Abstract
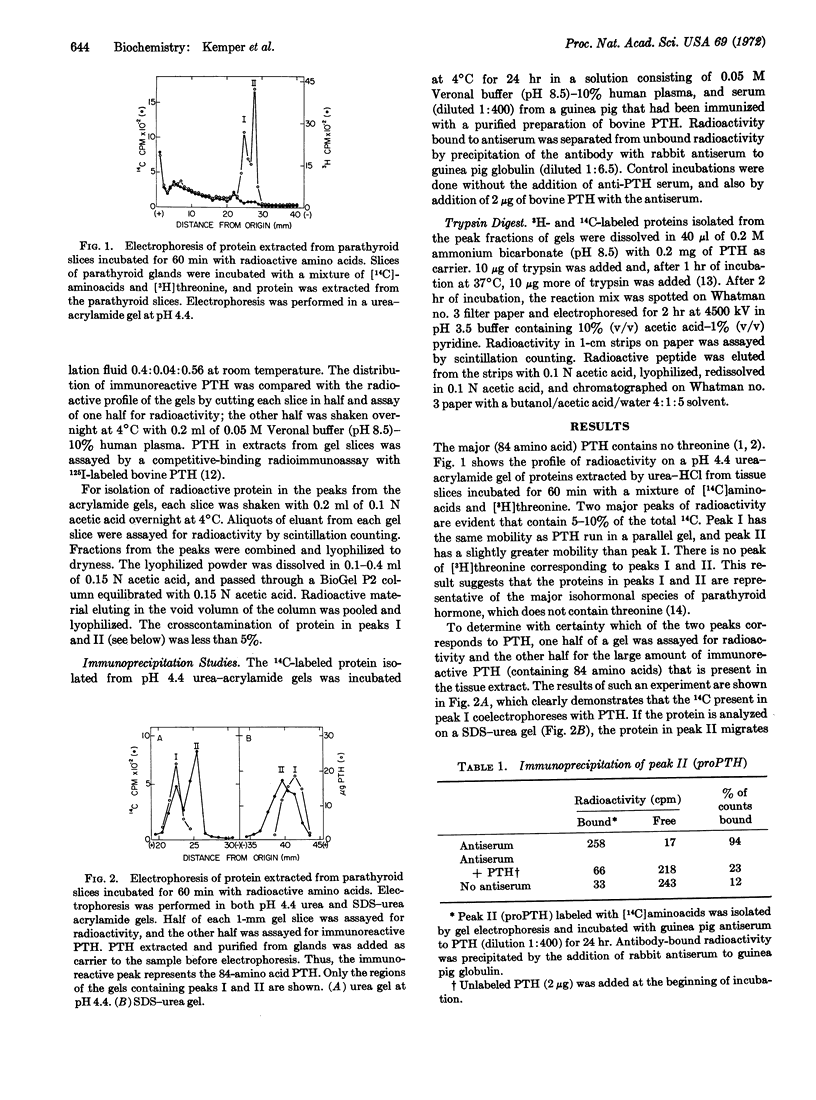
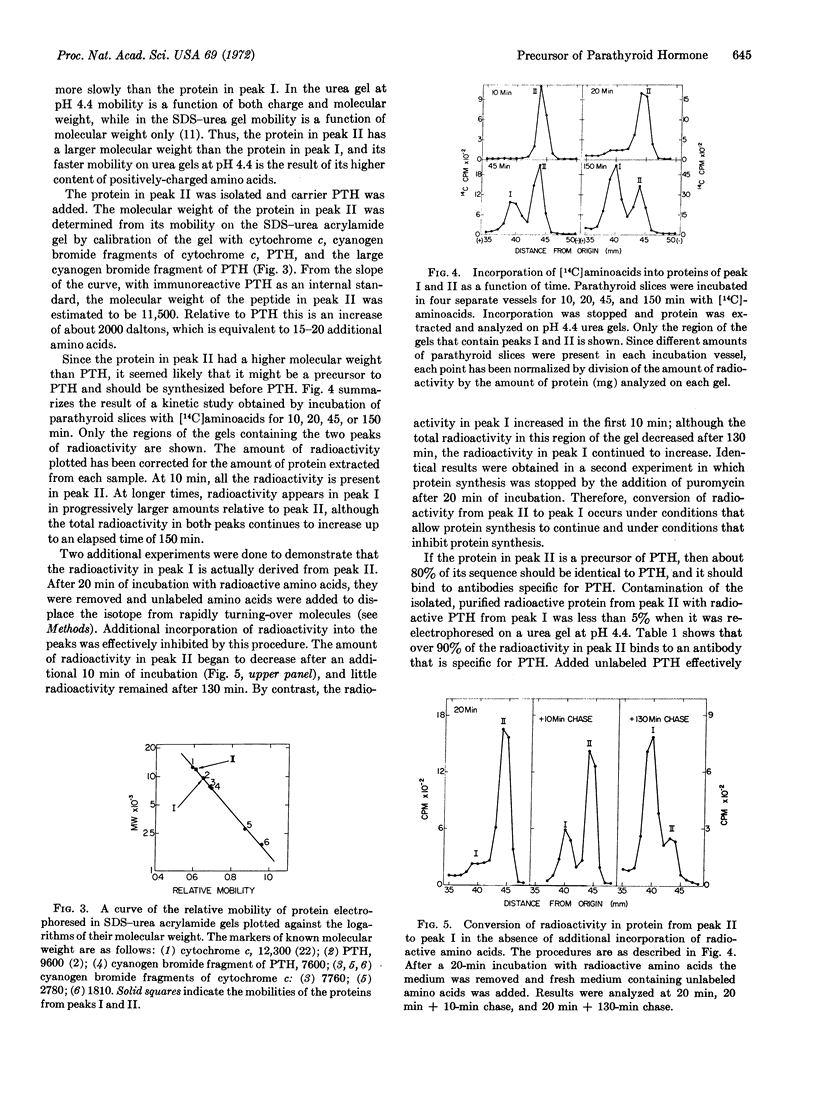
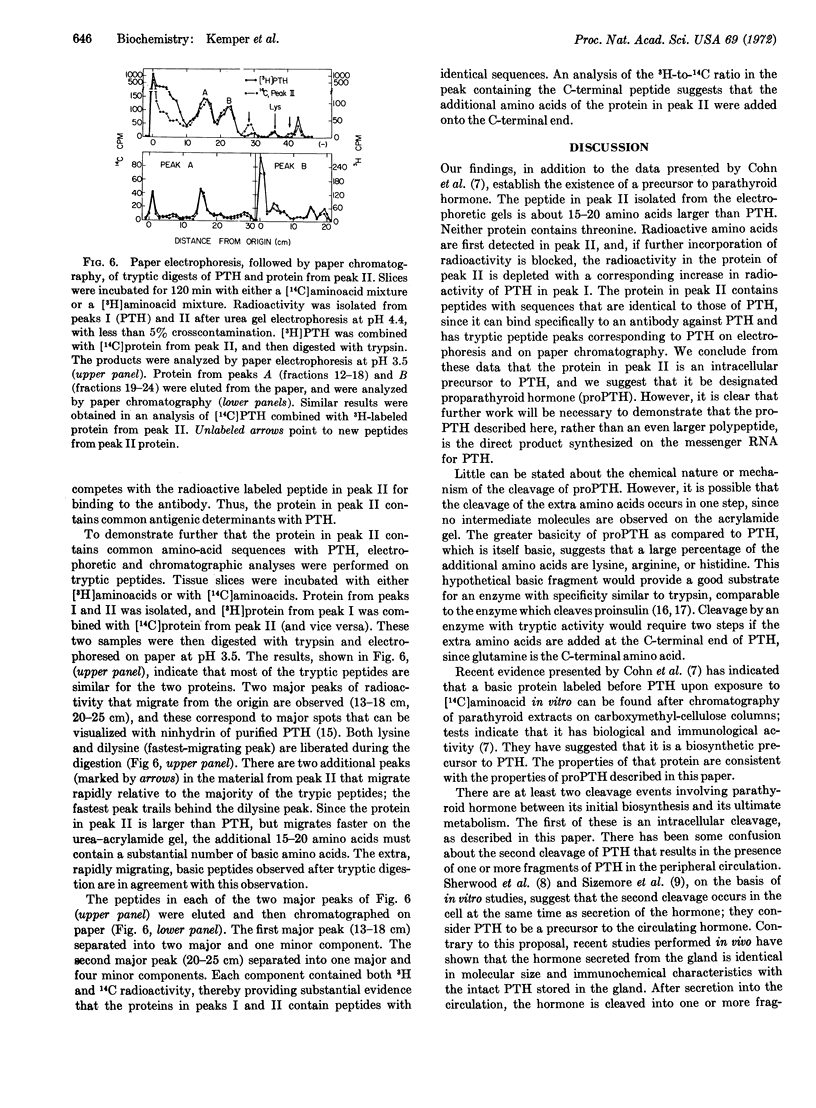
Biosynthesis of a precursor to bovine parathyroid hormone has been demonstrated in slices of parathyroid tissue incubated in vitro. The proparathyroid hormone is 15-20 amino acids larger than the bovine hormone, and has a molecular weight of about 11,500 as determined by polyacrylamide gel electrophoresis. Upon incubation of parathyroid slices with [14C]aminoacids, radioactivity is detected initially in the precursor. If incorporation of [14C]aminoacids is inhibited after a short incubation either by replacement of radioactive amino acids with unlabeled amino acids or by addition of puromycin, the amount of radioactivity in the precursor decreases, while the radioactivity in the hormone continues to increase. The precursor is bound by an antibody that is specific for parathyroid hormone, and its binding can be inhibited by addition of the hormone. Analysis of tryptic digests indicates that the precursor and the hormone have common tryptic peptides, and that there are at least two additional peptides in the precursor.
Keywords: tryptic peptides, molecular weight, protein synthesis, immunoassay
