Abstract
Aim
Multidisciplinary care (MDC) for patients with chronic kidney disease (CKD) may help to optimize disease care and improve clinical outcomes. Our study aimed to evaluate the effectiveness of pre-end-stage renal disease (ESRD) patients under MDC and usual care in Taiwan.
Method
In this 3-year retrospective observational study, we recruited 822 ESRD subjects, aged 18 years and older, initiating maintenance dialysis more than 3 months from five cooperating hospitals. The MDC (n = 391) group was cared for by a nephrologists-based team and the usual care group (n = 431) was cared for by sub-specialists or nephrologists alone more than 90 days before dialysis initiation. Patient characteristics, dialysis modality, hospital utilization, hospitalization at dialysis initiation, mortality and medical cost were evaluated. Medical costs were further divided into in-hospital, emergency services and outpatient visits.
Results
The MDC group had a better prevalence in peritoneal dialysis (PD) selection, less temporary catheter use, a lower hospitalization rate at dialysis initiation and 15% reduction in the risk of hospitalization (P < 0.05). After adjusting for gender, age and Charlson Comorbidity Index score, there were lower in-hospital and higher outpatient costs in the MDC group during 3 months before dialysis initiation (P < 0.05). In contrast, medical costs (NT$ 146 038 vs 79 022) and hospitalization days (22.4 vs 15.5 days) at dialysis initiation were higher in the usual care group. Estimated medical costs during 3 months before dialysis till dialysis initiation, the MDC group yielded a reduction of NT$ 59 251 for each patient (P < 0.001). Patient mortality was not significantly different.
Conclusion
Multidisciplinary care intervention for pre-ESRD patients could not only significantly improve the quality of disease care and clinical outcome, but also reduce medical costs.
Summary at a Glance.
This retrospective study clearly demonstrates that multidisciplinary care by nephrologists in dialysis patients provided better various outcomes and less medical costs. However, the patient mortality was not different.
Keywords: chronic kidney disease, hospitalization, medical costs, mortality, multidisciplinary care
The rapid increased incidence and prevalence of chronic kidney diseases (CKD) have been recognized as a global public health problem that consumes a large proportion of health care budgets. In Taiwan, the national prevalence of CKD is high, but disease awareness is inadequate. Only 3.5% of CKD patients are able to report their stage of disease, and awareness rates are closely related to disease severity.1,2 As one of the most rapidly aging countries with an increasing prevalence of diabetes mellitus, hypertension and subsequently CKD, Taiwan has the highest prevalence and incidence of end-stage renal disease (ESRD) in the world.3 According to the Bureau of National Health Insurance (BNHI) annual report, the dialysis costs of ESRD patients in Taiwan accounted for 5.0–7.52% of the total health-care resources in recent years.4
Medical resource utilization has become more frequent as disease progression and ESRD approach. According to a recent study, the medical cost of pre-ESRD patients increased sharply in the last 6 months prior to dialysis initiation.5 Service utilization and hospitalizations were the major components of cost during the period immediately before and after dialysis initiation.6 Co-morbidity, such as cardiovascular disease, is the major cause of mortality among CKD patients, and CKD patients with increasing co-morbidity may be responsible for the rapid escalation of medical expenditures.7,8
Optimal management of CKD may improve clinical outcome and decrease mortality, thus resulting in reduced hospitalization and medical cost. In 2002, the US National Kidney Foundation launched the promotion of clinical practice guidelines for the diagnosis, evaluation and monitoring of CKD within the Kidney Disease Outcomes Quality Initiative (NKF K/DOQI) in an effort to increase the awareness of optimal CKD care.9 Recent trials and studies also have proven the efficacy of several interventions such as early detection of CKD,10 prevention of kidney disease progression, early referral to nephrologists,11 promotion of pre-dialysis educations,12 timely preparation of renal replacement therapy (RRT), and care by a comprehensive, nephrology-based, multi-disciplinary team.13–15
The purpose of our study is to evaluate the effectiveness and association of clinical outcome, impact of dialysis modality selection, medical utilization and costs between pre-ESRD patients receiving MDC and usual care in Taiwan.
Materials and Methods
Study design and subjects
Our retrospective study was directly linked to the National Health Insurance (NHI) system with its outpatient and in-hospital database in the period from January 2005 to December 2009. Patients aged 18 years or older who were on maintenance renal replacement therapy (including haemodialysis and PD) and who had catastrophic illness cards had their medical expense covered by the NHI and were cared for in five cooperating hospitals in the northern, central and southern areas of Taiwan. Initially, 854 subjects were enrolled, but we excluded patients who received temporary dialysis due to acute deterioration of renal function, and those who died in the first 3 months after dialysis initiation. In the end, 822 subjects were recruited and further analyzed.
