doi: 10.1111/j.1526-4637.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 11.Cook SF, Bell TJ, Sweeney CT, Fehnel SE, Hollis K. Impact on quality of life of constipation-associated GI symptoms related to opioid treatment in chronic pain patients: Pac-qol results from the opioid survey. J Pain. 2007;8:S71. (Abstract 883) [Google Scholar]
- 12.Cook SF, Lanza L, Zhou X, Sweeney CT, Goss D, Hollis K, Mangel AW, Fehnel SE. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27:1224–32. doi: 10.1111/j.1365-2036.2008.03689.x. [DOI] [PubMed] [Google Scholar]
- 13.Tack J, Muller-Lissner S, Stanghellini V, Boeckxstaens G, Kamm MA, Simren M, Galmiche JP, Fried M. Diagnosis and treatment of chronic constipation–a European perspective. Neurogastroenterol Motil. 2011;23:697–710. doi: 10.1111/j.1365-2982.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzer P, Ahmedzai SH, Niederle N, Leyendecker P, Hopp M, Bosse B, Spohr I, Reimer K. Opioid-induced bowel dysfunction in cancer-related pain: causes, consequences, and a novel approach for its management. J Opioid Manag. 2009;5:145–51. doi: 10.5055/jom.2009.0015. [DOI] [PubMed] [Google Scholar]
- 15.De LA, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–15. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 16.McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control. 2004;11:3–9. doi: 10.1177/10732748040110S302. [DOI] [PubMed] [Google Scholar]
- 17.Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, Palaniappan K, Church A, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20:427–39. doi: 10.1517/13543784.2011.563236. [DOI] [PubMed] [Google Scholar]
- 18.Brock C, Olesen SS, Olesen AE, Frokjaer JB, Andresen T, Drewes AM. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs. 2012;72:1847–65. doi: 10.2165/11634970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De CF, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 20.Napp Pharmaceuticals Limited. 2013. Targinact Summary of Product Characteristics. Available at: http://www.medicines.org.uk.
- 21.Reimer K, Hopp M, Zenz M, Maier C, Holzer P, Mikus G, Bosse B, Smith K, et al. Meeting the challenges of opioid-induced constipation in chronic pain management - a novel approach. Pharmacology. 2009;83:10–7. doi: 10.1159/000165778. [DOI] [PubMed] [Google Scholar]
- 22.Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, Bell D. Low absolute bioavailability of oral naloxone in healthy subjects. Int J Clin Pharmacol Ther. 2012;50:360–7. doi: 10.5414/cp201646. [DOI] [PubMed] [Google Scholar]
- 23.Löwenstein O, Leyendecker P, Hopp M, Schutter U, Rogers PD, Uhl R, Bond S, Kremers W, et al. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Expert Opin Pharmacother. 2009;10:531–43. doi: 10.1517/14656560902796798. [DOI] [PubMed] [Google Scholar]
- 24.Löwenstein O, Leyendecker P, Lux EA, Blagden M, Simpson KH, Hopp M, Bosse B, Reimer K. Efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of moderate/severe chronic non-malignant pain: results of a prospectively designed pooled analysis of two randomised, double-blind clinical trials. BMC Clin Pharmacol. 2010;10:12. doi: 10.1186/1472-6904-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson K, Leyendecker P, Hopp M, Muller-Lissner S, Lowenstein O, De AJ, Troy FJ, Bosse B, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin. 2008;24:3503–12. doi: 10.1185/03007990802584454. [DOI] [PubMed] [Google Scholar]
- 26.Sandner-Kiesling A, Leyendecker P, Hopp M, Tarau L, Lejcko J, Meissner W, Sevcik P, Hakl M, et al. Long-term efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of non-cancer chronic pain. Int J Clin Pract. 2010;64:763–74. doi: 10.1111/j.1742-1241.2010.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Parliament and Council of the European Union. Clinical Trials Directive 2001/20/EC. 2001. Available at: http://eurlex.europa.eu/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF.
- 28.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. International Conference on Harmonisation; 1997. Guidelines on good clinical practice. Available at: http://ich.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 2002. Available at: http://www.wma.net/en/30publications/10policies/b3/17c.pdf. [DOI] [PubMed]
- 30.Rentz AM, Yu R, Muller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12:371–83. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 31.Ueberall MA, Muller-Lissner S, Buschmann-Kramm C, Bosse B. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res. 2011;39:41–50. doi: 10.1177/147323001103900106. [DOI] [PubMed] [Google Scholar]
- 32.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 33.Mundin GE, Smith KJ, Mysicka J, Heun G, Kramer M, Hahn U, Leuner C. Validated in vitro/in vivo correlation of prolonged-release oxycodone/naloxone with differing dissolution rates in relation to gastrointestinal transit times. Expert Opin Drug Metab Toxicol. 2012;8:1495–503. doi: 10.1517/17425255.2012.729578. [DOI] [PubMed] [Google Scholar]
- 34.Muller-Lissner S. Opioid-induced constipation - mechanisms, relevance and management. Eur Gastroenterol Hepatol Review. 2010;6:54–7. [Google Scholar]


