Abstract
Background & Aims
Environmental and genetic factors contribute to alcoholic cirrhosis onset. In particular, age at exposure to liver stressors has been shown to be important in progression to fibrosis in hepatitis C individuals. However, no definite data on the role of age at onset of at-risk alcohol consumption are available. Moreover, patatin-like phospholipase domain-containing protein 3 (PNPLA3) I148M (rs738409) variant has been associated with alcoholic cirrhosis, but only in cross-sectional studies. The aim of this study was to investigate the role of age at onset of at-risk alcohol consumption and PNPLA3 I148M variant on alcoholic cirrhosis incidence.
Methods
A total of 384 at-risk alcohol drinkers were retrospectively examined. The association among age at onset of at-risk alcohol consumption, PNPLA3 I148M variant and cirrhosis incidence was tested.
Results
A higher incidence of alcoholic cirrhosis was observed in individuals with an older (≥24 years) compared with a younger (<24) age at onset of at-risk alcohol consumption (P-value < 0.001). Moreover, PNPLA3 148M allele carriers showed an increased incidence of cirrhosis (P-value < 0.001). Both age at onset of at-risk alcohol consumption and PNPLA3 148M allele were independent risk factors for developing cirrhosis (H.R. (95% C.I.): 2.76 (2.18–3.50), P-value < 0.001; 1.53(1.07–2.19), P-value = 0.021 respectively). The 148M allele was associated with a two-fold increased risk of cirrhosis in individuals with a younger compared with an older age at onset of at-risk alcohol consumption (H.R. (95% C.I.): 3.03(1.53–6.00) vs. 1.61(1.09–2.38).
Conclusions
Age at onset of at-risk alcohol consumption and PNPLA3 I148M genetic variant are independently associated with alcoholic cirrhosis incidence.
Keywords: Age at onset, alcohol intake, alcoholic liver disease, genetics, incidence, patatin-like phospholipase domain-containing protein 3, polymorphism
Alcohol-related liver disease is a major cause of morbidity and premature mortality worldwide 1. In Europe, in the last decade, 38% of liver transplantations for cirrhosis were caused by alcohol-related disease 2. The progression from alcoholic steatosis to chronic hepatitis and to cirrhosis varies among ethnic groups and among different individuals from the same ethnic group, and it has been reported that some individuals, even if they have an excessive intake of alcohol for a long time, do not develop cirrhosis 1,3. This suggests that both environmental and genetic factors contribute to alcoholic cirrhosis onset 1,4. Among environmental factors related to alcohol, the quantity of alcohol consumption, the quality (drinking patterns) and the duration of at-risk alcohol consumption are considered when estimating the overall risk for cirrhosis 1. However, despite some studies showed the importance of age of exposure to liver stressors, such as hepatitis C virus (HCV), in determining the progression to cirrhosis 5,6, few data are available on the role of age at onset of at-risk alcohol consumption in determining alcoholic cirrhosis. A previous study showed that the risk of alcoholic cirrhosis onset was higher in individuals who started drinking after the age of 40 years 7. However, no other potential risk factors for alcoholic cirrhosis were investigated in that study.
Among the genetic factors, the patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409, a genetic variation consisting of an isoleucine to methionine substitution at position 148 (I148M) 8, has been associated with a wide spectrum of liver diseases, from hepatic steatosis 8,9, and liver damage 10,11 to fibrosis 12–15, cirrhosis 16,17 and hepatocellular carcinoma 16,18–21. Specifically, regarding alcohol-related liver disease, PNPLA3 I148M genetic variant has been found to be associated with an increased risk of both alcohol-related cirrhosis and cancer 17,20,22–25. However, no data are available on the effect of the PNPLA3 I148M genetic variant on alcoholic cirrhosis incidence.
The aim of this study was to investigate the effect of both environmental and genetic factors (particularly, the age at onset of at-risk alcohol consumption and the PNPLA3 I148M genetic variant) on alcoholic cirrhosis incidence in a population of at-risk drinkers.