A total of 391 subjects were placed into the MDC group who had received nephrologists-based CKD team care for more than 90 days before dialysis initiation. The other 431 subjects, who were under the intervention of sub-specialists alone such as endocrinologists, cardiologists and nephrologists without referring to the MDC, were defined as the usual care group (Fig. 1).
Fig 1.
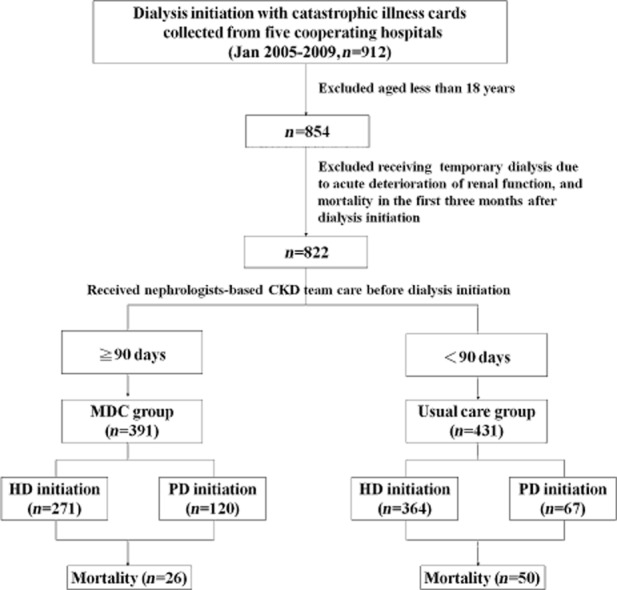
Participant flow chart.
Definition of multidisciplinary care and patient management
The multidisciplinary care (MDC) consisted of a nephrologist, nephrology nurse educator, renal dietician, social worker, pharmacy specialists, and surgeon for vascular access creation, tenchoff catheter implantation. For the standardized intervention of CKD in the MDC group, the management and education was dependent on the different stage of CKD and followed the NKF K/DOQI guidelines, Taiwan pre-ESRD care program and reimbursement policy of the NHI. CKD management in the MDC group focused both on medical management and lifestyle modification. The case-management nephrology nurse contacted patients to ensure regular follow-ups. The members of the MDC team met with and followed up on patients regularly to review and discuss patients' individualized therapy, medical recommendations for metabolic abnormality, diet assessment and co-morbidity.
Criteria for dialysis initiation
Criteria for dialysis initiation were mandated by the BNHI in Taiwan including:
Absolute criteria of serum creatinine levels above 10 mg/dL or creatinine clearance of less than 5 mL/min.
Relative criteria of serum creatinine levels above 6 mg/dL or creatinine clearance of less than 15 mL/min, and uremic conditions which threaten life or impair quality of life (not listed in detail).
Data collection and initial dialysis modality definition
Patients' basic characteristics were provided by the five cooperating hospitals. We directly linked to the NHI system and claim data to confirm 1st dialysis medical records and admission data to avoid incorrect data collection. If patients had ever experienced PD during the first 3 months after dialysis initiation, initial dialysis modality was defined as PD.
Definition of comorbidity diseases
Based on the definition of Dartmouth-Manitoba's Charlson Comorbidity Index (D-M's CCI),16 we used the International Classification of Diseases (ICD-9) code on the in-hospital database and double records in outpatient database to identify co-morbidities within one year before dialysis initiation. Subsequently, any instance of ICD-9 code once presented in the NHI database was counted as co-morbidity and weighted in the CCI score.
Service utilizations and medical costs, clinical outcome
Service utilization and patients' mortality were our clinical outcomes. Data were followed up until 31 December 2009. Medical service utilization and costs were analyzed from 3 months before dialysis until 6 months after dialysis initiation (defined as the observation period). Accident injuries or surgical conditions not related to CKD were excluded. Medical costs were further analyzed into outpatient visits, in-hospital, and emergency services. Service utilization included frequency of outpatient visits and hospitalization before and after dialysis initiation, percentage of hospitalization at dialysis initiation, and average length of stay (LOS). Service utilization and medical cost during observation period were further divided into ‘3 months before dialysis’, ‘at dialysis initiation’ and ‘6 months after dialysis initiation’. ‘At dialysis initiation’ was defined as the period of first time dialysis prescription (through hospitalization or outpatient). Dialysis costs were excluded in our study (there was no comparison of cost of PD vs haemodialysis).
