Abstract
The pharmacokinetics of marbofloxacin in pigs were evaluated as a function of dose and animal age following intravenous and intramuscular administration of a 16% solution (Forcyl®). The absolute bioavailability of marbofloxacin as well as the dose proportionality was evaluated in 27-week-old fattening pigs. Blood PK and urinary excretion of marbofloxacin were evaluated after a single intramuscular dose of 8 mg/kg in 16-week-old male pigs. An additional group of 12-week-old weaned piglets was used for the evaluation of age-related kinetics. The plasma and urine concentration of marbofloxacin was determined using a HPLC method. Pharmacokinetic parameters were calculated using noncompartmental methods. After intravenous administration in 27-week-old fattening pigs, the total body clearance was 0.065 L/h·kg. After intramuscular administration to the same animals, the mean observed Cmax was 6.30 μg/mL, and the AUCINF was 115 μg·h/mL. The absolute bioavailability was 91.5%, and dose proportionality was shown within the dose range of 4–16 mg/kg. The renal clearance was about half of the value of the total clearance. The total systemic clearance values significantly decreased as a function of age, being 0.092 L/h·kg and 0.079 L/h·kg in pigs aged 12 and 16 weeks, respectively.
Introduction
Marbofloxacin is a synthetic third-generation fluoroquinolone, with a broad spectrum of activity against many pathogens of veterinary importance. The pharmacokinetics of marbofloxacin in pigs have already been described after intravenous, oral and intramuscular administration (Petracca et al., 1993; Ding et al., 2010). The product Marbocyl® is licensed as a 2% solution for the treatment of respiratory infections and as a 10% solution for the treatment of the metritis–mastitis–agalactia syndrome in sows in the European Union. For both indications, the dosage regimen is 2 mg/kg once daily for 3–5 days. A new single dosage regimen of 8 mg/kg was developed for the treatment of respiratory diseases (Schneider et al., 2012) together with a more convenient 16% solution. The aim of the present work was to determine the disposition of marbofloxacin in pigs as a function of dose and age. Furthermore, because age effects can be related to changes in renal function, the urinary elimination of this drug was explored. In each study of the presented work, the recently approved 16% marbofloxacin solution (Forcyl®) was selected for both intravenous and intramuscular injections. For the determination of the basic parameters, a single intravenous and intramuscular dose of 8 mg/kg was administered to 10 fattening pigs. The dose proportionality of the kinetic response was tested with the same animals after additional single intramuscular doses of 4 and 16 mg/kg (study 1). To evaluate the effect of the age of the animals on the disposition of marbofloxacin, two other groups of animals were used. While in the first study, the age of the pigs was 27 weeks, the age of the animals in the two additional groups was 12 (study 2) and 16 weeks old (study 3), the youngest animals being postweaning piglets. A single intravenous and intramuscular dose of 8 mg/kg was given to both additional groups. To study the urinary elimination of marbofloxacin, the pigs aged 16 weeks were used and placed into metabolic cages (study 3). Urine samples should be collected without being contaminated with the faeces, although marbofloxacin should be excreted primarily in urine (European Medicines Agency, 1999). For this purpose, only male animals were used and they were fitted with a faeces collection bag maintained with nappies. Sex differences in pharmacokinetic parameters were not specifically evaluated, but previous studies did not highlight any difference. The urinary clearance of marbofloxacin was thus determined as well as the elimination of the metabolites, N-oxide-marbofloxacin and desmethyl-marbofloxacin.
Materials and Methods
Animals, treatments and sampling
Study 1: dose proportionality in fattening pigs
Ten cross-breed pigs (five male and five female animals) weighing 50–60 kg were used, and their age was about 27 weeks old. Before the last administration, about 2 months later, the pigs weighed between 67 and 80 kg. The animals were kept free in two straw-bedded pens of 4 × 3.5 m (five pigs per pen, sex separated), located in a room for large animals, and they were acclimatized to these housing conditions for about 2 weeks before study start. The food was composed of flattened barleycorn available ad libitum. Water was also available ad libitum. The experimental design was a nonrandomized four-period design with three periods dedicated to intramuscular dosing and one period dedicated to the intravenous dosing. For the intramuscular administration periods, three single doses of 4, 8 and 16 mg/kg were given in a cross-over design. The pigs were distributed by randomization on body weight between two groups of three animals and one group of four animals. For each period, each group received a different dose; the dosing sequence was not randomized. The intramuscular administrations were performed alternately into the right and the left side of the neck of each animal. The intravenous administration was given during the fourth period at a dose of 8 mg/kg into the auricular vein. A washout period of 2 weeks was observed between each treatment period. Marbofloxacin was administered as a 16 % solution for injection (Forcyl®, Vétoquinol, Lure, France). After intramuscular treatment, blood samples of about 5 mL were taken by puncture of the right or left jugular vein, in heparinized Vacutainer tubes at the following time points: 0, 20, 40 min, 1, 1.5, 2, 3, 4, 6, 10, 24, 32, 48, 56, 72, 80 and 96 h. After intravenous administration, the following time points were taken at the right or left jugular vein: 0, 5, 10, 20, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 10, 24, 32, 48, 56 and 72 h. The hour-0 sample was taken prior drug administration. The blood samples were centrifuged at about 1945 g for 10 min at about 5 °C. The plasma from each Vacutainer was divided into aliquots of about 0.6 mL which were deep frozen at about −75 °C until analysis.
