Abstract
Human reward pursuit is often assumed to involve conscious processing of reward information. However, recent research revealed that reward cues enhance cognitive performance even when perceived without awareness. Building on this discovery, the present functional MRI study tested two hypotheses using a rewarded mental‐rotation task. First, we examined whether subliminal rewards engage the ventral striatum (VS), an area implicated in reward anticipation. Second, we examined differences in neural responses to supraliminal versus subliminal rewards. Results indicated that supraliminal, but not subliminal, high‐value reward cues engaged brain areas involved in reward processing (VS) and task performance (supplementary motor area, motor cortex, and superior temporal gyrus). This pattern of findings is striking given that subliminal rewards improved performance to the same extent as supraliminal rewards. So, the neural substrates of conscious versus unconscious reward pursuit are vastly different—but despite their differences, conscious and unconscious reward pursuit may still produce the same behavioral outcomes. Hum Brain Mapp 35:5578–5586, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: reward, consciousness, motivation, cognition, task performance, ventral striatum, mental rotation
INTRODUCTION
Humans have a strong tendency to increase their performance when valuable outcomes—such as food, drink, or money—can be attained. One of the main driving forces behind this adaptive tendency is the ventral striatum (VS), a brain region that energizes behavior when people anticipate rewards [Bjork and Hommer, 2007; Knutson et al., 2008; Phillips et al., 2007; Pochon et al., 2002; Salamone et al., 2009; Vink et al., 2013]. Having extensive connections to many parts of the cortex [Haber and Knutson, 2009], the VS is related to many aspects of goal‐directed functioning, including the enhancement of performance during reward anticipation [Liljeholm and O'Doherty, 2012]. Importantly, and in contrast to what was previously thought, recent findings from neuroscience and psychology indicate that reward pursuit may occur even when people are not consciously aware of the reward value that is at stake [Bijleveld et al., 2009, 2010, 2012a; Childress et al., 2008; Pessiglione et al., 2007; Zedelius et al., 2011, in press]. Building on these developments, the present research addresses the possibility that the VS can be engaged by reward cues that are presented very briefly, below the threshold of conscious perception. Furthermore, we go beyond previous research by exploring potential differences in the neural substrates of unconscious versus conscious reward pursuit.
The possibility that the VS responds to reward cues even when these are perceived without awareness is suggested by a recent series of studies, in which participants were exposed to a reward cue that was on some trials presented very briefly, below the threshold of conscious awareness [i.e., subliminally; Bijleveld et al., 2010; Capa et al., 2011]. Such research indicated that even when they are briefly presented, high‐value reward cues can increase performance on various tasks (e.g., solving mathematical equations). This finding thus suggests that some kind of reward anticipation—albeit an unconscious kind—can be triggered by subliminal reward cues. On the brain level, this idea corresponds to the hypothesis that subliminal reward cues may trigger activity in the VS, and, perhaps subsequently [Haber and Knutson, 2009], engage cortical brain areas that are involved in task performance.
A previous functional MRI study seems to support this hypothesis, by showing that activity in the ventral pallidum increased due to very brief exposure to high‐value reward cues, which indicated that money could be earned by squeezing into a handgrip [Pessiglione et al., 2007]. This finding points to the involvement of the limbic loop, a network of brain structures that includes the VS [Smith et al., 2009]. Moreover, in line with the idea that briefly presented cues induced reward anticipation, participants also squeezed harder when cues signified rewards that were more valuable. It is important to note, however, that this previous study did not yield direct evidence of VS activation. Moreover, it is currently unclear whether subliminal reward cues increase activity in cortical areas that support task performance. Despite these considerations, and along with related research [Childress et al., 2008; Pessiglione et al., 2008], this previous study raises the possibility that reward anticipation in the VS may occur in the absence of awareness.
Although the VS may be engaged by both clearly visible and briefly presented high‐value (vs. low‐value) reward cues, there are also reasons to expect that the neural underpinnings of conscious versus unconscious reward pursuit are qualitatively distinct. That is, recent work discovered that the behavioral effects of unconscious and conscious reward pursuit diverge under some circumstances. For example, in tasks in which strategic decisions affect performance [Bijleveld et al., 2010, 2012b] and in tasks in which reward cues have the potential to distract attention away from the task [Zedelius et al., 2011], clearly visible versus briefly presented reward cues led to different performance outcomes. This suggests that different networks are involved in unconscious versus conscious reward pursuit [Bijleveld et al., 2012a]. Moreover, a recent EEG‐study that used a rewarded task‐switching paradigm investigated several neural correlates of clearly visible versus briefly presented reward cues. Although this study showed some similarities between the modes of presentation (e.g., both brief and visible reward cues enhanced behavioral performance; both decreased alpha band activity in the EEG signal), there were also important differences. Specifically, people focused more attention on the task in response to clearly visible stimuli only after they had been exposed to a clearly visible reward cue, as evidenced by an increased P3‐amplitudein response to task‐relevant stimuli [Capa et al., 2013]. Taken together, several studies indicate that exposure to conscious versus unconscious reward cues have different effects on neural activity.