Patients and methods
Study Population
A total of 753 consecutive individuals who were admitted to the Outpatient Clinics at the Department of Clinical Medicine, Policlinico Umberto I, Rome (Italy) for alcohol abuse or dependence between 2005 and 2010 were retrospectively examined. At the admittance, patients underwent a clinical examination with collection of detailed information about past and current health status and alcohol consumption. A daily at-risk alcohol consumption was defined as ≥3 and ≥2 alcohol units for men and women respectively. One unit of alcohol corresponds to 12 g of ethanol. The patterns and the amount of alcohol consumption from the onset of at-risk alcohol consumption were obtained by interview using the Lifetime Drinking History (LDH) 26. Particularly, the duration (in years) of at-risk alcohol consumption was calculated based on the reported age at onset of at-risk drinking. The amount of daily alcohol intake was extrapolated from the LDH and expressed as number of alcohol units per day. The diagnosis of alcoholic abuse or dependence was established by a psychiatrist according to the revised text of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria 27, which defines these two conditions as maladaptive patterns of alcohol use with different peculiar behavioural and psychological aspects, independently from the amount of alcohol intake (Table S1). The presence of cirrhosis was also assessed and the time of the cirrhosis diagnosis was reported. The diagnosis of cirrhosis was clinically defined by examining patients' past and present information on the health status. In particular, data from hospital discharge papers, physical examination, blood tests, imaging and endoscopy were analysed. Specifically, cirrhosis diagnosis was based on the presence of at least one of the following features: (i) current or past cirrhosis complications (e.g. ascites, gastrointestinal bleeding, hepatic encephalopathy); (ii) the presence of at least two parameters among hyperbilirubinaemia, hypoalbuminaemia, prolonged prothrombin time, low platelet count, irregular liver surface at ultrasound/CT, reduced portal vein flow at ultrasound, gastroesophageal varices at endoscopy. In the absence of overt cirrhosis, individuals with positivity of only one of these parameters were excluded from the current analyses.
Individuals with other aetiological factors (n = 141) of liver disease (i.e. hepatitis B and C infection, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilson's disease, hemochromatosis, Budd Chiari syndrome), with incomplete LDH data (n = 98), incomplete clinical/biochemical data to achieve a diagnosis of alcoholic cirrhosis and/or a defined age at cirrhosis diagnosis (n = 110), or with poor DNA quality (n = 20) were excluded from the analyses. Thus, a total of 384 individuals were analysed in the current report.
Blood samples were collected after an overnight fast and analysed for biochemical parameters. DNA was collected from all the individuals. Written informed consent was obtained from all the individuals and this study was approved by the local ethical committee.
Genotyping of the PNPLA3 I148M (rs738409) variant
Fluorogenic 5′-nucleotidase allelic discrimination assay (TaqMan® Applied Biosystems, Foster City, CA, USA) for the PNPLA3 rs738409 sequence variant was used to genotype, as previously described with a success rate of 99% 10.
Statistical analyses
Variables are shown as median and interquartile range or proportions. Genotype, allele and categorical variable distribution were compared by χ2 test. Non-normally distributed continuous variables were log-transformed before entering the statistical models.
An additive genetic model was tested. The differences in the continuous variables were assessed using a linear regression analysis after adjusting for age, gender, and BMI and, when appropriate, the presence of cirrhosis.
The incidence of cirrhosis was examined. Time of progression to cirrhosis was examined by Kaplan–Meier estimates of cumulative incidence rates. The beginning of the Kaplan–Meier curves was considered the reported age at onset of at-risk alcohol consumption. The follow-up time refers to the years of exposure to the at-risk alcohol consumption. It corresponds to the years from the age at onset of at-risk alcohol consumption to the age at cirrhosis onset or at the clinical examination, reflecting the period of exposure to the pathogenic agent (i.e. at-risk alcohol consumption).
Log-rank test was used to analyse differences in cumulative incidence between age at onset of at-risk alcohol consumption subgroups and across PNPLA3 I148M genotypes. Cox proportional-hazards models were used to evaluate the effect of age at onset of at-risk alcohol consumption and PNPLA3 I148M genotype on cirrhosis incidence after adjusting for confounding factors (i.e. gender, BMI, diabetes status, amount of daily alcohol consumption, alcohol behaviour). Analyses were carried out using the Statistical Package for Social Sciences (spss, version 18, Inc. Chicago, IL, USA). P-values <0.05 were considered significant.