Study 2: absolute bioavailability in weaned piglets
Ten cross-breed piglets (five females and five males) weighing 20–30 kg were used, and their age was about 12 weeks old. The housing and feeding of the animals were identical to the animals of study 1, and they were acclimatized to these housing conditions for about 2 weeks before study start. The intramuscular and intravenous injections at a dose of 8 mg/kg were given as a two period's cross-over design. In a first period, the intravenous administration was performed into the right jugular vein, and the intramuscular administration was given into the right side of the neck in a second period. Treatments were separated by a 2-week washout interval. Marbofloxacin was administered as a 16 % solution for injection (Forcyl®, Vétoquinol, Lure, France). After intramuscular and intravenous treatment, blood samples of about 5 mL were taken by puncture of the right or left jugular vein (left jugular vein after intravenous administration until the time point of 1 h after administration), in heparinized Vacutainer tubes. The time points were the following: 0, 20, 40 min, 1, 1.5, 2, 3, 4, 6, 10, 24, 32, 48, 56, 72, 80 and 96 h after intramuscular administration and 0, 5, 10, 20, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 10, 24, 32, 48, 56, 72, 80 and 96 h after intravenous administration. The plasma samples were prepared and stored in the same way as in study 1.
Study 3: urinary excretion in adult pigs
Ten cross-breed male pigs weighing between 29 and 35 kg were used, and they were about 16 weeks old. The animals were housed individually in stainless steel metabolism cages, and they were acclimatized to these housing conditions 1 day before the first administration. The animals were fed with flattened barleycorn. Water and food were available ad libitum. In a first period, the pigs received an intravenous dose of 8 mg/kg into the right auricular vein. The intramuscular dose of 8 mg/kg was performed into the right side of the neck during the second period. Treatments were separated by a 2-week washout interval. After intravenous administration, blood samples were taken at the following time points: 0, 5, 15, 30 min, 1, 2, 4, 6, 8, 12, 24, 32, 48, 56, 72, 80, 96 and 104 h. After intramuscular administration, blood samples were taken at the following time points: 0, 30 min, 1, 2, 4, 8, 12, 24, 32, 48, 56, 72, 80, 96 and 104 h. The plasma samples were prepared and stored in the same way as in study 1. For urine sampling, previous experiments showed that female animals could not be used because sample collection was not reliable and very impractical due to proximity between vulva and anus. Therefore, only male animals were used and to avoid a faecal contamination, they were fitted with a faeces collecting bag maintained by a nappy, both being changed once or twice a day. The total amount of the excreted urine was collected from the metabolism cage at the following time points for each treatment period: 0, 4, 8, 12, 24, 32, 48, 52, 56, 72, 80, 96 and 104 h. Urine samples were weighed, filtered and divided into six aliquots of about 1.1 mL poured in labelled polypropylene tubes. The samples were then stored at about −75 °C until analysis.
Sample analysis
Determination of marbofloxacin in the plasma samples was performed using a HPLC method adapted from Vallé et al. (2012). The difference was linked to the analytical column which was a Gemini-NX C18 column (5 μm, 150 × 4.6 mm), fitted with a Gemini-NX C18 guard column (5 μm, 4 × 3 mm) (Phenomenex, Torrance, CA, USA). The retention time of marbofloxacin was 5.5–6.5 min and of the internal standard was 7–8 min. The lower limit of quantification of the method was 0.005 μg/mL, and the upper quantification limit was 5 μg/mL. The intra- and inter-assay coefficients of variation were <2.5% and <4.0%, respectively, for the quality control (QC) samples close to the limit of quantification. Accuracy of these QC samples was within the range of 96–103%. The coefficients of correlation of the calibration curves were >0.999.
For urine samples, the method was very close to the method of Vallé et al. (2012). The only difference was the absence of protein precipitation before extraction of the sample. The lower limit of quantification of the method was 0.075 μg/mL, and the upper limit of quantification was 4 μg/mL. The intra- and inter-assay coefficients of variation were <2.1% and <3.0%, respectively, for the lower QC samples. The accuracy of the QC samples was within the range of 97–101%. The coefficients of correlation of the calibration curves were >0.999.