Statistical analysis
Frequency (n) and percentage (%) were used to determine the distribution of gender, dialysis modality selection, and temporary catheter use, hospitalization at dialysis initiation, frequency of hospitalization, co-morbidity and mortality. Mean and standard deviation (SD) were used to describe the distribution of age, hospital days, and medical costs on outpatient visits, in-hospital and emergency services. We also used median to describe the distribution of medical costs. The differences in categorical variables were analyzed by χ2 test (or Fisher's exact test). A generalized linear model (GLM) was used to evaluate medical costs between the MDC and usual care group after adjusting for gender, age and CCI score. The Tobit regression model was used to estimate the adjusted medical costs associated with the MDC and usual care group after controlling for other covariates. The Kaplan–Meier survival curve and log-rank statistics were used to describe hospitalization risk and patient survival. A multivariate Cox regression model was performed to predict hospitalization risk and patient mortality after adjusting for gender, age and CCI score. A two-tailed P-value less than 0.05 was considered statistically significant. All statistical analyses were conducted using the statistical package for Window, SAS 9.2 (SAS Institute, Cary, NC, USA) and SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics and co-morbidity
In total, 822 subjects (usual care, n = 431; MDC, n = 391) with mean age of 62.8 years (male, 51.5%) were included in our study. Patient's characteristics, dialysis modality, percentage of hospitalization at dialysis initiation and co-morbidity are shown in Table 1. The MDC group had a higher percentage of PD modality selection, lower temporary catheter use, and a lower hospitalization rate at dialysis initiation (P < 0.05). The CCI score in the MDC group was lower as compared to the usual care group (P = 0.02).
Table 1.
General characteristic of our subjects
| MDC (n = 391) | Usual care (n = 431) | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender, male | 210 | 53.7 | 213 | 49.4 | 0.22 |
| Dialysis choice, PD† | 120 | 30.7 | 67 | 15.6 | <0.001 |
| Temporary catheter use | 222 | 56.8 | 328 | 76.1 | <0.001 |
| Initial dialysis admission | 0.05 | ||||
| In-hospital | 327 | 83.6 | 369 | 85.6 | |
| Outpatient | 42 | 10.7 | 28 | 6.5 | |
| Emergency | 22 | 5.6 | 34 | 7.9 | |
| Hospitalization rate‡ | 347 | 88.7 | 402 | 93.3 | 0.03 |
| CCI score§ | (2.0 ± 1.6) | (2.2 ± 1.8) | 0.02 | ||
| Age | (63.0 ± 13.9) | (62.6 ± 15.5) | 0.72 | ||
If patients had the experience of the peritoneal dialysis (PD) model from dialysis initiation to after a 3 months period, it was calculated.
If patients were transferred from outpatient services or emergency services to hospitalization at dialysis initiation, it was calculated.
Comorbidity diseases were based on Dartmouth-Manitoba's CCI (D-M's CCI). Values are expressed as (mean ± SD). CCI, Charlson Comorbidity index; GFR, glomerular filtration rate; SD, standard deviation.
Decline of renal function and eGFR at dialysis initiation
Table 2 further illustrates the baseline estimated glomerular filtration rate (eGFR) one year before dialysis initiation, at dialysis initiation, and the decline of renal function based on the valid subjects provided by five cooperating hospitals. We found the patients of the MDC group had more compliance to outpatients follow-up and more detail laboratory data record (data not shown). The decline of renal function was slower in the MDC group (7.6 vs 11.1 mL/min per 1.73 m2, P < 0.001), also eGFR at the dialysis initiation was lower as compared to the usual care group (5.1 vs 5.9 mL/min per 1.73 m2, P < 0.001).
Table 2.
Baseline estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) and eGFR change (ΔeGFR) between multidisciplinary care (MDC) and usual care group
| MDC (n = 391) | Usual care (n = 431) | P | Adjusted P | |||
|---|---|---|---|---|---|---|
| n# | Mean ± SD | n # | Mean ± SD | |||
| Baseline eGFR 1 year before dialysis | 315 | 12.1 ± 7.0 | 218 | 17.0 ± 11.7 | <0.001 | <0.001 |
| eGFR at dialysis initiation | 315 | 5.1 ± 0.7 | 218 | 5.9 ± 0.7 | <0.001 | <0.001 |
| ΔeGFR | 315 | 7.0 ± 6.3 | 218 | 11.1 ± 11.0 | <0.001 | <0.001 |
| ΔeGFR/month | 315 | 0.6 ± 0.6 | 218 | 1.1 ± 1.4 | <0.001 | <0.001 |
ΔeGFR (estimated glomerular filtration rate) as baseline eGFR 1 year before dialysis – eGFR at dialysis initiation. Adjust P for gender, age and CCI score; eGFR provided by five cooperating hospitals based on valid subjects (some subjects lost to follow-up and initiation renal replacement therapy [RRT] outside the study hospitals).