Results
Demographic and clinical characteristics of the study population
A total of 384 individuals with at-risk alcohol consumption in the absence of other risk factors for chronic liver disease were included in the current analyses. Demographic and clinical characteristics are shown in Table 1. The vast majority of the individuals were of male gender (76.3%), with a median age of 46 years. They were all at-risk drinkers (median alcohol consumption was 12 alcohol units per day). The median age at onset of at-risk alcohol consumption was 24 years and the median duration of the at-risk consumption was 19 years. Cirrhosis was present in 22% of individuals.
Table 1.
Demographic and clinical characteristics of the overall study population
| n | 384 |
| Age (years) | 46 (38–56) |
| Male Gender, n (%) | 293 (76.3) |
| BMI (kg/m2) | 25.3 (23.0–28.2) |
| Diabetes, n (%) | 30 (7.8) |
| Alcohol abuse, n (%) | 23 (6.0) |
| Alcohol dependence, n (%) | 361 (94.0) |
| Age at onset of at-risk alcohol consumption (years) | 24 (18–32) |
| Daily alcohol consumption (units) | 12 (8–18) |
| Duration of at-risk alcohol consumption (years) | 19 (10–28) |
| I.N.R. | 1 (0.92–1.07) |
| Serum bilirubin (mg/dl) | 0.6 (0.5–1.0) |
| Serum creatinine (mg/dl) | 0.9 (0.7–1.0) |
| Serum AST (U/L) | 30 (20–51) |
| Serum ALT (U/L) | 29 (19–51) |
| Cirrhosis, n (%) | 84 (21.9) |
| Age at diagnosis of cirrhosis (years) | 55 (47–61) |
| Child-Pugh class, n (%)* | |
| A | 29 (34.5) |
| B | 36 (42.9) |
| C | 19 (22.6) |
| MELD score* | 13 (9–17) |
| PNPLA3, n (%) | |
| II | 164 (42.7) |
| IM | 165 (43.0) |
| MM | 55 (14.3) |
Data are expressed as median and interquartile range or as proportions. P-values for continuous variables were calculated using linear regression model adjusting for age, gender and BMI. Non-normally distributed variables were log-transformed before entering the model. Categorical variables' distribution was compared by χ2 test. Genotype and allele frequencies were in Hardy–Weinberg equilibrium (P > 0.05).
Child-Pugh classes and MELD score were assessed only in individuals with cirrhosis.
n, number; BMI, body mass index; I.N.R., international normalized ratio; AST, aspartate transferase; ALT, alanine transferase; MELD, model for end-stage liver disease; PNPLA3, patatin-like phospholipase domain-containing 3; II, individuals with two 148I alleles; IM, heterozygotes; MM, individuals with two 148M alleles.
Alcoholic cirrhosis incidence according to age at onset of at-risk alcohol consumption and PNPLA3 I148M genotypes
To examine whether the age at onset of at-risk alcohol consumption affects cirrhosis incidence, the study population was stratified by the median value (24 years of age) of this parameter. After stratifying individuals according to this parameter, a higher incidence of alcoholic cirrhosis was observed in individuals with an older compared with a younger age at onset of at-risk alcohol consumption (Log-rank P-value < 0.001; Fig. 1). In a sensitivity analysis, the incidence of cirrhosis was examined after stratifying the population by tertiles of age at onset of at-risk alcohol consumption and the same result was observed (Log-rank P-value < 0.001; Fig. S1).
Figure 1.