The analytical method for the assay of the metabolites of marbofloxacin in urine was quite different from that of Vallé et al. (2012). One millilitre of urine sample was mixed with 100 μL of internal standard solution (enrofloxacin, Sigma–Aldrich, Saint Quentin Fallavier, France) and centrifuged at about 15 875 g for 10 min at about 5 °C. Supernatant was transferred into an amber glass tube, and after addition of 1 mL of pH 7 buffer, the mixture was loaded onto an extraction cartridge (Oasis, Waters, USA) which was previously primed with 3 mL of methanol and about 3 mL of ultrapure water. The cartridge was washed with 3 mL of ultrapure water and vacuum dried for about 1 min. The metabolites were eluted with 3 mL of methanol, and the cartridge was again vacuum dried for 1 min. The methanol was evaporated into dryness under a nitrogen flow at about 38 °C. The dry extract was solubilized in 200 μL of a mixture of mobile phase which was transferred into a screw-capped vial which was placed into a refrigerated autosampler, and 50 μL of the solution were injected into the chromatographic system. The analytical column was the same as the column used for marbofloxacin. The mobile phase was a mixture of pH 2.7 buffer (phase A) and methanol/acetonitrile (80/20 v/v) (phase B) and was delivered at a flow rate of 1 mL/min using a gradient mode. At start of analysis, mobile phase was a mixture of 90% of phase A and 10% of phase B during 5 min. The mixture was subsequently changed to one of 80% of phase A and 20% of phase B over the next 10-min period before changing back to the initial conditions (90/10). The retention time of desmethyl-marbofloxacin was 13–16 min, 17–21 min for N-oxide-marbofloxacin and 20–26 min for the internal standard (enrofloxacin). The lower limit of quantification of desmethyl-marbofloxacin and N-oxide-marbofloxacin was 0.05 and 0.2 μg/mL, respectively. The upper limit of quantification for both metabolites was 5 μg/mL. The intra- and inter-assay coefficients of variation were <3.0% and <4.0%, respectively, for the lower QC samples. The accuracy of the QC samples was within the range of 96–104%. The coefficients of correlation of the calibration curves were >0.997.
Pharmacokinetic evaluation
The plasma pharmacokinetic parameters were determined using noncompartmental analysis with the winnonlin software version 5.0.1 (Pharsight Corporation, St Louis, MO, USA). The area under the plasma concentration–time curve until the last measurable time point (AUClast) was calculated using the linear trapezoidal method. The maximum plasma concentration (Cmax) and the occurrence time of Cmax (Tmax) for each animal were taken directly from the concentration data. The rate constant associated with the slope of the terminal elimination phase (λz) was determined using linear regression. The plasma terminal half-life (T½λz) was calculated by the formula: T½λz = ln(2)/ λz. The area under the plasma concentration–time curve extrapolated to infinity (AUCINF) was calculated using the formula: AUCINF = AUClast + Clast/λz with Clast being the last measurable concentration. The absolute bioavailability (F) was determined with the formula: F = ((AUCINFim/AUCINFiv) × (Div/Dim)) with AUCINFim, the AUCINF obtained after intramuscular administration, AUCINFiv, the AUCINF obtained after intravenous administration, Div, the actual administered intravenous dose, Dim, the actual administered intramuscular dose. The total body clearance, Cl, was calculated as the actual administered dose after intravenous administration, Div, divided by the AUCINF (Div/AUCINF). The volume of distribution at steady state, Vss, was obtained with the following equation: Vss = Cl × MRTINF with MRTINF the mean residence time extrapolated to infinity obtained after intravenous administration.
The urine pharmacokinetic parameters were determined using noncompartmental analysis with excretion rate data and mid-point of collection interval for marbofloxacin and its two metabolites. The urine terminal half-life (T½λz) was calculated with the same formula used for plasma data. The renal clearance, ClR, was calculated after intravenous administration with the following equation: ClR = Xu [Δt]/AUC[Δt] with Xu[Δt] being the total excreted amount of marbofloxacin recovered in urine during the collection interval and AUC[Δt] the area under the plasma concentration–time curve for the same interval. Clearance value was thereafter normalized by the body weight. The classical way of calculating renal clearance could not be used because the total amount of marbofloxacin eliminated in urine (Xu∞) is not known. However, as the cumulative excreted-amount reached a plateau at the last sampling time point, the calculation of the clearance was deemed acceptable.
Statistical analyses
Dose proportionality was evaluated with respect to both Cmax and AUCINF obtained in pigs when marbofloxacin was administered within a dose range of 4–16 mg/kg (Study 1). Prior to evaluation of Cmax and AUCINF estimates, parameter values were dose corrected. To evaluate dose proportionality, a linear regression analysis was used to estimate the slope and intercept of the lines obtained for each pharmacokinetic parameters vs. the corresponding theoretical doses. Thereafter, a theoretical line was generated using the origin (0,0) and the PK parameter values obtained at the lowest dose (4 mg/kg). The slope of this theoretical line was estimated by a linear regression analysis. Slopes and intercepts of both observed and theoretical regression lines were subsequently compared using a two-way analysis of variance.
The effect of the age of the animals on the pharmacokinetic parameters was evaluated by comparing the following parameters within the three age groups of pigs (12, 16 and 27 weeks old): total body clearance, volume of distribution and terminal half-life (after intravenous administrations), Cmax and AUCINF (after intramuscular administrations). For all parameters, a one-way analysis of variance was used.