Hospitalization risk and patient mortality
During an average follow-up time of 33.6 months, a total of 104 (24.1%) patients in the usual care and 67 (17.1%) patients in the MDC group died (P = 0.01). The usual care group had a higher hospitalization rate during the observation periods (usual care 75.4% vs MDC 69.3%, P = 0.05), and even 6 months after dialysis initiation (usual care 58.7% vs MDC 50.6%, P = 0.02). The MDC group had better patient survival and lower risks of hospitalization in the Kaplan–Meier analysis (log-rank test, P < 0.05) (Figs 3). After adjusting for gender, age and CCI, the MDC group was still associated with a 15% reduction in the risk of hospitalization (hazard ration [HR], 0.85; 95% confidence interval [CI], 0.72–0.99, P < 0.05). However, there was no significant difference in patient mortality between the two groups (HR, 0.79; 95% CI, 0.58–1.08, P = 0.14).
Fig 3.

Kaplan–Meier curve of patient survival between multidisciplinary care (MDC) and usual care group in follow-up period.
Fig 2.
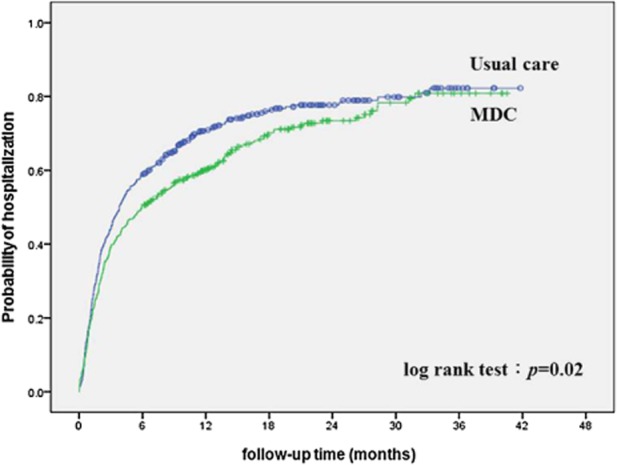
Kaplan–Meier curve of time to first hospitalization between multidisciplinary care (MDC) and usual care group in the follow-up period.
Medical costs and frequency of service utilization
The average medical cost for ‘3 months before dialysis initiation’, ‘at dialysis initiation’ and ‘6 months after dialysis initiation’ were NT$ 43 329, NT$ 114 161 and NT$ 224 624, respectively (data not shown). The mean LOS of ‘at dialysis initiation’ and ‘6 months after dialysis initiation’ were 19.1 days and 13.7 days, respectively (data not shown).
Medical cost for the usual care group ‘at dialysis initiation’ was significantly higher (NT$ 146 038 vs NT$ 79 022 NT, P < 0.001) (Table 3). The MDC group had a shorter LOS of hospitalization for both ‘at dialysis initiation’ (15.5 days vs 22.4 days, P < 0.001) and ‘6 months after dialysis initiation’ (11.2 days vs 16.0 days, P < 0.05) (Table 3).
Table 3.
Medical costs† and hospital utilization in our subjects
| MDC (n = 391) | Usual care (n = 431) | P | |||
|---|---|---|---|---|---|
| Mean (median)‡ | SD | Mean (median)‡ | SD | ||
| Medical costs, NT | |||||
| Before 3 months | 41 013 (31 374) | 41 111 | 45 430 (27 029) | 54 503 | 0.19 |
| At dialysis initiation | 79 022 (53 302) | 117 118 | 146 038 (63 327) | 259 431 | <0.001 |
| After 6 months | 218 684 (197 092) | 185 792 | 230 014 (195 463) | 243 206 | 0.46 |
| Hospital days | |||||
| At dialysis initiation | 15.5 | 12.9 | 22.4 | 24.2 | <0.001 |
| After 6 months | 11.2 | 21.8 | 16.0 | 31.2 | 0.01 |
Excluding dialysis cost; before 3 months and after 6 months, costs were based on total subjects, while at dialysis initiation, they were based on the number of valid subjects.
Median was calculated based on the number of all the subjects. MDC, multidisciplinary care; SD, standard deviation.
3 months before dialysis initiation
There was a higher frequency of outpatient visits (46.6 vs 38.1, P < 0.001) and higher average medical costs in the MDC group (NT$ 26 629 vs NT$ 20 768, P < 0.001); however, frequency of hospitalization (0.8 vs 1.4, P < 0.001) and in-hospital (NT$ 12 265 vs NT$ 21 519, P < 0.01), emergency costs (NT$ 2119 vs NT$ 3143, P < 0.05) were lower in the MDC group (Table 4).
Table 4.
