Abstract
Background
Access to tissue, difficulties with dissection, and poor visibility of enteric ganglia have hampered electrophysiological recordings of human enteric neurons. Here, we report a method to combine intracellular recording with simultaneous morphological identification of neurons in the intact myenteric plexus of human colon ex vivo.
Methods
Specimens of human colon were dissected into flat-sheet preparations with the myenteric plexus exposed. Myenteric neurons were impaled with conventional microelectrodes containing 5% 5,6-carboxyfluorescein in 20 mM Tris buffer and 1 M KC.
Key Results
Electrophysiological recordings identified myenteric neurons with S and AH type properties (n = 13, N = 7) which were dye filled and classified during the recording as Dogiel type I (n = 10), Dogiel type II (n = 2), or filamentous (n = 1) cells. This classification was confirmed after fixation, in combination with immunohistochemical characterization.
Conclusions & Inferences
This method allows electrophysiological characterization with simultaneous identification of morphology. It can be used to identify recorded cells immediately after impalement and greatly facilitates recordings of human myenteric neurons in freshly dissected specimens of tissue. It can also be combined with immunohistochemical labeling of recorded cells.
Keywords: carboxyfluorescein, electrophysiology, human colon, myenteric neurons
Key Messages.
The advanced method described in this study allows electrophysiological characterization of human enteric neurons with simultaneous identification of their morphology which greatly facilitates recordings of human myenteric neurons.
The aim of the study was to develop the technique using a fluorescent dye carboxyfluorescein in microelectrodes to record from human enteric neurons and identify their morphology in situ.
Myenteric neurons were identified with carboxyfluorescein as Dogiel type I, II, or filamentous; their morphologies were matched with S and AH type electrophysiological properties. This classification was confirmed after fixation, in combination with immunohistochemical characterization.
Introduction
Electrophysiological recording from human myenteric neurons is a significant challenge due to the thickness of the intestinal wall, the amount of connective tissue, and the inability to visualize ganglia and internodal strands. Intracellular microelectrode recordings have been restricted to two studies in freshly dissected myenteric plexus preparations1 and in cultured fetal myenteric neurons.2 Human submucosal neurons have been recorded using voltage-sensitive3 and calcium-sensitive dyes,4,5 however, recordings from human myenteric neurons required either direct injection of ganglia with dyes or culturing of preparations.4 Dye recordings allow simultaneous monitoring of multiple cells, but have limited signal to noise ratios and are restricted by the kinetics of the dye. While immunohistochemical labeling of recorded cells can be performed,3 detailed soma-dendritic morphology is not revealed by dye recording techniques. Because functional properties of enteric neurons can change significantly in disorders, correlating electrophysiological properties and cell morphology will be important in studies of human enteric pathology.6 In this study, we developed the use of a fluorescent dye in microelectrodes that facilitates recordings from human enteric neurons in situ. This dye does not impair recording quality and allows morphological characterization during the recording period. Labeling can then be enhanced by immunohistochemical localization of carboxyfluorescein using a fluorescent-labeled primary antiserum.
Materials and Methods
Specimens of human colon were provided by the Victorian Cancer Biobank (N = 4) and Flinders Medical Centre (N = 3) (approved by the Victoria University Human Research and Southern Adelaide Clinical Research Ethics Committees). Written informed consent was obtained from patients (47–79 years, two female) prior to partial colectomy for removal of non-obstructive carcinoma; six specimens were taken proximal and one distal to the tumor site. Distal colon (N = 5) and proximal colon (N = 2) tissues were taken from healthy margins. Full thickness flat-sheet preparations (∼1 cm2) were dissected in Krebs solution at room temperature (mM: NaCl 118, KCl 4.6, CaCl2 3.5, MgSO4 1.2, NaH2PO4 1, NaHCO3 25, D-Glucose 11, bubbled with 95% O2 and 5% CO2) in a Sylgard-lined Petri dish (Dow Corning, Midland, Michigan, USA). Serosa, fat, and mesentery were removed, the mucosa and submucosa were dissected off7 and circular muscle was peeled away to expose the myenteric plexus. Nerve cell bodies containing lipofuscin were identified by pale pigmentation.1 Specimens were re-pinned in a recording chamber with 50 μm tungsten pins; extra pins were added to outline ganglia for recording. The chamber was superfused with Krebs solution (35 °C) containing 1 μM atropine and 1 μM nicardipine, while mounted on a Zeiss Axiovert-200 inverted microscope (Oberkochen, Germany) and allowed 1 h to equilibrate.