Results
The main pharmacokinetic parameters obtained in study 1 are presented in Table 1. The predose concentration of all individual samples was below the lower limit of quantification. After intravenous administration, the total body clearance, Cl, with a value of 0.065 L/h·kg (SD = 0.011 L/h·kg) could be qualified as low according to Toutain and Bousquet-Melou (2004). The volume of distribution at steady state, Vss, was 1.58 L/kg (SD = 0.266 L/kg), and the terminal half-life, T½λz, was 18.4 h (SD = 3.86 h). The AUCINF was 127 μg·h/mL (SD = 22.1 μg·h/mL), with <10% of this value contributed by the extrapolated portion of the curve. Following an intramuscular dose of 8 mg/kg, the mean observed Cmax was 6.30 μg/mL (SD = 1.81 μg/mL) which occurred at an observed Tmax of 0.95 h (SD = 0.83 h). The AUCINF was 115 μg·h/mL (SD = 18.7 μg·h/mL), with the extrapolated component being <10% of the total area. The absolute bioavailability (F) was 91.5% (SD = 13.7%). As shown in Table 1 and in Fig.1, Cmax and AUCINF increased in a dose-proportional manner. The statistical analysis confirmed that the theoretical regression line was not significantly different from the observed regression line (P = 0.48 for the slopes of the lines calculated with Cmax and P = 0.95 for the slopes of the lines calculated with AUCINF). The linear plots of both PK values vs. administered dose are presented in Fig.2.
Table 1.
Mean pharmacokinetic parameters of marbofloxacin in 27-week-old pigs (standard deviations in brackets) after a single intravenous dose of 8 mg/kg and single intramuscular doses of 4, 8 and 16 mg/kg
| Parameter | IV 8 mg/kg | IM 4 mg/kg | IM 8 mg/kg | IM 16 mg/kg |
|---|---|---|---|---|
| Cl (L/h·kg) (SD) | 0.065 (0.011) | – | – | – |
| Vss (L/kg) (SD) | 1.58 (0.266) | – | – | – |
| T½λz (h) (SD) | 18.4 (3.86) | 15.4 (5.32) | 15.1 (4.16) | 15.2 (2.01) |
| AUCINF (μg·h/mL) (SD) | 127 (22.1) | 56.9 (20.8) | 115 (18.7) | 228 (32.9) |
| AUC%Extrap (%) (SD) | 7.10 (4.02) | 2.56 (3.20) | 1.39 (1.45) | 1.26 (0.65) |
| Cmax (μg/mL) (SD) | – | 3.38 (0.866) | 6.30 (1.81) | 15.5 (8.45) |
| Tmax (h) (SD) | – | 1.18 (0.46) | 0.95 (0.83) | 1.06 (0.91) |
| F (%) (SD) | – | 90.0 (28.2) | 91.5 (13.7) | 90.1 (10.2) |
IV: intravenous; IM: intramuscular; SD: standard deviation; Cl: total body clearance; Vss: volume of distribution at steady state; T½λz: last elimination half-life; AUCINF: area under the concentration–time curve extrapolated to infinity; AUC%Extrap: extrapolated part of the AUCINF; Cmax: maximum plasma concentration; Tmax: occurrence time of the maximum plasma concentration; F: absolute bioavailability.
Figure 1.

Mean concentration–time profiles of marbofloxacin in plasma of 27-week-old pigs after single intramuscular doses of 4, 8 and 16 mg/kg.
Figure 2.

Linear regression plots between administered dose and Cmax values and between administered dose and AUCINF values. Mean values and standard deviations are represented in the plots.
In study 1, male and female animals were used, whereas the two other studies were carried out with only male pigs. The parameters obtained in male and female animals were very similar (mean Cl was 0.063 and 0.067 L/h·kg for male and female animals, respectively), indicating that there was no sex effect. Therefore, based on an assumption of no age by gender interaction, the results obtained across all three studies were compared.
The results obtained in the two other groups of pigs are presented in Table 2. For both study 2 and study 3, predose concentration of marbofloxacin was below the lower limit of quantification. After intravenous administration, the total body clearance in the youngest piglets was 0.092 L/h·kg (SD = 0.004 L/h·kg), and in the 16 weeks old pigs, it was 0.079 L/h·kg (SD = 0.009 L/h·kg). Both values were higher than that observed in the 27-week-old animals. Mean concentration–time profiles are shown in Fig.3. The clearance values of the three groups were significantly different (P < 0.0001). Thus, as shown in Table 2 and Fig.3, the clearance decreased as pigs matured, that is, the elimination of marbofloxacin was lower in older animals.
Table 2.