Medical costs† of medical service admission during the observation periods
| MDC (n = 391) | Usual care (n = 431) | Adj. P* | |||
|---|---|---|---|---|---|
| Mean (median)‡ | SD | Mean (median)‡ | SD | ||
| Before 3 months, NT | |||||
| Outpatient | |||||
| Visit | 46.6 (42.0) | 25.5 | 38.1 (34.0) | 25.7 | <0.001 |
| Cost | 26 629 (24 429) | 17 421 | 20 768 (17 715) | 18 405 | <0.001 |
| In-hospital | |||||
| Visit | 0.8 (0.0) | 1.2 | 1.4 (1.0) | 1.9 | 0.001 |
| Cost | 12 265 (0) | 35 606 | 21 519 (0) | 45 634 | 0.005 |
| Emergency | |||||
| Visit | 1.4 (1.0) | 2.3 | 1.9 (1.0) | 3.0 | 0.52 |
| Cost | 2 119 (0) | 4 200 | 3 143 (0) | 5 964 | 0.03 |
| At dialysis initiation, NT | |||||
| Outpatient | |||||
| Cost | 764 (0) | 3 906 | 499 (0) | 2 941 | 0.39 |
| In-hospital | |||||
| Cost | 77 896 (52 860) | 117 674 | 144 825 (62 892) | 259 905 | <0.0001 |
| Emergency | |||||
| Cost | 362 (0) | 1 751 | 714 (0) | 3 173 | 0.07 |
| After 6 months, NT | |||||
| Outpatient | |||||
| Visit | 21.4 (20.0) | 11.5 | 20.0 (18.0) | 11.7 | 0.05 |
| Cost | 147 976 (157 056) | 112 893 | 126 659 (74 516) | 118 702 | 0.01 |
| In-hospital | |||||
| Visit | 0.9 (1.0) | 1.2 | 1.1 (1.0) | 1.5 | 0.005 |
| Cost | 66 840 (9 129) | 165 036 | 96 902 (23 859) | 233 118 | 0.03 |
| Emergency | |||||
| Visit | 1.0 (0.0) | 1.7 | 1.2 (1.0) | 1.9 | 0.06 |
| Cost | 3 867 (0) | 8 805 | 6 453 (311) | 14 456 | 0.002 |
Adjusting for gender, age and Charlson Comorbidity Index (CCI) score.
Excluding dialysis cost; before 3 months and after 6 months, costs were based on total subjects, while at dialysis initiation, they were based on the number of valid subjects.
Median was calculated based on the number of allthe subjects. SD, standard deviation.
At dialysis initiation
Medical costs at dialysis initiation were mainly attributed to in-hospital costs. The MDC group incurred lower in-hospital costs after adjusting for gender, age and CCI score (NT$ 77 896 vs NT$ 144 825, P < 0.05) (Table 4). However, there was no difference on outpatient and emergency costs between the two groups.
6 months after dialysis initiation
Outpatient visits (21.4 vs 20.0, P = 0.05) and costs (NT$ 147 976 dollars vs NT$ 126 659, P = 0.01) were higher in the MDC group, whereas frequency of hospitalization (0.9 vs 1.1, P = 0.005) and in-hospital (NT$ 66 840 vs NT$ 96 902, P = 0.03) and emergency costs (NT$ 3867 vs NT$ 6453, P = 0.002) were lower in the MDC group (Table 4 and Fig. 4).
Fig 4.
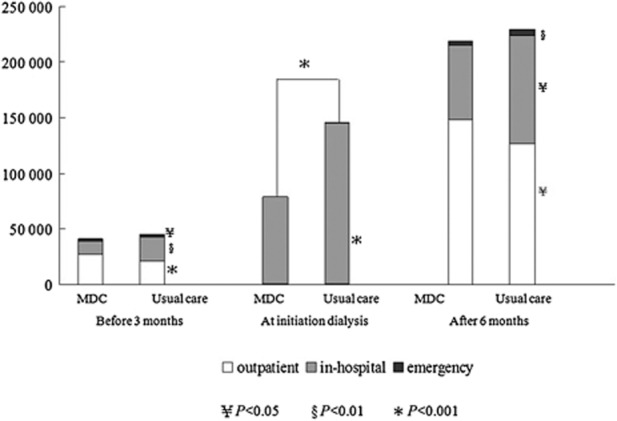
Medical costs (excluding dialysis costs) of multidisciplinary care (MDC) and usual care group in observation periods. Before 3 months and after 6 months were based on the total number of subjects. At dialysis initiation, costs were based on the number of valid subjects (adjusting for gender, age and CCI score). □, outpatient;  , in-hospital; ■, emergency.
, in-hospital; ■, emergency.
Medical costs by Tobit regression
Age, CCI score, temporary catheter use and hospitalization at dialysis initiation had significantly positive effects on medical costs (Table 5). Adjusted medical costs were significantly lower in the MDC group and were reduced up to NT$ 59 251 for each patient during 3 months before dialysis until dialysis initiation (P < 0.001).
Table 5.