Intracellular recording
Neurons were impaled with conventional glass micropipettes, filled with 5% 5,6-carboxyfluorescein in 20 mM Tris buffer and 1 M KCl (pH 7.0),8 with resistances of 100–150 MΩ. Carboxyfluorescein was iontophoresed by hyperpolarizing current pulses for 2 min (0.5 nA, 0.2 s duration at 2.5 Hz). Labeled cells were visualized in situ using fluorescence, and later photographed. Recordings made using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA, USA), digitized at 1–10 kHz with a Digidata 1440A interface (Molecular Devices, Sunnyvale, CA, USA) and stored using PClamp 10.0 (Molecular Devices, Sunnyvale, CA, USA). Cells which had resting membrane potentials (RMPs) more negative than −40 mV, and which were filled adequately with carboxyfluorescein were analyzed. Intracellular hyperpolarizing current pulses (500 ms, 100–500 pA) were used to determine input resistance (Rin). A tungsten electrode (10–50 μm tip diameter, 1 mm circumferential to the recording micropipette) was connected to an ISO-Flex stimulator controlled by a Master-8 pulse generator (AMPI, Jerusalem, Israel). Fast excitatory postsynaptic potentials (EPSPs) were evoked by single-shot stimuli (0.4 ms; 10–60 V), while slow postsynaptic potentials were evoked by trains of pulses at 10–20 Hz. Data were analyzed with Axograph X software and presented as mean ± SD.
Following recording, preparations were fixed in Zamboni's fixative (N = 7) and processed for immunohistochemistry (N = 6), by 3 × 10 min, in dimethylsulfoxide, 3 × 10 min in phosphate-buffered saline (PBS), 48 h incubation in primary antibodies, rinsed in PBS, then 24 h incubation in secondary antisera (Table1). Tissues were viewed using IX71 Olympus microscope (Tokyo, Japan) or Nikon Eclipse Ti confocal microscope (Tokyo, Japan).
Table 1.
Primary and secondary antibodies used in this study
| Primary antisera | Secondary antisera | |||||
|---|---|---|---|---|---|---|
| Antigen | Species | Source | Concentration | Secondary antisera | Source | Concentration |
| Fluorescein/Oregon Green -Alexa Fluor 488 | Rabbit | A-11090; Molecular Probes | 1 : 400 | Donkey anti-rabbit FITC | 711-095-152; Jackson ImmunoResearch | 1 : 200 |
| Hu | Mouse | A21271; Molecular Probes | 1 : 500 | Donkey anti-mouse CY5 | 715-175-150; Jackson ImmunoResearch | 1 : 200 |
| Neuronal Nitric Oxide Synthase (nNOS) | Sheep | Gift from Dr P Emson | 1 : 500 | Donkey anti-sheep AMCA | 713-155-003; Jackson ImmunoResearch | 1 : 200 |
FITC, Fluorescein Isothiocyanate; Cy5, indodicarbocyanine; AMCA, aminomethylcoumarin.
Drugs
All drugs and 5,6-carboxyfluorescein were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Nicardipine and hexamethonium were stored in aqueous solution (10−2 M and 1 M). Atropine was stored in ethanol at 10−2 M.
Results
Impaled cells were labeled with carboxyfluorescein; recordings and morphological identification were achieved in 13 neurons in seven preparations. In 10/13 neurons, a single axon extended from a cell body with short lamellar dendrites characteristic of Dogiel type I morphology (Fig.1A).9,10 Two neurons had large cell bodies and two axons (Dogiel type II morphology, Fig.1B). One neuron had multiple thick branching dendrites and a single bifurcating axon (filamentous morphology, Fig.2A). Micrographs of carboxyfluorescein-labeled cells were captured immediately after withdrawing the microelectrode (Fig.1A), after fixation, and again after processing for immunohistochemistry (Fig.1A', B, B'). Carboxyfluorescein fluorescent signaling could be enhanced using an antifluorescein antibody, (Fig.1A', B, B') but this extra step was not required for routine visualization of cells (Fig.2A).