Mean pharmacokinetic parameters of marbofloxacin in piglets (standard deviations in brackets) of 12 and 16 weeks old after a single intravenous and intramuscular dose of 8 mg/kg
| Parameter | 12 weeks | 16 weeks | ||
|---|---|---|---|---|
| IV | IM | IV | IM | |
| Cl (L/h·kg) (SD) | 0.092 (0.004) | – | 0.079 (0.009) | – |
| Vss (L/kg) (SD) | 1.58 (0.238) | – | 1.75 (0.124) | – |
| T½λz (h) (SD) | 13.5 (1.37) | 13.2 (1.20) | 18.0 (2.63) | 16.7 (2.13) |
| AUCINF (μg·h/mL) (SD) | 88.6 (4.54) | 79.9 (4.48) | 102 (13.1) | 106 (10.7) |
| AUC%Extrap (%) (SD) | 0.67 (0.30) | 0.62 (0.25) | 1.63 (0.82) | 1.31 (0.63) |
| ClR (L/h·kg) (SD) | – | – | 0.041 (0.007) | – |
| Cmax (μg/mL) (SD) | – | 5.55 (2.88) | – | 5.86 (0.666) |
| Tmax (h) (SD) | – | 0.93 (0.86) | – | 1.15 (0.63) |
| F (%) (SD) | – | 89.6 (7.88) | – | 105 (12.0) |
IV: intravenous; IM: intramuscular; SD: standard deviation; Cl: total body clearance; Vss: volume of distribution at steady state; T½λz: last elimination half-life; AUCINF: area under the concentration–time curve extrapolated to infinity; AUC%Extrap: extrapolated part of the AUCINF; ClR: renal clearance; Cmax: maximum plasma concentration; Tmax: occurrence time of the maximum plasma concentration; F: absolute bioavailability.
Figure 3.

Mean concentration–time profiles of marbofloxacin in plasma of pigs of different ages after a single intravenous dose of 8 mg/kg.
On the other hand, the volume of distribution was not significantly changed (P = 0.16). The plasma terminal half-life, T½λz, tended to increase with age (which is consistent with a decrease in Cl) although the differences between the three age groups were not statistically significantly different (P > 0.05). After intramuscular administration, the observed Cmax was 5.55 μg/mL (SD = 2.88 μg/mL) in the youngest animals and 5.86 μg/mL (SD = 0.666 μg/mL) in the 16-weeks-old pigs. The Cmax values tended to increase with the age of the pig although the difference was not statistically significant. A similar outcome was obtained for the AUCINF values. The absolute bioavailability (F) remained very high in both groups. As growth is very fast in the youngest animals (12 weeks of age), the clearance might have decreased already for the intramuscular administration. Thus, the absolute bioavailability obtained in these piglets may be slightly overestimated. As shown in Fig.4, high marbofloxacin urinary excretion rates were observed in 16-week-old pigs after intravenous injection. The maximal urinary excretion rate was 6.03 mg/h (SD = 2.46 mg/h) at 9.20 h (SD = 7.25 h) postdose. The terminal half-life in urine was 19.0 h (SD = 4.34 h). This value is the same as the value obtained in plasma. The renal clearance of marbofloxacin was 0.041 L/h·kg (SD = 0.007 L/h·kg) which is about half of the total body clearance. This is confirmed by the part of the dose excreted unchanged in urine which was 51.6% (SD = 4.75%). The maximal excretion rate of N-oxide-marbofloxacin was 0.65 mg/h (SD = 0.24 mg/h), and the fraction of dose excreted in urine was 4.9% (SD = 1.2%). For desmethyl-marbofloxacin, the maximal excretion rate was 0.19 mg/h (SD = 0.11 mg/h), and the fraction of the dose excreted in urine was 1.6% (SD = 0.59%). Thus, the excreted amount of marbofloxacin and its metabolites in urine was about 60% of the administered dose. These results are consistent with previously published radiometric study data (European Medicines Agency, 1999). After intramuscular administration, the maximal excretion rate of marbofloxacin was 7.54 mg/h (SD = 3.31 mg/h), and it occurred 8.60 h (SD = 9.34 h) after drug injection. Both values are very close to those obtained after intravenous administration. The fraction of dose excreted unchanged in urine was 56.7% (SD = 4.70%). Figures 4 and 5 show that for the excreted cumulative amount of marbofloxacin and its metabolites, a plateau was reached as early as 3 days after administration.
Figure 4.
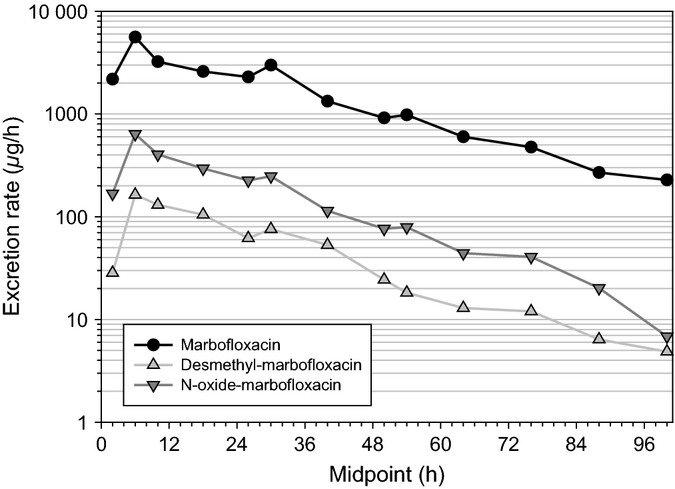
Mean excretion rate–time profiles of marbofloxacin, N-oxide-marbofloxacin and desmethyl-marbofloxacin in pig urine after a single marbofloxacin intravenous dose of 8 mg/kg in 16-week-old animals.
Figure 5.