In line with the line of reasoning addressed above, the present research has two main aims. First, we test the hypothesis that, just like clearly visible reward cues, briefly presented high‐value (vs. low‐value) reward cues engage the VS. Second, we explore potential differences in neural responses to clearly visible versus briefly presented high‐value reward cues. Specifically, we predict that clearly visible reward cues, more than briefly presented reward cues, trigger cortical brain areas that are involved with higher‐level aspects of reward processing and task performance [Bijleveld et al., 2012a; Capa et al., 2013; Dehaene et al., 2006].
To test these expectations, we used a rewarded mental‐rotation task (Fig. 1). This task was designed to unravel how conscious versus unconscious rewards affect (a) activity in reward processing areas (VS), (b) activity in spatial processing areas directly involved in task performance (parietal cortex and superior temporal gyrus), and (c) activity in motor areas involved in response preparation and execution (motor cortex and supplementary motor area). During each trial of this task, participants are exposed to a high‐value or a low‐value coin, a proportion of which they can earn by quickly and accurately responding to a mental‐rotation stimulus. Importantly, this coin was presented subliminally on half of the trials, preventing subjects from consciously perceiving it [Pessiglione et al., 2007]. In previous research, this procedure has successfully been used to discern between the effects of unconscious versus conscious reward pursuit, both on the behavioral and the brain level [Bijleveld et al., 2010; Capa et al., 2013]. The mental‐rotation stimuli were taken from Shepard and Cooper [1982]; performing this task is known to be related to activity in the various parietal areas, superior temporal gyrus, (pre)motor cortex, and supplementary motor area [Gauthier et al., 2002; Milivojevic et al., 2009; Zacks, 2008].
Figure 1.
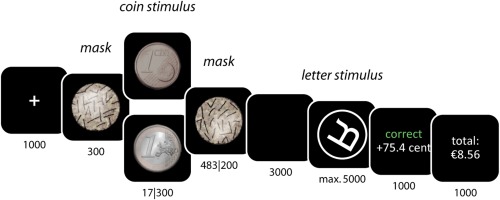
Schematic display of the task. Numbers refer to presentation durations in milliseconds. In all conditions, the duration of the coin and the masks added up to 800 ms. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MATERIALS AND METHODS
Subjects
Twenty‐three subjects (14 males and 9 females; mean age, 23.8 ± 2.2 years) participated in the study. Exclusion criteria included history of heart disease, epilepsy, claustrophobia, and pregnancy in women. Informed consent was obtained from all subjects and the study was approved by the local ethics committee of University Medical Center Utrecht, The Netherlands. Participants received the amount of money they earned in the experiment, which was on average €22 (SD = 4.0).
Task
The study used a 2(Reward: Euro vs. Cent) × 2(Presentation: Supraliminal vs. Subliminal) within‐subjects design. On each trial, subjects were exposed to a coin (a Euro vs. a Eurocent), which they could earn by accurately indicating whether a rotated letter was presented in its normal or its mirror‐image (backward) form. The amount of money they received for each trial was contingent on their response latency: the faster they were, the more money they earned. On some trials, coins were clearly visible; on other trials, coins were presented for only 17 ms, preventing subjects from consciously perceiving them. Subjects completed 72 trials in total, 18 repetitions per condition. These experimental trials were preceded by 18 practice trials, during which no money could be earned.
The sequence of events during each trial was as follows (Fig. 1). First, subjects saw a fixation cross (1,000 ms). Then, they saw the coin stimulus (17 or 300 ms), presented in between masks. The duration of the premask was 300 ms; the duration of the postmask was varied such that the coin and the masks were always on screen for 800 ms in total. Then, subjects saw a blank screen (3,000 ms), followed by the letter stimulus to which they had to respond. Specifically, by pressing either of two buttons on a response pad, subjects indicated whether the letter was in its normal or its mirror‐image form. Letter stimuli were encircled letters (L, F, G, Q, P, and R; normal or mirror‐image form) that were rotated at various angles (120°, 180°, and 240°). The letter stimulus disappeared after the subject's response or after 5,000 ms, whichever came first.