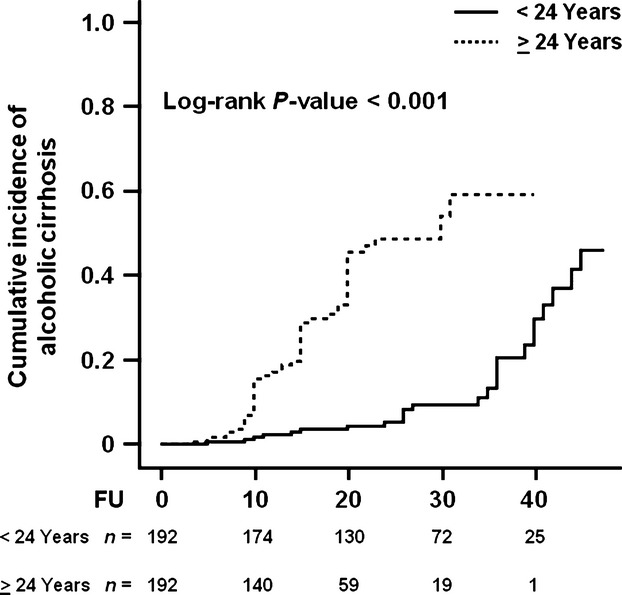
Cumulative incidence of alcoholic cirrhosis in individuals stratified by age at onset of at-risk alcohol consumption. Individuals were stratified by the median value of the age at onset of at-risk alcohol consumption, which was equal to 24 years. FU indicates the duration (years) of at-risk alcohol consumption, which was defined as a daily intake of ≥3 and ≥2 alcohol units for men and women respectively. Number of events: <24 Years, n = 25; ≥24 Years, n = 59. The number at the bottom indicates the number of individuals at risk at the following time points: 0, 10, 20, 30 and 40 years. Abbreviations: FU, follow-up.
Demographic and clinical characteristics of the population stratified by median age at onset of at-risk alcohol consumption are shown in Table S2. Individuals with age at onset of at-risk alcohol consumption ≥24 years were older, had a shorter duration of at-risk alcohol consumption and a lower daily alcohol intake. In addition to a higher incidence of cirrhosis, individuals with an older age at onset of at-risk alcohol consumption showed a more severe liver disease assessed by Child-Pugh class (P-value = 0.004) and MELD score (P-value = 0.009), compared with those with a younger age at onset of at-risk alcohol consumption (Table S2). In those with older age at onset of at-risk alcohol consumption, there was also a higher prevalence of diabetes (P-value = 0.001; Table S2).
Next the effect of PNPLA3 I148M genetic variant on alcoholic cirrhosis incidence was examined. Demographic and clinical characteristics of the population stratified by PNPLA3 genotype are depicted in Table S3. A higher cumulative incidence of cirrhosis was observed in the PNPLA3 148M allele carriers (Log-rank P-value < 0.001; Fig. 2).
Figure 2.
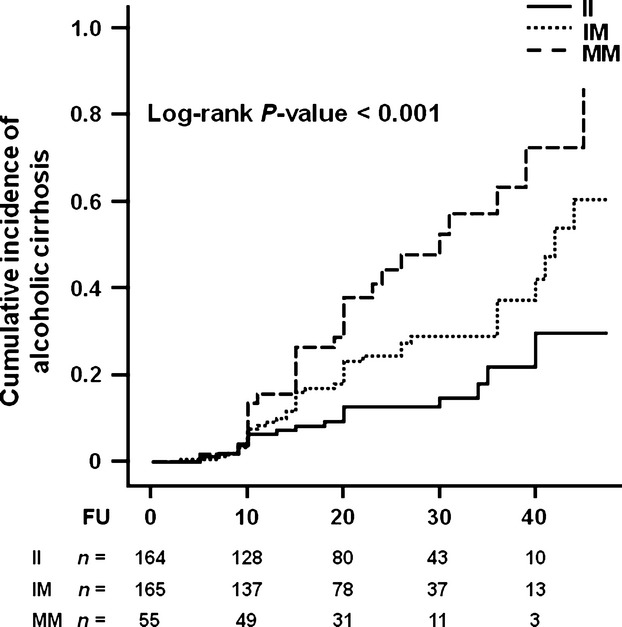
Cumulative incidence of alcoholic cirrhosis in individuals according to PNPLA3 I148M genotype. Individuals were stratified by the PNPLA3 I148M genotype. FU indicates the duration (years) of at-risk alcohol consumption, which was defined as a daily intake of ≥3 and ≥2 alcohol units for men and women respectively. Number of events: II, n = 19; IM, n = 39; MM, n = 26. The number at the bottom indicates the number of individuals at risk at the following time point: 0, 10, 20, 30 and 40 years. Abbreviations: PNPLA3, patatin-like phospholipase domain-containing 3; II, individuals with two 148I alleles; MM, individuals with two 148M alleles; IM, heterozygotes; FU, follow-up.