Tobit model analysis on adjusted medical costs†: before 3 months to initial dialysis periods
| Total (n = 822) | MDC (n = 391) | Usual care (n = 431) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | P | β | 95%CI | P | β | 95%CI | P | ||||
| Low | high | Low | High | Low | High | |||||||
| Intercept | −81 501 | −177 274 | −4 273 | 0.10 | −53 235 | −125 152 | 18 682 | 0.15 | −177 367 | −351 407 | −3 327 | 0.05 |
| MDC versus non-MDC | −59 251 | −91 477 | −27 025 | <0.001 | — | — | — | — | — | — | — | — |
| Male versus female | 1 299 | −30 303 | 32 902 | 0.94 | −2 198 | −27 510 | 23 114 | 0.86 | 5 457 | −49 868 | 60 783 | 0.85 |
| Age | 1 924 | 827 | 3 022 | <0.001 | 1 345 | 420 | 2 270 | 0.004 | 2 350 | 501 | 4 198 | 0.01 |
| CCI score | 28 798 | 19 255 | 38 342 | <0.001 | 18 799 | 10 892 | 26 706 | <0.001 | 36 011 | 19 743 | 52 279 | <0.001 |
| Temporary catheter use‡ | 64 479 | 28 752 | 100 206 | <0.001 | 65 664 | 39 294 | 92 035 | <0.001 | 67 096 | −1 453 | 135 646 | 0.06 |
| Hospitalization rate‡ | 76 068 | 17 858 | 134 278 | 0.01 | 45 657 | 4 428 | 86 885 | 0.03 | 124 185 | 7 024 | 241 347 | 0.04 |
| Scale | 229 560 | 218 727 | 240 929 | 125 821 | 117 304 | 134 956 | 291 996 | 273 139 | 312 154 | |||
Excluding dialysis cost.
Events were at dialysis initiation. CCI, Charlson Comorbidity Index; MDC, multidisciplinary care.
Discussion
Several studies have evaluated the effectiveness of comprehensive, nephrology-based, multi-disciplinary care (MDC) and have affirmed their substantial benefits, such as better laboratory parameters and clinical outcomes, slower renal function declines, more functional vascular access and shorter LOS at dialysis initiation, and reduction in medical costs and service utilization.13–15,17 However, only few studies have simultaneously focused on medical service utilization, cost and clinical outcomes for the MDC on pre-ESRD patients.17 From our large-scale population and multi-hospital collaborative study that was directly linked to the NHI system and database in Taiwan, we confirmed the effectiveness of the MDC for pre-ESRD patients not only for improving clinical outcomes, but also for reducing medical costs.
Declines of renal function and characteristic at dialysis initiation
Consensus guidelines for CKD management emphasize the administration of nephroprotective agents (RAAS blockade), reduction of cardiovascular risk, screening and intervention for CKD-MBD and anaemia. Unfortunately, some physicians may be reluctant to prescribe renoprotective medications such as RAAS blockage due to hyperkalaemia or haemodynamic mediated increase in serum creatinine level. MDC membranes could help and support additional effort to conduct medications side effects follow-up. Due to combination of lifestyle modifications and more effective medical prescription according to K/DOQI and consensus guidelines, then eGFR declines in the MDC group was slower than the usual care group. Also in our study, there was no earlier initiation of dialysis therapy and CKD patients in the MDC group in Taiwan were more likely to initiate dialysis rather than face mortality.18,19
Previous research has shown that a reduction in temporary catheter use is associated with a lower infection rate, avoidance of emergency dialysis, lower risk of hospitalization, and a decrease in mortality, which results in a reduction of medical cost.20,21 Also the timing of referral and pre-dialysis education has been shown to influence the selection of dialysis modality and compliance to therapy prescription.22,23 In Taiwan, the proportion of dialysis patients undergoing PD increased from 6.1 to 7.8% between 2001 and 2005, but the proportion opting for PD remains low. MDC may help CKD patients to receive well-balanced presentations of all renal replacement therapy options. Educational intervention could increase in patient self-care ability and the provision of adequate and good quality information of dialysis, making patients willing to undergo PD and self-care dialysis. Our study demonstrated the same results as previous studies insofar as the MDC group had lower temporary catheter use (56.8% vs 76.1%, P < 0.001), a lower rate of emergency visit at dialysis initiation (5.6% vs 7.9%, P < 0.05) and double the odds of selecting the PD modality (30.7% vs 15.6%, P < 0.001).
Service utilizations and medical costs
Studies have indicated that resource utilization has become more frequent as ESRD approaches.6,24–26 A substantial proportion of the sharp increase in hospitalization rates in the 3 months before and after the initiation of dialysis was attributed to vascular access and related complications.25 Several other factors also confirmed the increased risk of hospitalization, such as later referral, temporary catheter use, advanced age, lower albumin and hematocrit levels, and an increased number of co-morbidity.5,25,27,28 The MDC group could facilitate the early creation of functional dialysis access in the outpatient setting, which obviated the requirement of temporary catheter use and decreased associated complications, thus decreasing medical service utilization and expenditure.