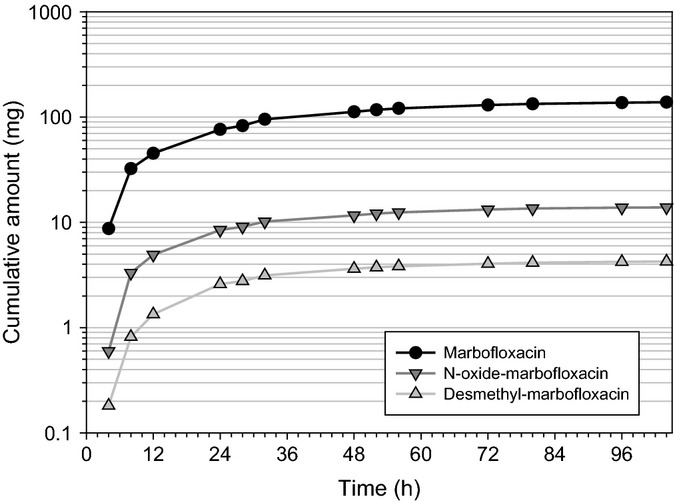
Mean cumulative amount of marbofloxacin, N-oxide-marbofloxacin and desmethyl-marbofloxacin excreted in pig urine after a single marbofloxacin intravenous dose of 8 mg/kg in 16-week-old animals.
Discussion
The first aim of the presented work was to determine the absolute bioavailability of marbofloxacin in a 16% solution given at a single intramuscular dose of 8 mg/kg in pigs of different ages. The obtained value, about 90%, was very high and was close to the value obtained in swine with a 2% solution at a dose of 2.5 mg/kg in 14 to 15-weeks-old male pigs (Ding et al., 2010). This finding indicates that, within the range of doses examined, the high absolute bioavailability of marbofloxacin injectable solution is not affected by the drug concentration (cross-study comparison), dose (within and between study comparison) or by the injection volume (within study comparison). A high absolute bioavailability of about 95% was also obtained in pigs following intramuscular administration of difloxacin (Ding et al., 2008). Somewhat lower values were obtained following intramuscular administration of other fluoroquinolones: 75% for enrofloxacin (Anadón et al., 1999), 76% for danofloxacin (Mann & Frame, 1992) and 54% for norfloxacin (Anadón et al., 1995).
The estimated clearance values were somewhat different from those previously published. Petracca et al. (1993), obtained values of about 0.12–0.2 L/h/kg in pregnant and lactating sows after administration of a dose of 2 mg/kg. These values are about twice those obtained in our study in 27-week-old pigs. This difference may be explained by the pregnancy where clearance of drugs is increased especially for drugs predominantly eliminated in urine (Dvorchik, 1982). As we observed that 60% of the administered marbofloxacin dose is eliminated in urine, its clearance could be affected by pregnancy. Ding et al. (2010) published a clearance value of 0.12 L/h/kg in 10-week-old pigs. This estimate is similar to the clearance estimate of 0.092 L/h/kg which we obtained in our study in 12-week-old animals. Thus, values obtained in both studies can be considered comparable, especially when considering that the animals in the study of Ding et al. (2010) were even younger than the 12-week-old animals in the present work. The clearance of marbofloxacin is lower than that estimated for enrofloxacin (0.1–0.45 L/h·kg, Anadón et al., 1999; Post et al., 2002, 2003), difloxacin (0.16 L/h·kg, Ding et al., 2008), danofloxacin (0.63 L/h·kg, Mann & Frame, 1992) and norfloxacin (0.66 L/h·kg, Anadón et al., 1995).
According to Toutain and Bousquet-Melou (2004), an overall drug extraction ratio (E) can be qualified as low when its value is below 0.05. The overall drug extraction ratio is the ratio of total body clearance divided by the cardiac output. Taking the animals of study 1 with an average weight of 75 kg, their allometrically scaled cardiac output was 79.25 mL/min/kg using the following relationship: Cardiac output = 180 × (Weight)−0.19 (Toutain & Bousquet-Melou, 2004). With an estimated clearance of 0.065 L/h/kg, the extraction ratio was 0.014. Thus, this value can be qualified as low.
The second aim of the work to evaluate pharmacokinetic dose proportionality was statistically confirmed within a dose range of 4–16 mg/kg. Bioavailability was not affected by either injection volume or the amount of drug at the injection site. In other words, the extent and rate of absorption was not altered within the tested dose range. To the best of our knowledge, such a finding was not described in pigs with other fluoroquinolones. Among other antibiotics, the dose proportionality of tildipirosin in pigs (within the range of 2–6 mg/kg) was confirmed for the extent of exposure (AUC) but not for Cmax (Rose et al., 2013).