Finally, the incidence of cirrhosis was examined in the population stratified by both age at onset of at-risk alcohol consumption and PNPLA3 I148M genotypes. Carriers of the PNPLA3 148M allele had a higher incidence of cirrhosis, irrespective of the age at onset of at-risk alcohol consumption (Log-rank P-value < 0.001; Fig. 3). Individuals with the lowest incidence of cirrhosis were those with lower age at onset of at-risk alcohol intake and without the PNPLA3 148M allele. Conversely, those with the highest incidence of cirrhosis were the individuals with higher age at onset of at-risk alcohol intake carrying the 148M allele (Fig. 3).
Figure 3.
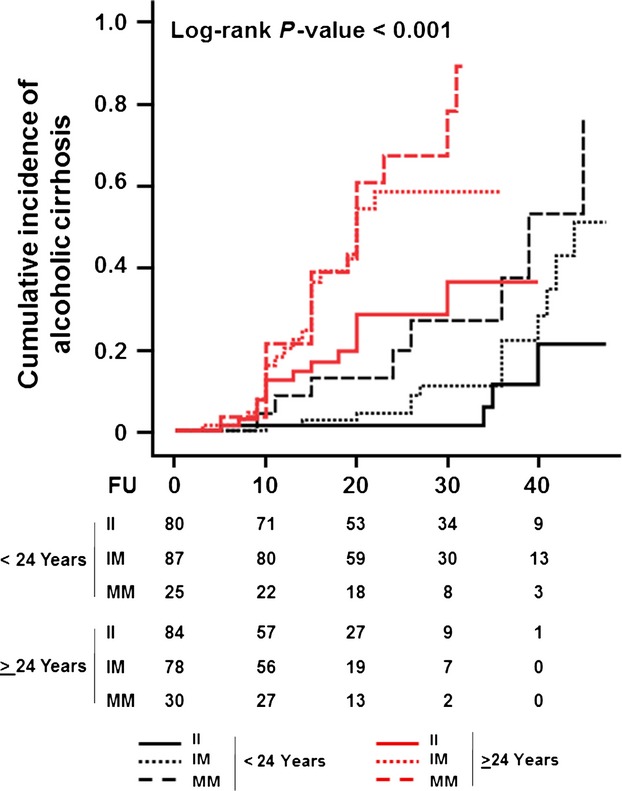
Cumulative incidence of alcoholic cirrhosis in individuals stratified by age at onset of at-risk alcohol consumption and PNPLA3 I148M genotype. Individuals were stratified by the median value of the age at onset of at-risk alcohol consumption, which was equal to 24 years, into two groups. Next, each group was divided according to the PNPLA3 I148M genotype. FU indicates the duration (years) of at-risk alcohol consumption, which was defined as a daily intake of ≥3 and ≥2 alcohol units for men and women respectively. The number of events was as follows: II n = 4, IM n = 13, MM n = 8 (<24 Years); II n = 15, IM n = 26, MM n = 18 (≥24 Years). The number at the bottom indicates the number of individuals at risk at the following time points: 0, 10, 20, 30 and 40 years. Abbreviations: PNPLA3, patatin-like phospholipase domain-containing 3; II, individuals with two 148I alleles; MM, individuals with two 148M alleles; IM, heterozygotes; FU, follow-up.
Risk of developing alcoholic cirrhosis
Next, the risk of developing alcoholic cirrhosis was examined. Both the age at onset of at-risk alcohol consumption (H.R. estimated for each decade (10 years) (95% C.I.): 2.76 (2.18–3.50); P-value<0.001) and PNPLA3 148M allele (H.R. 1.53 (1.07–2.19); P-value = 0.021) were independently associated with an increased risk of developing alcoholic cirrhosis (Table 2).
Table 2.
Risk of developing alcoholic cirrhosis
| H.R. | 95% C.I. | P-value | |
|---|---|---|---|
| Gender (male) | 1.34 | 0.72–2.56 | 0.349 |
| BMI (kg/m2) | 1.05 | 0.98–1.12 | 0.154 |
| Diabetes | 1.42 | 0.70–2.90 | 0.335 |
| Daily alcohol consumption (unit) | 1.03 | 1.00–1.06 | 0.041 |
| Alcohol behaviour* | 1.41 | 0.49–4.04 | 0.522 |
| Age at onset of at-risk alcohol consumption† | 2.76 | 2.18–3.50 | <0.001 |
| PNPLA3 148M allele | 1.53 | 1.07–2.19 | 0.021 |
Abuse vs. Dependence.