Next, subjects received feedback on their accuracy and on the amount of money they earned (1,000 ms). When they were incorrect, they received no money. When they were correct, they received a proportion of the value of the coin. That is, the more time they took to give a correct response, the less money they earned. Specifically, the amount of money subjects earned per trial was computed as , with E ≥ 0, where E is the amount of money earned, V is the value of the coin that was presented (in cents), T is the response latency, and A is the time by which the original reward (i.e., 100 or 1 cent) would decay to nothing. A, in turn, was computed based on subjects' performance during the practice trials. This was done to make sure that all subjects would earn approximately the same amount of money regardless of their ability. Specifically, A was computed as A = 2 × (M RT + 2 × SDRT), where M RT and SDRT are the mean and the standard deviation of the response latencies of the practice trials on which subjects were accurate. We chose these specific values (i.e., 2 × [M + 2SD]) to ensure subjects would earn at least 50% of the original amount of money, even on their slower trials. This was done to encourage participants to actually mentally rotate the letters, rather than to adopt a guessing strategy.
Finally, subjects saw another feedback screen that indicated the total (cumulative) amount of money they had earned in the experiment. The duration of the intertrial interval, during which the screen was blank, was varied such that each trial started exactly 12.8 s after the start of the previous one.
Coin Visibility
To examine whether the coins were indeed subliminal, a visibility check was conducted directly after the session, while subjects underwent the anatomical scan. In this test, subjects were exposed to the same coin stimuli as in the experiment (i.e., Euros vs. Cents, presented for 17 vs. 300 ms; 72 coins in total). This time, however, they merely indicated the identity of the stimulus,—that is, they reported whether they saw a Euro or a Cent. Specifically, on each trial of this visibility test, participants first saw a fixation cross (1,000 ms). Next, they saw the premask, the coin stimulus, and the postmask, presented in exactly the same way as in the main experiment (800 ms in total). Then they saw a blank screen (1,000 ms), after which they were prompted for their response. When they responded, they saw another blank screen (1,000 ms), after which the next trial started. Visibility check data from one subject was not stored due to software failure; analyses were conducted on data from the remaining 22 subjects. Results confirmed that subjects could detect the identity of the coin above chance when it was presented for 300 ms (accuracy = 90.9%, P < .001),1 but not when it was presented for 17 ms (accuracy = 50.7%, P = .75).
Image Acquisition
Data were acquired on a 3T Philips Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands). Foam padding was used to restrict head motion. Functional scans were acquired using a 2D‐EPI sequence and SENSE factor 2.4 (anterior‐posterior), with the following parameters: TE = 23 ms, TR = 1,600 ms, voxel size = 4 mm isotropic, flip angle = 72.5°, reconstructed matrix = 64×64, 36 axial slices per volume, and field of view 192×256×96. A total of 640 functional volumes were acquired in about 17 min. Fast field echo T1 weighted structural image was acquired for within‐subject registration purposes. The parameters were as follows: voxel size: 1 mm isotropic; RT = 25 msec; TE = 2.4 msec; field of view 256 × 150 × 204; flip angle 30°; 150 slices.
Functional MRI Analysis
Preprocessing
Preprocessing and statistical analyses were performed using Statistical Parametric Mapping (SPM) (http://www.fil.ion.ucl.ac.uk/spm/). Functional scans were realigned using rigid‐body affine transformation. The anatomical scan was coregistered to the functional scans, and both the anatomical and functional scans were normalized to match the MNI‐152 T1‐template. Finally, the functional scans were smoothed using a full‐width‐half‐maximum 8 mm Gaussian kernel.
Individual analyses
For each individual subject, regression‐coefficients for each voxel were obtained from a General Linear Model regression analysis, using a factor matrix that contained factors modeling hemodynamic changes that were event‐related to the anticipation phase of each trial (i.e., the period of time from the onset of the cue to the onset of the rotated letter; duration 3,500 ms). In line with the design, four conditions were modeled (Euro's vs. Cents, subliminal vs. supraliminal presentation). The onset of the factors modeling reward anticipation was aligned with the onset of the coin stimulus. Additional factors were included to model hemodynamic changes associated with the task performance phase (duration depending on task performance, range 255–2,926 ms) and the feedback phase of each trial (duration 2,000 ms). To take residual head motion effects into account, realignment parameters were included as regressors of no interest. Furthermore, a standard high‐pass filter was included to model out low‐frequency drifts in the signal (cutoff 128 s).
Group analyses
Group analyses were performed to identify brain activation related to the anticipation of reward (contrast Euro > Cent), for the subliminal and supraliminal coins separately. Maps resulting from these analyses were tested for significance using cluster‐level inference (cluster‐defining threshold, P < 0.001, cluster probability of P < 0.05, family‐wise error corrected for multiple comparisons). These parameters were determined using SPM and a script (CorrClusTh.m, http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm), which uses estimated smoothness (estimated Full Width at Half Maximum: 3.56×3.65×3.46 voxels) and Random Field Theory to find these corrected thresholds.