In our retrospective cohort study from 2005 to 2009 based on the NHI database, we found that the MDC group did have a significantly lower risk of hospitalization and LOS. The major medical costs were in-hospital costs at dialysis initiation, and there was a 50% reduction of medical costs after the MDC intervention (NT$ 77 896 vs NT$ 144 825). Also, CCI score, temporary catheter use and hospitalization at dialysis initiation are major independent risk factors for medical expenditure. After the Tobit model analysis adjustment, the estimated medical costs reduction for each MDC patient during 3 months before until dialysis initiation could be up to NT$ 59 251. In light of the 8000 incident dialysis patients who all received care from the MDC in 2009, we could estimate that saved medical expenditure can reach 475 million NT dollars each year, which accounts for 1.4‰ of the 400 billion NT-dollar fiscal budgets for the NHI.29
Clinical outcome
Nephrology care 6 months before dialysis and the consistency of care are strong predictors of mortality.30,31 MDC could provide pre-ESRD patients with enhanced disease knowledge and awareness, a more positive attitude, better lifestyle modifications with cardiovascular risk reduction, and more effective medical prescription according to K/DOQI and consensus guidelines; all of these may contribute to a decrease in all-causes mortality.
As expected, our finding confirmed the effectiveness of MDC in decreasing the risk of hospitalization, but indicated no significantly better survival benefits. We speculate that ESRD patients who died within 90 days after dialysis initiation were excluded from our study. Also due to the accessibility of dialysis therapy in Taiwan, pre-ESRD patients under MDC intervention are most likely to initiate the dialysis electively and experience optimal dialysis therapy before reaching mortality.
There are still some limitations in our study. First, due to our study design, we could not provide detailed cause of hospitalization and mortality (garbled data provided by the NHI). Also this was a retrospective observational study of secondary data analysis, so we could not provide detailed biochemistry differences between the two groups. These limited items used as clinical parameters and incomplete information due to primary data errors were neither avoided nor overlooked. Second, we only analyzed medical costs, and as such, could not evaluate differences in patients' quality of life, society resource utilization and expenditure. Third, the fixed reimbursement policy of the NHI restrained laboratory parameter measurement and medical utilization (such as haemoglobin correction by the ESA administration). Furthermore, the NHI system in Taiwan is a unique health insurance coverage system, and our results may not apply to other countries that conduct similar analyses. The Bureau of Health Promotion in Taiwan has launched pre-ESRD care initiatives since 2002. The MDC program in Taiwan proposed a standard care protocol and annual reporting system. Since 2008 the Taiwan Society of Nephrology committee also conducted a surveillance program and improvement of the quality of pre-ESRD care. The propagation of the MDC might partially explain the stabilization of increase in incident ESRD patients in Taiwan.
Conclusion
Pre-ESRD patients under the MDC intervention, especially for those at 3 months prior to initiating dialysis, had a higher percentage of functional vascular access and better prevalence in PD selection. Moreover, both LOS at dialysis initiation as well as the risk of hospitalization was reduced. Finally, our study confirmed the effectiveness of MDC not only in improving clinical outcome but also in reducing medical expenditure.
Acknowledgments
This study was supported by the Bureau of Health Promotion, Department of Health, R.O.C. (97-HP-1103, 98-HP-1112, 99-HP-1108, 100-HP-1104). Yu Yang was responsible for the study conception and execution. Chiou, Chang, Chen, Lin and Ferng were responsible for plan coordination and cooperation. Wang was responsible for data analysis and the drafting of the manuscript. Chou assisted with implementing the study and data collection. We thank the cooperation of the hospital CKD nurses, e.g. Jui-Hsin Chen, Shu-Chi Lu, Jay-Jen Lin, Ya-Hsueh Shih, Chih-Ying Huang in the collection of the subjects' data.