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berson S. A., Yllow R. S. Nature of immunoreactive gastrin extracted from tissues of gastrointestinal tract. Gastroenterology. 1971 Feb;60(2):215–222. [PubMed] [Google Scholar]
- Brewer H. B., Jr, Ronan R. Bovine parathyroid hormone: amino acid sequence. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1862–1869. doi: 10.1073/pnas.67.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Powell D., Murray T. M., Mayer G. P., Potts J. T., Jr Parathyroid hormone: secretion and metabolism in vivo. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2986–2991. doi: 10.1073/pnas.68.12.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Keutmann H. T., Aurbach G. D., Dawson B. F., Niall H. D., Deftos L. J., Potts J. T., Jr Isolation and characterization of the bovine parathyroid isohormones. Biochemistry. 1971 Jul 6;10(14):2779–2787. doi: 10.1021/bi00790a020. [DOI] [PubMed] [Google Scholar]
- Melani F., Ryan W. G., Rubenstein A. H., Steiner D. F. Proinsulin secretion by a pancreatic beta-cell adenoma. Proinsulin and C-peptide secretion. N Engl J Med. 1970 Oct 1;283(14):713–719. doi: 10.1056/NEJM197010012831401. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Keutmann H., Sauer R., Hogan M., Dawson B., Aurbach G., Potts J., Jr The amino acid sequence of bovine parathyroid hormone I. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1586–1588. [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence for glucagon biosynthesis involving a protein intermediate in islets of the anglerfish (Lophius americanus). Endocrinology. 1971 Sep;89(3):642–651. doi: 10.1210/endo-89-3-642. [DOI] [PubMed] [Google Scholar]
- Nolan C., Margoliash E., Peterson J. D., Steiner D. F. The structure of bovine proinsulin. J Biol Chem. 1971 May 10;246(9):2780–2795. [PubMed] [Google Scholar]
- Potts J. T., Jr, Aurbach G. D., Sherwood L. M., Sandoval A. Structural basis of biological and immunological activity of parathyroid hormone. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1743–1751. doi: 10.1073/pnas.54.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Rodman J. S., Lundberg W. B. Evidence for a precursor to circulating parathyroid hormone. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1631–1638. doi: 10.1073/pnas.67.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]
- Yip C. C. A bovine pancreatic enzyme catalyzing the conversion of proinsulin to insulin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1312–1315. doi: 10.1073/pnas.68.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]