Brain‐behavior correlations
Finally, we explored how individual differences in brain activation due to subliminal Euros (vs. Cents) related to behavioral performance in response to these same subliminal Euros (vs. Cents). To this aim, we obtained average activation levels (i.e., regression coefficients for the subliminal Euros vs. Cents contrast) from the brain regions whose voxels were above threshold during the anticipation of supraliminal Euros (vs. Cents). We chose to use these regions (i.e., the ones activated by supraliminal Euros vs. Cents) as the basis for this analysis, because we did not find above‐threshold activations for subliminal Euros (vs. Cents; see Results, Imaging data).
RESULTS
Behavioral Data
Mean accuracy data were submitted to a 2(Reward: Euro vs. Cent) × 2(Presentation: Supraliminal vs. Subliminal) × 3(Rotation: 120° vs. 180° vs. 240°) repeated‐measures Analysis of Variance (ANOVA). This analysis revealed a main effect of Rotation, F(1, 22) = 19.6, P < 0.001, indicating that people made most mistakes when the letter stimulus was fully rotated (i.e., 180°). Moreover, there was a main effect of Reward, F(1, 22) = 6.3, P = 0.020, indicating that people made less errors when a Euro (vs. a Cent) was at stake. Also, there was a Rotation × Reward interaction, F(1, 22) = 6.7, P = 0.017, indicating that the reward effect was present especially on the most demanding trials (i.e., 180°). None of the other effects were significant, F's < 2.4, suggesting that supraliminal and subliminal coins affected accuracy in the same way. Most notably, there was no hint of a Reward × Presentation interaction, F(1, 22) < 1, P = 0.367, indicating absence of evidence for the idea that supraliminal rewards produced different (stronger) performance effects than subliminal rewards. Means and more specific tests are reported in Table 1.
Table 1.
Overview of behavioral findings
| Cent | Euro | t‐test of the difference | ||
|---|---|---|---|---|
| Accuracy (%) | Supraliminal | |||
| 120° | 88 ± 3 | 94 ± 3 | 1.4 | |
| 180° | 72 ± 3 | 83 ± 4 | 3.0a | |
| 240° | 91 ± 2 | 88 ± 3 | 0.9 | |
| Subliminal | ||||
| 120° | 94 ± 2 | 89 ± 4 | 1.1 | |
| 180° | 76 ± 5 | 86 ± 3 | 3.0a | |
| 240° | 94 ± 2 | 94 ± 2 | 0.3 | |
| Response latency (ms) | Supraliminal | |||
| 120° | 834 ± 34 | 824 ± 31 | 0.4 | |
| 180° | 976 ± 48 | 930 ± 31 | 1.4 | |
| 240° | 793 ± 26 | 821 ± 30 | 1.6 | |
| Subliminal | ||||
| 120° | 773 ± 23 | 804 ± 27 | 1.5 | |
| 180° | 902 ± 36 | 902 ± 37 | <0.1 | |
| 240° | 775 ± 23 | 749 ± 28 | 1.3 | |
Note: Values are given as Mean ± SEM.
P < 0.01.
Mean response latencies, computed over trials in which the subject was accurate, were analyzed with the same ANOVA. This analysis revealed a main effect of Rotation, F(1, 22) = 29.0, P < 0.001, indicating that people were slowest when the letter stimulus was fully rotated (i.e., 180°). Moreover, there was a main effect of Presentation, F(1, 22) = 17.0, P < 0.001, indicating that people were faster when the coin was presented subliminally (vs. supraliminally).2 No other effects were significant, F's < 3.3, suggesting that reward value had no effect on response latencies. Means are reported in Table 1. Taken together, these results indicate that subliminal and supraliminal rewards increased performance in the same way, specifically, both increased accuracy on the trials that were most demanding (i.e., trials in which the letter stimulus was fully rotated).
To further explore whether coin visibility affected performance, we explored whether speed and accuracy on the mental‐rotation task correlated with subjects' ability to detect briefly‐presented coins (as measured with the visibility check, reported above). To that end, we computed two contrasts that reflected the extent to which people were faster and more accurate, respectively, for subliminal Euros compared to subliminal Cents. People who were better able to consciously detect briefly‐presented coins were neither more accurate, r(20) = −0.25, P = 0.26, nor faster, r(20) = 0.26, P = 0.25. When the same analysis was done specifically for the condition in which the behavioral effect was found (i.e., the 180° trials), there were again no significant correlations, neither for accuracy, r(20) = −0.14, P = 0.54, nor for speed, r(20) = 0.20, P = 0.36. If anything, these results show that people who were better able to detect the coins performed worse, not better, when Euros were at stake. More importantly, the absence of significant correlations is consistent with the idea that visibility of the reward cues did not play an important role in enhancing subjects' performance.