Figure 1.
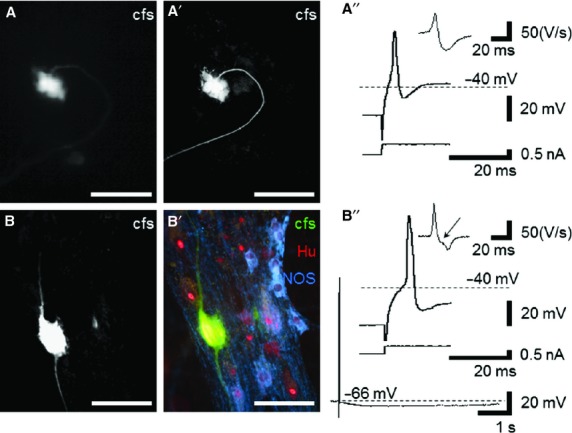
Morphological and electrophysiological properties of human myenteric neurons labeled with carboxyfluorescein. (A) Using fluorescence microscopy, a carboxyfluorescein-labeled Dogiel type I neuron could be identified in situ. (A') Labeling was maintained with overnight fixation and enhanced with antifluorescein primary antisera (standard fluorescent microscopy). (A”) This cell had typical S neuron electrical properties. Action potentials were evoked with depolarizing current and the repolarizing phase lacked an inflection (top: action potential; bottom: depolarizing current; subset: differential trace). (B) Confocal image of a Dogiel type II neuron. (B') The carboxyfluorescein-filled neuron among other neurons labeled with pan-neuronal marker Hu, some of which were nNOS immunoreactive (standard fluorescent microscopy). (B”) The repolarizing phase of the action potential in this neuron had an inflection highlighted in the differential trace (top: action potential; middle: depolarizing current; subset: differential trace with arrow highlighting inflection). A long after-hyperpolarization followed the action potential in this cell, a typical characteristic of AH neurons. Scale bars for micrographs = 100 μm.
Figure 2.
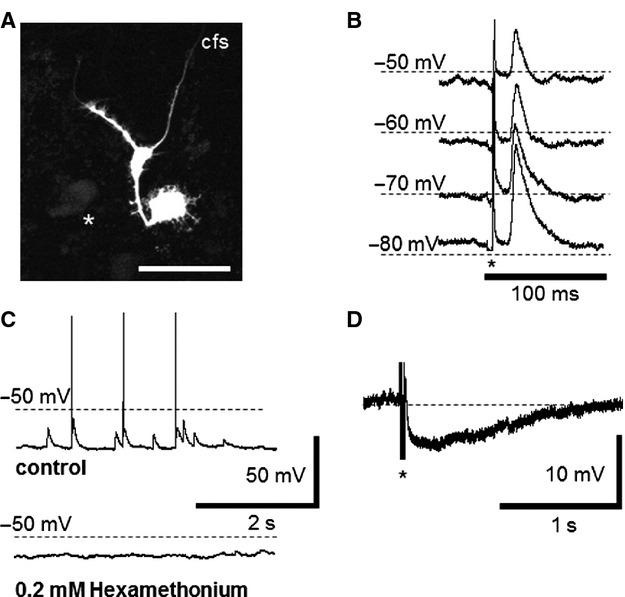
Synaptic inputs to Dogiel type I and filamentous myenteric neurons from the human colon. Spontaneous and evoked EPSPs were recorded in Dogiel type I, and in a filamentous neuron (A, scale bar 100 μm). (B) Fast EPSPs induced by focal electrical stimulation of nearby ganglia or internodal strands (n = 5) increased in amplitude when the membrane potentials were offset to more negative levels. (C) Spontaneous fast EPSPs were recorded in eight neurons. These EPSPs provided sufficient depolarization to evoke action potentials in two neurons (C top trace) and were inhibited by addition of hexamethonium (0.2 mM, C bottom trace). (D) An IPSP evoked by electrical stimulation (3 pulses, 20 V) in one neuron.