It refers to the risk for each 10 years of age.
H.R., hazard ratio; C.I., confidence interval; BMI, body mass index; PNPLA3, patatin-like phospholipase domain-containing 3.
To examine if the effect of the PNPLA3 148M allele was different in the individuals with a younger (<24 years) or older (≥ 24 years) age at onset of at-risk alcohol consumption, we examined the risk of developing cirrhosis in these two groups separately. A two-fold increase risk for the 148M allele was observed in individuals with a younger compared with an older age at onset of at-risk alcohol consumption (H.R. (95% C.I.): 3.03 (1.53–6.00) vs. 1.61 (1.09–2.38), respectively; Table 3). In individuals with older age at onset of at-risk alcohol consumption, the presence of type 2 diabetes and higher BMI were also associated with the risk of cirrhosis onset (Table 3).
Table 3.
Risk of developing alcoholic cirrhosis in individuals stratified by age at onset of at-risk alcohol consumption
| Age at onset of at-risk alcohol consumption | ||||||
|---|---|---|---|---|---|---|
| <24 years | ≥24 years | |||||
| H.R. | 95% C.I. | P-value | H.R. | 95% C.I. | P-value | |
| Gender (male) | 1.28 | 0.39–4.19 | 0.680 | 1.86 | 0.85–4.10 | 0.121 |
| BMI (kg/m2) | 1.01 | 0.89–1.14 | 0.905 | 1.10 | 1.02–1.19 | 0.021 |
| Diabetes | 2.04 | 0.50–8.40 | 0.323 | 2.76 | 1.26–6.04 | 0.011 |
| Daily alcohol intake (unit) | 1.03 | 0.99–1.08 | 0.094 | 1.04 | 0.98–1.09 | 0.179 |
| Alcohol behaviour* | 3.11 | 0.63–15.30 | 0.162 | 1.37 | 0.32–5.86 | 0.674 |
| PNPLA3 148M allele | 3.03 | 1.53–6.00 | 0.001 | 1.61 | 1.09–2.38 | 0.017 |
Abuse vs. Dependence.
H.R., hazard ratio; C.I., confidence interval; BMI, body mass index; PNPLA3, patatin-like phospholipase domain-containing 3.
Discussion
This study shows for the first time that age at onset of at-risk alcohol consumption is an independent risk factor for alcoholic cirrhosis and this is independent by the PNPLA3 I148M genetic variant in at-risk drinkers.
In the current report, we examined the incidence of alcoholic cirrhosis in a cohort of individuals with at-risk alcohol consumption from Italy, according to environmental and genetic factors.
To investigate whether the age at the beginning of at-risk alcohol consumption affects cirrhosis onset, we analysed the cumulative incidence of this disease in our population stratified by the median of the distribution of this variable. A higher incidence of alcoholic cirrhosis was observed in those individuals with an older (≥24 years) compared with a younger (<24 years) age at onset of at-risk alcohol consumption (Log-rank P-value: <0.001). Consistently, a higher risk of alcoholic cirrhosis onset was observed for older age at the beginning of at-risk alcohol consumption. Interestingly, individuals with an older age at onset of at-risk alcohol consumption showed also a more severe cirrhosis stage, despite a lower daily alcohol intake and a shorter duration of at-risk alcohol consumption. Thus, even if exposed for a shorter period to the pathogenic agent (excess alcohol intake), these individuals presented a higher incidence of cirrhosis and a more severe liver disease. This finding is in line with previous data showing the importance of the age at exposure to the pathogenic agent or stressor on the HCV-related chronic liver diseases 5,6,28 and of ageing on liver transplant outcome 29. Specifically, the older age of exposure to HCV infection or to ischaemia-reperfusion injury (for liver grafts during transplantation) leads to a faster HCV-related fibrosis progression in chronic HCV infection or to a worse graft function after liver transplantation. With regard to alcohol, the increased liver sensitivity to this pathogenic agent with age may be ascribed to an increased sensitivity of the liver to its chronic effects with age 30, as a consequence of age-related reduced alcohol metabolism in the liver and of cellular senescence with impaired hepatocyte function 31,32.