Imaging Data
Results from the group analyses are presented in Figure 2 and Table 2. As expected, supraliminal presentation of Euros (vs. Cents) resulted in greater activation in brain areas typically associated with reward anticipation (VS), as well as regions related to task performance (parietal regions, supplementary motor area, motor cortex).3 The same contrast yielded no significant activations for the subliminal presentation of Euros (vs. Cents). Next, we performed a Region of Interest analysis (which is more liberal than a whole‐brain analysis) to test whether those clusters for which we found greater activation for supraliminal Euros (vs. supraliminal Cents; Table 2) were also activated by subliminal Euros (vs. subliminal Cents). Interestingly, we found no significant activations in these regions of interest. Next, we tested the Reward × Presentation interaction directly, to identify brain areas where activation due to supraliminal Euros (vs. Cents) was different from activation due to subliminal Euros (vs. Cents). By contrast to what we found for the behavioral data, this analysis (Table 2, bottom, and Fig. 2, bottom) indicated that the VS, supplementary motor area (SMA), motor cortex, and parietal regions were significantly more activated by supraliminal Euros (vs. Cents) than by subliminal Euros (vs. Cents).
Figure 2.
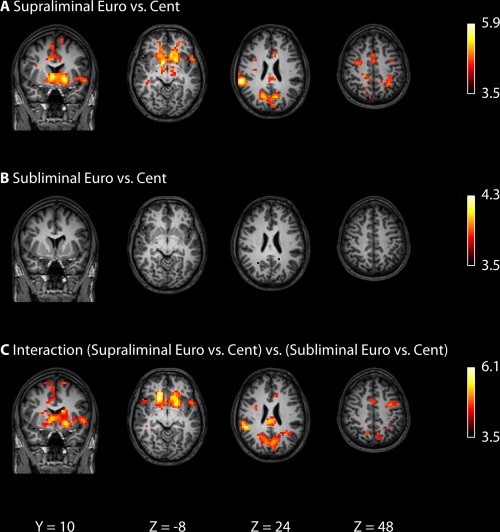
Imaging results, depicting the effects of supraliminal and subliminal Euros versus Cents. All brain activation maps are thresholded at a family‐wise error‐corrected cluster level of P < 0.05. See Table 2 for details.
Table 2.
Overview of brain areas activated in response to supraliminal and subliminal reward cues
| Region | BA | Side | Number of voxels | X | Y | Z | Max t‐value |
|---|---|---|---|---|---|---|---|
| Supraliminal Euro versus Cent (300 ms) | |||||||
| Superior temporal gyrus | 22 | R | 228 | 64 | −36 | 20 | 5.88 |
| Striatum | R | 576 | 12 | 4 | −8 | 5.87 | |
| Ventral striatum | L | −8 | 20 | −4 | 5.30 | ||
| Posterior cingulate cortex | 31 | R | 207 | 16 | −68 | 28 | 5.13 |
| Cuneus | L | 158 | −28 | −48 | 36 | 5.00 | |
| SMA | 6 | R | 286 | 8 | 8 | 56 | 4.98 |
| Dorsal ACC | 32 | R | 12 | 16 | 32 | 4.64 | |
| Superior frontal gyrus | 6/8 | R | 28 | −4 | 60 | 3.86 | |
| MCC | 28 | R | 98 | 4 | −28 | 40 | 4.40 |
| Insula | L | 35 | −52 | 12 | −8 | 4.18 | |
| Motor cortex | L | 40 | −32 | −20 | 68 | 4.39 | |
| Subliminal Euro versus Cent (17 ms) | |||||||
| — | |||||||
| Interaction (supraliminal Euro vs. Cent) versus (subliminal Euro vs. Cent) | |||||||
| Ventral striatum | R | 687 | 20 | 28 | −12 | 6.12 | |
| R | 16 | 20 | −8 | 5.99 | |||
| Ventral striatum | L | −32 | 16 | 4 | 5.40 | ||
| Parietal cortex | 7 | L | 553 | −24 | −60 | 32 | 5.90 |
| Cingulum | R | 16 | −32 | 32 | 5.14 | ||
| Posterior cingulum | 23 | R | 4 | −28 | 24 | 5.12 | |
| Insular cortex | 13 | R | 133 | 52 | −40 | 24 | 5.67 |
| Temporal midbrain | 21 | R | 44 | 36 | −4 | 4.88 | |
| Supramarginal gyrus | 40 | R | 60 | −32 | 24 | 4.70 | |
| Dorsal ACC | 32 | R | 142 | 12 | 16 | 32 | 4.93 |
| SMA | 6 | L | −4 | −4 | 64 | 4.44 | |
| −12 | 4 | 72 | 4.20 | ||||
| Motor cortex | L | 58 | −36 | −4 | 44 | 4.68 | |
| −40 | −8 | 60 | 3.67 | ||||
Note: Cluster‐defining threshold of P < 0.001 and a P < 0.05 family‐wise error‐corrected critical cluster size of 33 voxels.