References
- 1.Wen CP, Cheng TYD, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 2.Hsu C-C, Hwang S-J, Wen C-P, et al. High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am. J. Kidney Dis. 2006;48:727–738. doi: 10.1053/j.ajkd.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Hwang S-J, Lin M-Y, Chen H-C, et al. Increased risk of mortality in the elderly population with late-stage chronic kidney disease: A cohort study in Taiwan. Nephrol. Dial. Transplant. 2008;23:3192–3198. doi: 10.1093/ndt/gfn222. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health. National Health Insurance Annual Statistical Report 1996. Department of Health: Taiwan; 2004. 2004: . [Cited 25 August 2014.] Available from URL: http://www.nhi.gov.tw. [Google Scholar]
- 5.Arora P, Kausz AT, Obrador GT, et al. Hospital utilization among chronic dialysis patients. J. Am. Soc. Nephrol. 2000;11:740–746. doi: 10.1681/ASN.V114740. [DOI] [PubMed] [Google Scholar]
- 6.St Peter WL, Khan SS, Ebben JP, Pereira BJG, Collins AJ. Chronic kidney disease: The distribution of health care dollars. Kidney Int. 2004;66:313–321. doi: 10.1111/j.1523-1755.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 8.Gullion CM, Keith DS, Nichols GA, Smith DH. Impact of comorbidities on mortality in managed care patients with CKD. Am. J. Kidney Dis. 2006;48:212–220. doi: 10.1053/j.ajkd.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 9.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 10.Levin A, Stevens PE. Early detection of CKD: The benefits, limitations and effects on prognosis. Nat. Rev. Nephrol. 2011;7:446–457. doi: 10.1038/nrneph.2011.86. [DOI] [PubMed] [Google Scholar]
- 11.Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: A systematic review. Am. J. Med. 2011;124:1073–1080.e1072. doi: 10.1016/j.amjmed.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Mason J, Khunti K, Stone M, Farooqi A, Carr S. Educational interventions in kidney disease care: A systematic review of randomized trials. Am. J. Kidney Dis. 2008;51:933–951. doi: 10.1053/j.ajkd.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Hemmelgarn BR, Manns BJ, Zhang J, et al. Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J. Am. Soc. Nephrol. 2007;18:993–999. doi: 10.1681/ASN.2006080860. [DOI] [PubMed] [Google Scholar]
- 14.Wu IW, Wang S-Y, Hsu K-H, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality – a controlled cohort study based on the NKF/DOQI guidelines. Nephrol. Dial. Transplant. 2009;24:3426–3433. doi: 10.1093/ndt/gfp259. [DOI] [PubMed] [Google Scholar]
- 15.Curtis BM, Ravani P, Malberti F, et al. The short- and long-term impact of multi-disciplinary clinics in addition to standard nephrology care on patient outcomes. Nephrol. Dial. Transplant. 2005;20:147–154. doi: 10.1093/ndt/gfh585. [DOI] [PubMed] [Google Scholar]
- 16.Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J. Clin. Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 17.Wei S-Y, Chang Y-Y, Mau L-W, et al. Chronic kidney disease care program improves quality of pre-end-stage renal disease care and reduces medical costs. Nephrology. 2010;15:108–115. doi: 10.1111/j.1440-1797.2009.01154.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen YR, Yang Y, Wang SC, et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: A 3-year prospective cohort study. Nephrol. Dial. Transplant. 2013;28:671–682. doi: 10.1093/ndt/gfs469. [DOI] [PubMed] [Google Scholar]
- 19.Hwang S-J, Yang W-C, Lin M-Y, Mau L-W, Chen H-C Taiwan Society of N. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: A national cohort study in Taiwan. Nephrol. Dial. Transplant. 2010;25:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 20.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am. J. Kidney Dis. 2009;53:475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J. Am. Soc. Nephrol. 2011;22:1113–1121. doi: 10.1681/ASN.2010111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol. Dial. Transplant. 2005;20:1842–1847. doi: 10.1093/ndt/gfh905. [DOI] [PubMed] [Google Scholar]
- 23.Manns BJ, Taub K, Vanderstraeten C, et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: A randomized trial. Kidney Int. 2005;68:1777–1783. doi: 10.1111/j.1523-1755.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J. Am. Soc. Nephrol. 2004;15:1300–1306. doi: 10.1097/01.asn.0000125670.64996.bb. [DOI] [PubMed] [Google Scholar]
- 25.Mix TCH, St Peter WL, Ebben J, et al. Hospitalization during advancing chronic kidney disease. Am. J. Kidney Dis. 2003;42:972–981. doi: 10.1016/j.ajkd.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Hunsicker LG. The consequences and costs of chronic kidney disease before ESRD. J. Am. Soc. Nephrol. 2004;15:1363–1364. doi: 10.1097/01.asn.0000126069.68755.99. [DOI] [PubMed] [Google Scholar]
- 27.Bessette RW, Carter RL. Predicting hospital cost in CKD patients through blood chemistry values. BMC Nephrol. 2011;12:65. doi: 10.1186/1471-2369-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laliberte F, Bookhart BK, Vekeman F, et al. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: A managed care perspective. J. Manag. Care Pharm. 2009;15:312–322. doi: 10.18553/jmcp.2009.15.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M-S, Wu I-W, Shih C-P, Hsu K-H. Establishing a platform for battling end-stage renal disease and continuing quality improvement in dialysis therapy in Taiwan. Acta Nephrol. 2011;25:148–153. [Google Scholar]
- 30.Dogan E, Erkoc R, Sayarlioglu H, Durmus A, Topal C. Effects of late referral to a nephrologist in patients with chronic renal failure. Nephrology. 2005;10:516–519. doi: 10.1111/j.1440-1797.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 31.Huisman RM. The deadly risk of late referral. Nephrol. Dial. Transplant. 2004;19:2175–2180. doi: 10.1093/ndt/gfh409. [DOI] [PubMed] [Google Scholar]