In a previous study, Poynard et al. showed that age at onset of at-risk alcohol consumption greater than 40 years was associated with an increased risk of cirrhosis 7. However, this study was performed in subjects whose daily alcoholic consumption, at least of 50 g during the year preceding their enrolment, was not further detailed and no information on other risk factors (environmental or genetic causes) were available. Moreover, other previous studies focused on age of the patients rather than the age at exposure to the liver stressor 33,34.
Next, we examined the effect of PNPLA3 I148M variant on the incidence of alcoholic cirrhosis. Previous studies showed an association between PNPLA3 I148M genetic variant and alcoholic cirrhosis only in cross-sectional studies 17,20–25. However, to date, no studies have investigated the role of this genetic variant on alcoholic cirrhosis incidence. In this study, we found that PNPLA3 148M allele was associated with a higher incidence of alcoholic disease (Log-rank P-value < 0.001). A 50% increase in the risk of cirrhosis onset was observed for each 148 M allele. Interestingly, the effect of the PNPLA3 148M allele was even more pronounced in individuals with a younger compared with an older age at onset of at-risk alcohol consumption. In the former group of subjects, the risk of developing cirrhosis was two-fold higher compared with individuals in the latter group carrying the 148 M allele (H.R. (95% C.I.): 3.03 (1.53–6.00) vs. 1.61 (1.09–2.38) respectively). This finding suggests that the genetic background has a stronger effect in younger individuals. In older individuals, other metabolic factors (i.e. diabetes and obesity) play also an important role in the development of alcoholic cirrhosis. In these individuals, the chronic liver damage may be possibly ascribed to the combined effect of alcoholic and metabolic steatohepatitis.
The novelty of the present report resides in examining, for the first time, the effect of PNPLA3 genetic variant combined with the age at onset of at-risk alcohol consumption on the alcoholic cirrhosis incidence.
A limitation is that the retrospective study design may have led to selection bias. However, prospective studies investigating on the effect of alcohol consumption would be too long and unfeasible, whereas randomized investigations cannot be performed for obvious ethical reasons. Another limitation of the present report is that no data were available on factors influencing the liver damage, such as smoking, coffee and cannabis consumption, frequency of drinking during the day and relationship with food intake.
In conclusion, the present report shows that older age at onset of at-risk alcohol consumption and the PNPLA3 148 M allele are independent risk factors for alcoholic cirrhosis incidence.
Acknowledgments
Financial support: This study was supported by grants from the ‘Fondazione Onlus Parioli’, Rome, Italy and from the Swedish Research Council (K2010-55X-11285-13, K2008-65X-20753-01-4, K2013-99X-22230-01-4), the Hjärt-Lungfonden (Project number: 20120533), the Swedish Foundation for Strategic Research to Sahlgrenska Centre for Cardiovascular and Metabolic Research, the Swedish federal government under the LUA/ALF agreement and the Sahlgrenska Academy.
Conflict of interest: The authors do not have any disclosures to report.
Glossary
- ALT
alanine transferase
- AST
aspartate transferase
- BMI
body mass index
- C.I.
confidence interval
- H.R.
hazard ratio
- I.N.R.
international normalized ratio
- MELD
model for end-stage liver disease
- PNPLA3
patatin-like phospholipase domain-containing protein 3
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Cumulative incidence of alcoholic cirrhosis in individuals stratified by tertiles of distribution of the age at onset of at-risk alcohol consumption.
DSM-IV-TR diagnostic criteria for alcohol abuse and dependence.
Table S2. Demographic and clinical characteristics of the study population stratified by the age at onset of at-risk alcohol consumption.
Table S3. Demographic and clinical characteristics of the study population stratified by PNPLA3 I148M genotypes.