ACC, anterior cingulate cortex; L, left; MCC, middle cingulate cortex; R, right; SMA, supplementary motor area.
As mentioned, and as can be seen in Figure 2 and Table 2, we found no significant activity in the VS, neither left nor right, in response to subliminal Euros (vs. Cents). As this is a remarkable null finding, we plotted reward‐induced VS activity as a function of Presentation (Fig. 3), to explore the possibility that subliminal coins triggered some VS activation, but that this activation was too weak to reach statistical significance (e.g., due to insufficient power). However, inspection of Figure 3 indicates that there was no VS activation at all in response to subliminal coins.
Figure 3.
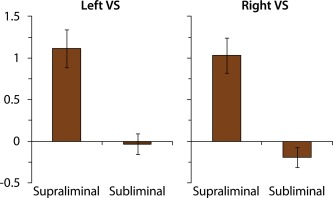
Imaging results, depicting the effects of supraliminal and subliminal Euros versus Cents on brain activation in the left and right ventral striatum (VS). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Brain‐Behavior Correlations
The lack of observed brain activation in response to subliminal Euros (vs. Cents) is striking especially because subliminal Euros improved performance to the same extent as supraliminal Euros (vs. Cents). Because of this discrepancy, we explored whether the reward effect on performance (computed as accuracy in response to subliminal Euros versus Cents, across all trials) was correlated with activation in the regions of interest involved in reward processing and task performance (the same contrast: brain activation in response to subliminal Euros versus Cents, across all trials3). Better performance was related to greater activation in task performance areas, including the motor cortex, r(21) = 0.49, P = 0.018, right superior frontal gyrus, r(21) = 0.47, P = 0.023, right supplementary motor area, r(21) = 0.58, P = 0.004, and right superior temporal gyrus, r(21) = 0.47, P = 0.025. Better performance was not related to greater activation in the VS, r's < 0.28, P's > 0.21.
DISCUSSION
The present research was designed to address two main questions. First, we tested the idea that the VS are involved in reward pursuit even when this occurs without awareness. We found no evidence for this idea. Second, we examined differences between unconscious versus conscious reward pursuit. Strikingly, we found that several structures involved in reward processing (VS) and task performance (motor and premotor cortex and inferior parietal lobe) were engaged due to supraliminal but not subliminal high‐value (vs. low‐value) reward cues, even though the behavioral effects of both types of cues were the same.
We started out by examining whether our mental‐rotation task was suitable for tapping the effects of rewards in the first place, by examining the effects of supraliminal (conscious) reward cues on brain activity and performance. Results indicated that high‐value (vs. low‐value) reward cues engaged several structures involved in reward processing and task performance, including the VS, supplementary motor area, motor cortex, and superior temporal gyrus. Moreover, in line with this pattern of brain activation and fitting previous research [Bijleveld et al., 2012b], there was also an effect of supraliminal reward cues on performance: high‐value (vs. low‐value) rewards facilitated performance, specifically on demanding trials. These results support the idea that our task is suitable for detecting effects of reward cues.
Then, we tested whether the same structures were also activated by subliminal high‐value (vs. low‐value) coins. This was clearly not the case. In fact, subliminal high‐value coins triggered significantly less activation than supraliminal high‐value coins, in several reward‐ and task performance‐related brain structures. This finding is rather striking, especially since subliminal high‐value (vs. low‐value) reward cues did facilitate performance, even in the same way and to the same extent as the supraliminal high‐value (vs. low‐value) reward cues.
One might be tempted to conclude from this finding that fMRI, as a technique, is simply not suitable to detect brain activation triggered by subliminal stimuli. However, a recent meta‐analysis (of fMRI studies that used subliminal paradigms) showed that at least some subliminal stimuli (e.g., emotional faces) reliably trigger activity in subcortical areas [e.g., the amygdala; Brooks et al., 2012]. So, in principle, examining the effects of subliminal reward cues on VS activation with fMRI seems realistic. Still, the present study indicates that subliminal reward cues instigate some brain process that (a) can remain undetected with fMRI but that (b) at the same time detectably boosts performance. What, then, is the nature of this process? In our view, there are two possibilities.