Age-dependent pharmacokinetics of fluoroquinolones in pigs are not described in the literature. However, they are described for other antibiotic drugs in piglets within the age range of 1–74 days (Friis, 1981; Tagawa et al., 1994; Kinoshita et al., 1995). For example, these studies report that the pig clearance of sulphachlorpyridazine, trimethoprim and erythromycin increased as a function of animal age. This is in contrast to the findings in our current investigation where marbofloxacin clearance decreased as a function of animal age. Tagawa et al. (1994) and Kinoshita et al. (1995) attributed the increased clearance of trimethoprim and erythromycin to decreased blood concentrations of alpha1-acid glycoprotein in older piglets. Both drugs being bound to this acute phase protein, its decreased concentration induced an increased volume of distribution and hence an increased apparent clearance (note that protein binding does not impact intrinsic clearance in a linear system). In the present study, volume of distribution is not significantly affected by the age of the animals, excluding potential changes in protein binding as a mechanism for age-related changes in marbofloxacin pharmacokinetics in pigs. Friis (1981) explained that the increased clearance of sulphachlorpyridazine in older animals is related to the maturation of excretory organs such as the kidneys. He also stated that the glomerular filtration rate and various tubular functions have reached adult values at 8 weeks of age. Marbofloxacin being eliminated mostly in urine (European Medicines Agency, 1999) could exhibit increased systemic clearance due to the maturation-induced changes in kidneys' function. However, as the youngest piglets in our study were 12 week old, it can be considered that all the animals have reached an adult status and that the decrease of the clearance may be rather related to the decreased function of the kidneys and liver in older animals. This is a well-known phenomenon in man (Rowland & Tozer, 2011) where clearance of creatinine increases in the first months after birth and then decreases steadily with age. One can think that, as in human beings, the decreased function takes place as soon as the kidneys have reached the adult status which is the case in our study. In two independent publications (Anadón et al., 1999; Post et al., 2003), total body clearance was determined for enrofloxacin after a single intravenous dose of 2.5 and 5 mg/kg, respectively. Age of the animals was not cited but, in the first paper, the weight of the pigs was 76–86 kg, and in the second paper, it was 25–35 kg. It can be assumed that the animals in the first paper were older than in the second paper. The clearance of enrofloxacin was 0.10 L/h·kg in the older pigs and 0.45L/h·kg in the younger piglets. Although dose was not the same, it can be reasonably assumed that similar to that seen with marbofloxacin, enrofloxacin clearance decreases with age of the pigs.
To clarify which elimination pathway, kidney or liver is involved in the decreased total body clearance of marbofloxacin, the renal clearance should have been determined in the different age groups. In the present work, renal clearance was determined in the 16-week-old animals. Thus, it was not possible to confirm our interpretation of an age-related change in renal clearance to a difference in drug urinary excretion. The obtained renal clearance of 0.041 L/h·kg accounted for about half of the total body clearance.
In comparison to the renal Cl observed with marbofloxacin, the following swine renal Cl estimates have been reported with other fluoroquinolones
a) The renal clearance of enrofloxacin is not published, but information on its urinary elimination is available (Post et al., 2002). About 45% of the administered dose was recovered in urine as enrofloxacin and its main metabolite, ciprofloxacin. Thus, the value obtained for marbofloxacin, about 60%, was close to the value for enrofloxacin.
b) For danofloxacin, about 60% of the daily dose was excreted into urine as unchanged danofloxacin (80%), N-oxide-danofloxacin (10–14%), N-desmethyl-danofloxacin (2–3%) and β-glucuronide of danofloxacin (3%) (European Medicines Agency, 1998a). This urinary elimination pattern is very close to the elimination pattern of marbofloxacin.
c) Less than 20% of the administered dose of difloxacin was excreted in urine as unchanged difloxacin, sarafloxacin and N-oxide-difloxacin were also recovered in urine (European Medicines Agency, 1998b).
The results of this work show that the pharmacokinetic profile of marbofloxacin contained in a new 16% concentrated solution (Forcyl®) was proportional to the administered dose within a range of 4–16 mg/kg. About 60% of the administered dose is eliminated in urine mainly as unchanged drug, while metabolites (desmethyl-marbofloxacin and N-oxide-marbofloxacin) are accounting for about 6.5% of the dose. The total body clearance of the drug is a function of the age of the pigs as it decreases when the animals are becoming older. This alteration of the clearance may be related to maturational changes of the kidneys which are the main excretory organ for marbofloxacin.
References
- Anadón A, Martinez-Larrañaga MR, Diaz MJ, Fernandez R, Martinez MA. Fernandez MC. Pharmacokinetics and tissue residues of norfloxacin and its N-desethyl- and oxo-metabolites in healthy pigs. Journal of Veterinary Pharmacology and Therapeutics. 1995;18:220–225. doi: 10.1111/j.1365-2885.1995.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Anadón A, Martínez-Larrañaga MR, Díaz MJ, Fernández-Cruz ML, Martínez MA, Frejo MT, Martínez M, Iturbe J. Tafur M. Pharmacokinetic variables and tissue residues of enrofloxacin and ciprofloxacin in healthy pigs. American Journal of Veterinary Research. 1999;60:1377–1382. [PubMed] [Google Scholar]
- Ding HZ, Yang GX, Huang XH, Chen ZL. Zeng ZL. Pharmacokinetics of difloxacin in pigs and broilers following intravenous, intramuscular, and oral single-dose applications. Journal of Veterinary Pharmacology and Therapeutics. 2008;31:200–204. doi: 10.1111/j.1365-2885.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- Ding H, Li Y, Chen Z, Rizwan-UL-Haq M. Zeng Z. Plasma and tissue cage fluid pharmacokinetics of marbofloxacin after intravenous, intramuscular and oral single-dose application in pigs. Journal of Veterinary Pharmacology and Therapeutics. 2010;33:507–510. doi: 10.1111/j.1365-2885.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Dvorchik BH. Drug disposition during pregnancy. International Journal of Biological Research in Pregnancy. 1982;3:129–137. [PubMed] [Google Scholar]
- European Medicines Agency. Danofloxacin (Extension to pigs): Summary report (1) – Committee for Veterinary Medicinal Products. 1998a. European Public MRL Assessment Report No. EMEA/MRL/458/98-FINAL.