References
- 1.EASL. Clinical practical guidelines management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A Report From The European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–88. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Stinson FS, Grant BF, Dufour MC. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001;25:1181–7. [PubMed] [Google Scholar]
- 4.Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150–9. doi: 10.1136/gutjnl-2011-301239. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, Bedossa P, Opolon P for the OBSVIRC, METAVIR, CLINIVIR, DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 6.Pradat P, Voirin N, Tillmann HL, Chevallier M, Trépo C. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver Int. 2007;27:335–9. doi: 10.1111/j.1478-3231.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, Mathurin P, Lai CL, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–65. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 8.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenti L, Maggioni P, Piperno A, et al. Patatin-like phospholipase domain containing-3 gene i148 m polymorphism, steatosis, and liver damage in hereditary hemochromatosis. World J Gastroenterol. 2012;18:2813–20. doi: 10.3748/wjg.v18.i22.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Sentinelli F, Dash S, et al. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes (Lond) 2010;34:190–4. doi: 10.1038/ijo.2009.216. [DOI] [PubMed] [Google Scholar]
- 11.Romeo S, Sentinelli F, Cambuli VM, et al. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010;53:335–8. doi: 10.1016/j.jhep.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–94. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 13.Petta S, Grimaudo S, Cammà C, et al. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J Hepatol. 2012;56:1356–62. doi: 10.1016/j.jhep.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Rotman Y, Koh C, Zmuda JM, et al. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–17. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 16.Valenti L, Rumi M, Galmozzi E, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791–9. doi: 10.1002/hep.24123. [DOI] [PubMed] [Google Scholar]
- 17.Stickel F, Buch S, Lau K, et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 18.Burza MA, Pirazzi C, Maglio C, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037–41. doi: 10.1016/j.dld.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Ginanni Corradini S, Burza MA, Molinaro A, Romeo S. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology. 2011;53:1776. doi: 10.1002/hep.24244. [DOI] [PubMed] [Google Scholar]
- 20.Trépo E, Guyot E, Ganne-Carrie N, et al. PNPLA3 (rs738409 C>G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. 2012;55:1307–8. doi: 10.1002/hep.25518. [DOI] [PubMed] [Google Scholar]
- 21.Falleti E, Fabris C, Cmet S, et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011;31:1137–43. doi: 10.1111/j.1478-3231.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 22.Tian C, Stokowski RP, Kershenobich D, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–3. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 23.Trépo E, Gustot T, Degré D, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–12. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Seth D, Daly AK, Haber PS, Day CP. Patatin-like phospholipase domain containing 3: a case in point linking genetic susceptibility for alcoholic and nonalcoholic liver disease. Hepatology. 2010;51:1463–5. doi: 10.1002/hep.23606. [DOI] [PubMed] [Google Scholar]
- 25.Nischalke HD, Berger C, Luda C, et al. The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis C cirrhosis. PLoS One. 2011;6:e27087. doi: 10.1371/journal.pone.0027087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner HA, Sheu WJ. Reliability of alcohol use indices. the lifetime drinking history and the mast. J Stud Alcohol. 1982;43:1157–70. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Task Force on DSM-IV. Substance use disorders. Appendix F: DSM-IV-TR Diagnostic Criteria for Alcohol Abuse and Dependence. In: American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology. 2011;54:1127–34. doi: 10.1002/hep.24503. [DOI] [PubMed] [Google Scholar]
- 29.Keswani RN, Ahmed A, Keeffe EB. Older age and liver transplantation: a review. Liver Transpl. 2004;10:957–67. doi: 10.1002/lt.20155. [DOI] [PubMed] [Google Scholar]
- 30.Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care. 2008;11:21–6. doi: 10.1097/MCO.0b013e3282f30564. [DOI] [PubMed] [Google Scholar]
- 31.Schmucker DL. Age-related changes in liver structure and function: implications for disease? Exp Gerontol. 2005;40:650–9. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Hoare M, Das T, Alexander G. Ageing, telomeres, senescence, and liver injury. J Hepatol. 2010;53:950–61. doi: 10.1016/j.jhep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–8. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 34.Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–11. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cumulative incidence of alcoholic cirrhosis in individuals stratified by tertiles of distribution of the age at onset of at-risk alcohol consumption.
DSM-IV-TR diagnostic criteria for alcohol abuse and dependence.
Table S2. Demographic and clinical characteristics of the study population stratified by the age at onset of at-risk alcohol consumption.
Table S3. Demographic and clinical characteristics of the study population stratified by PNPLA3 I148M genotypes.