First, it may be the case that the VS is specifically involved in creating associations between cues and responses, in this case, between high‐value reward cues and the recruitment of resources in the service of task performance. It is a possibility that these associations are made only in the presence of conscious awareness of the cue [Hofmann et al., 2010]. Since subliminal trials were intermixed with supraliminal trials in the present experiment, it could be the case that the cue‐response associations were formed during the supraliminal trials (hence VS activation) and merely used during the subliminal trials (hence no VS activation). It should be noted, though, that this possibility is contradicted by research showing that subliminal stimuli may lead to VS activation in learning paradigms [Pessiglione et al., 2008], and that people may readily learn associations to subliminal cues [Seitz et al., 2009].
A second possibility is that subliminal reward cues trigger VS activation, but that this occurs in a quicker and more transient way compared to supraliminal reward cues. This possibility is in line with the idea that conscious awareness of a stimulus keeps information carried by that stimulus active over a sustained period of time [Dehaene and Naccache, 2001]. If true, this accounts for why we did not find VS activity after subliminal high‐value reward cues, as it may be the case that VS activation (due to subliminal high‐value vs. low‐value reward cues, specifically) occurs very quickly and transiently, making it difficult to detect.
The latter explanation makes sense given that this study used a shorter presentation time for our coin stimuli (17 ms) compared to previous work that aimed to explore effects of subliminal value‐related stimuli on activation in subcortical brain areas [33 ms; Childress et al., 2008; Pessiglione et al., 2008]. Pessiglione et al. [2007] indeed suggest that differences in timing can be crucial: they found pallidal activation when coins were presented for 50 ms, but not 17 ms. However, only in the 17 ms condition, participants were unable to detect the value of the coin above chance. When considered together, the study by Pessiglione et al. [2007] and this study suggest that becoming more conservative regarding stimulus visibility by decreasing presentation duration, makes it much more difficult to detect effects on brain activation (with fMRI, in this specific case) even though behavioral effects may still surface.
With regard to the interpretation of the behavioral data, we inferred that subliminal and supraliminal high‐value reward cues improved performance to the same extent, as coin visibility did not interact with coin value. Indeed, there was no hint of such an effect (F < 1). Nevertheless, we should note that inspection of Table 1 suggests that supraliminal rewards seemed to increase performance not only on high‐demanding but also a bit on low‐demanding trials. Although also these increments were far from significant (t's < 1.4), the questions of when and how performance effects of supraliminal versus subliminal rewards can diverge remains an important issue for further investigation [Bijleveld et al., 2012a].
CONCLUSIONS
The present research allows for two key conclusions with regard to how reward pursuit can occur with and without conscious awareness. First, our study indicates that—with respect to their neural underpinnings—conscious and unconscious reward pursuit can clearly be dissociated. Fitting previous research [Bijleveld et al., 2012a], brain structures involved are activated to a vastly different extent and perhaps also following a different time course. So, this study shows that conscious and unconscious reward pursuit are different with respect to their underlying neural dynamics. Second, this study shows that even though the neural underpinnings are different, conscious and unconscious reward pursuit may still have the same effects on performance. This speaks to the striking suggestion that, at least under some circumstances, the neural substrates of conscious awareness are functionally redundant [Lau and Rosenthal, 2011]. This work thus sets the stage for future studies that aim to increase our understanding of the role of consciousness in reward pursuit.
Footnotes
For stimuli presented for 300 ms, a detection rate of 90.9% seems low (see Bijleveld et al., 2012bb). Inspection of detection data revealed that three participants in particular had low scores (<60%; all others M = 96.6%), suggesting that these participants might have misheard the (verbal) instructions for the detection task. Exclusion of these participants would not change the statistical significance of any of our analyses.
This effect may well be due to the fact that supraliminal coins are processed more deeply, and therefore, have some processing cost of their own (regardless of their value). This may explain why RT's for the subsequent stimulus (i.e., the rotated letter) are longer.
One could argue that we should analyze not all trials of the experiment, but only the trials on which the letter stimulus was fully rotated. After all, the behavioral effect emerged specifically on these trials. In our view, however, this analysis would not be informative, as our study was designed to measure brain activation during reward anticipation, that is, the time period between the onset of the coin and the onset of the letter stimulus. Importantly, during this period, participants were not yet exposed to the letter stimulus, making it impossible for them to know anything about the demands of the (upcoming) task. So, the rotation factor could not have affected any brain activity during the time at which we measured brain activation. For this reason, we chose to include all trials in the imaging analyses.