- European Medicines Agency. Difloxacin (Extension to swine and cattle): Summary report – Committee for Veterinary Medicinal Products. 1998b. European Public MRL Assessment Report No. EMEA/MRL/525/98-FINAL.
- European Medicines Agency. Marbofloxacin: Summary report (2) – Committee for Veterinary Medicinal Products. 1999. European Public MRL Assessment Report No. EMEA/MRL/693/99-FINAL.
- Friis C. Postnatal development of renal function in piglets: changes in excretory pattern of sulphachlorpyridazine. Acta Pharmacologica et Toxicologica. 1981;48:409–417. doi: 10.1111/j.1600-0773.1981.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Son DS, Shimoda M. Kokue E. Impact of age-related alteration of plasma alpha 1-acid glycoprotein concentration on erythromycin pharmacokinetics in pigs. American Journal of Veterinary Research. 1995;56:362–365. [PubMed] [Google Scholar]
- Mann DD. Frame GM. Pharmacokinetic study of danofloxacin in cattle and swine. American Journal of Veterinary Research. 1992;53:1022–1026. [PubMed] [Google Scholar]
- Petracca K, Riond JL. Wanner M. Pharmacokinetics of the gyrase inhibitor marbofloxacin: influence of pregnancy and lactation in sows. Journal of Veterinary Medicine. 1993;40:73–79. doi: 10.1111/j.1439-0442.1993.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Post LO, Cope CV, Farrell DE, Baker JD. Myers MJ. Influence of porcine Actinobacillus pleuropneumoniae infection and dexamethasone on the pharmacokinetic parameters of enrofloxacin. Journal of Pharmacology and Experimental Therapeutics. 2002;301:217–222. doi: 10.1124/jpet.301.1.217. [DOI] [PubMed] [Google Scholar]
- Post LO, Farrell DE, Cope CV, Baker JD. Myers MJ. The effect of endotoxin and dexamethasone on enrofloxacin pharmacokinetic parameters in swine. Journal of Pharmacology and Experimental Therapeutics. 2003;304:889–895. doi: 10.1124/jpet.102.042416. [DOI] [PubMed] [Google Scholar]
- Rose M, Menge M, Bohland C, Zschiesche E, Wilhelm C, Kilp S, Metz W, Allan M, Röpke R. Nürnberger M. Pharmacokinetics of tildipirosin in porcine plasma, lung tissue, and bronchial fluid and effects of test conditions on in vitro activity against reference strains and field isolates of Actinobacillus pleuropneumoniae. Journal of Veterinary Pharmacology and Therapeutics. 2013;36:140–153. doi: 10.1111/j.1365-2885.2012.01397.x. [DOI] [PubMed] [Google Scholar]
- Rowland M. Tozer TN. Lippincott Williams & Wilkins . Clinical Pharmacokinetics and Pharmacodynamics, Concepts and Applications. Baltimore: Lippincott Williams & Wilkins; 2011. Change in physiologic functions and drug disposition with age; pp. 380–385. 4th edn. [Google Scholar]
- Schneider M, Galland D, Giboin H. Woehrlé F. Proceedings of the 12th International Congress of the European Association for Veterinary Pharmacology and Toxicology. Noordwijkerhout, the Netherlands; 2012. Pharmacokinetic/pharmacodynamic testing of marbofloxacin administered as a single injection for the treatment of porcine respiratory disease; pp. 192–193. 8–12 July. [Google Scholar]
- Tagawa Y, Kokue E, Shimoda M. Son DS. Alpha 1-acid glycoprotein-binding as a factor in age-related changes in the pharmacokinetics of trimethoprim in piglets. The Veterinary Quarterly. 1994;16:13–17. doi: 10.1080/01652176.1994.9694408. [DOI] [PubMed] [Google Scholar]
- Toutain PL. Bousquet-Melou A. Plasma clearance. Journal of Veterinary Pharmacology and Therapeutics. 2004;27:415–425. doi: 10.1111/j.1365-2885.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Vallé M, Schneider M, Galland D, Giboin H. Woehrlé F. Pharmacokinetic and pharmacodynamic testing of marbofloxacin administered as a single injection for the treatment of bovine respiratory disease. Journal of Veterinary Pharmacology and Therapeutics. 2012;35:519–528. doi: 10.1111/j.1365-2885.2011.01350.x. [DOI] [PubMed] [Google Scholar]