REFERENCES
- Bijleveld E, Custers R, Aarts H (2009): The unconscious eye‐opener: Pupil size reveals strategic recruitment of resources upon presentation of subliminal reward cues. Psychol Sci 20:1313–1315. [DOI] [PubMed] [Google Scholar]
- Bijleveld E, Custers R, Aarts H (2010): Unconscious reward cues increase invested effort, but do not change speed‐accuracy tradeoffs. Cognition 115:330–335. [DOI] [PubMed] [Google Scholar]
- Bijleveld E, Custers R, Aarts H (2012a): Human reward pursuit: From rudimentary to higher‐level functions. Curr Dir Psychol Sci 21:194–199. [Google Scholar]
- Bijleveld E, Custers R, Aarts H (2012b): Adaptive reward pursuit: How effort requirements affect unconscious reward responses and conscious reward decisions. J Exp Psychol Gen 141:728–742. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW (2007): Anticipating instrumentally obtained and passively‐received rewards: A factorial fMRI investigation. Behav Brain Res 177:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Savov V, Allzén E, Benedict C, Fredriksson R, Schiöth HB (2012): Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: A systematic meta‐analysis of fMRI studies. NeuroImage 59:2962–2973. [DOI] [PubMed] [Google Scholar]
- Capa RL, Bustin GM, Cleeremans A, Hansenne M (2011): Conscious and unconscious reward cues can affect a critical component of executive control: (Un)Conscious updating? Exp Psychol 58:370–375. [DOI] [PubMed] [Google Scholar]
- Capa RL, Bouquet CA, Dreher J‐C, Dufour A (2013): Long‐lasting effects of performance‐contingent unconscious and conscious reward incentives during cued task‐switching. Cortex 49:1943–1954. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O'Brien CP (2008): Prelude to passion: Limbic activation by “unseen” drug and sexual cues. PLoS One 3:e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Naccache L (2001): Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 79:1–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux J‐P, Naccache L, Sackur J, Sergent C (2006): Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10:204–211. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Hayward WG, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (2002): BOLD activity during mental rotation and viewpoint‐dependent object recognition. Neuron 34:161–171. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2009): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacol 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, De Houwer J, Perugini M, Baeyens F, Crombez G (2010): Evaluative conditioning in humans: A meta‐analysis. Psychol Bull 136:390–421. [DOI] [PubMed] [Google Scholar]
- Knutson B, Delgado MR, Phillips PEM (2008): Neuroeconomics: Decision Making and the Brain. Representation of subjective value in the striatum In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors. Oxford: Oxford University Press. [Google Scholar]
- Lau H, Rosenthal D (2011): Empirical support for higher‐order theories of conscious awareness. Trends Cogn Sci 15:365–373. [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O'Doherty JP (2012): Contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn Sci 16:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic B, Hamm JP, Corballis MC (2009): Functional neuroanatomy of mental rotation. J Cogn Neurosci 21:945–959. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD (2007): How the brain translates money into force: A neuroimaging study of subliminal motivation. Science 316:904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Petrovic P, Daunizeau J, Palminteri S, Dolan RJ, Frith CD (2008): Subliminal instrumental conditioning demonstrated in the human brain. Neuron 59:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Walton M, Jhou T (2007): Calculating utility: Preclinical evidence for cost–benefit analysis by mesolimbic dopamine. Psychopharmacol 191:483–495. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B (2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99:5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M (2009): Dopamine, behavioral economics, and effort. Front Behav Neurosci 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T (2009): Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron 61:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, Cooper LA (1982): Mental Images and Their Transformations. Cambridge, MA: MIT Press. [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009): Ventral pallidum roles in reward and motivation. Behav Brain Res 196:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Pas P, Bijleveld E, Custers R, Gladwin TE (2013): Ventral striatum is related to within‐subject learning performance. Neurosci 250:408–416. [DOI] [PubMed] [Google Scholar]
- Zacks JM (2008): Neuroimaging studies of mental rotation: A meta‐analysis and review. J Cogn Neurosci 20:1–19. [DOI] [PubMed] [Google Scholar]
- Zedelius CM, Veling H, Aarts H (2011): Boosting or choking—How conscious and unconscious reward processing modulate the active maintenance of goal‐relevant information. Conscious Cogn 20:355–362. [DOI] [PubMed] [Google Scholar]
- Zedelius CM, Veling H, Custers R, Bijleveld E, Chiew KS, Aarts H (2014): A new perspective on human reward research: How consciously and unconsciously perceived reward information influences performance. Cogn Affect Behav Ne 14:493–508. [DOI] [PubMed] [Google Scholar]