The RMP of Dogiel type I neurons averaged −58.0 ± 8.5 mV and the Rin averaged 59.2 ± 22.5 MΩ (n = 10, N = 7). Depolarizing current pulses evoked 1–3 action potentials in 9/10 cells (Fig.1A”), but one neuron fired up to 22 action potentials during longer depolarizations. Action potential amplitudes ranged from 37 to 79 mV with durations at half amplitude of 0.9–1.8 ms. The filamentous neuron (n = 1) had RMP = −76.0 mV, Rin = 20.1 MΩ and fired once at the onset of depolarization. Action potential amplitudes averaged 46.6 ± 2.7 mV with duration at half peak amplitude of 1.1 ± 0.0 ms (mean ± STD). Dogiel type II neurons (n = 2) had RMP = −63.7 ± 7.6 mV and Rin = 31.4 ± 25.4 MΩ. It fired one action potential to depolarization (40.0 ± 14.9 mV amplitude; duration 2.2 ± 0.2 ms). Action potentials in these neurons had an inflection on the falling phase, clearly identified in the differentiated trace (Fig.1B”). One Dogiel type II neuron had a slow after-hyperpolarization (8.1 ± 3.3 mV amplitude and 3.1 ± 1.8 ms duration).
Fast EPSPs were recorded in 9/10 Dogiel type I cells and in the filamentous neuron. Their amplitude increased during hyperpolarizing current pulses (Fig.2B), and decreased during depolarization, ranging from 3.5 to 16.5 mV. By plotting amplitude against holding potential, the reversal potential for EPSPs was estimated as −30.3 ± 7.9 mV (n = 5, N = 5). The mean amplitude of spontaneous fast EPSPs recorded at −70 mV holding potential averaged 5.5 ± 21.3 mV (range 1.8–15.8 mV; n = 8 cells), with durations at half amplitude of 19.9 ± 3.9 ms (range 7.5–51.5 ms, Fig.2C). In two cells, fast EPSPs triggered action potentials. Fast EPSPs were blocked by hexamethonium (0.2 mM) in 3/3 cells tested. Repetitive electrical stimulation evoked inhibitory postsynaptic potentials (IPSPs) in 1/6 cells tested, but slow EPSPs were not observed. At RMP of −70 mV, the IPSP averaged −7.0 ± 0.5 mV and a lasted for 3.9 ± 0.6 s (Fig.2D).
Discussion
Human myenteric neurons have been recorded previously in intact ganglia in vitro, with intracellular microelectrodes.1,2 While several studies using calcium and voltage-sensitive dyes have added considerable data,3–5 our understanding of the basic physiology of the human enteric nervous system remains limited. The major hindrances to electrophysiological recording of human enteric neurons are the poor visibility and difficulty of penetrating myenteric ganglia. The addition of 5,6-carboxyfluorescein to microelectrodes does not directly reduce these problems. However, because this dye is fluorescent, it reveals cell morphology within seconds of achieving an impalement. This provides certainty about whether the electrode tip has actually penetrated the ganglion, which type of cell is being recorded and even which part of the cell has been impaled. This is invaluable in deciding whether to persist with a borderline impalement or attempt again. Therefore, this approach greatly improves productivity and overall success rate.
The addition of carboxyfluorescein to the electrode definitively pairs the morphology of every impaled cell with its electrical properties. The majority of the neurons in this study were identified as Dogiel type I neurons with S type electrophysiology. Two neurons had Dogiel type II morphology and one had a filamentous soma-dendritic morphology. A slow after-hyperpolarization was readily evoked in one of the two Dogiel type II neurons. The apparent scarcity of AH neurons in the human colon is consistent with a previous report of human enteric neurons, which did not include morphological identification of most of the cells.1 Although enteric neurons with slow after-hyperpolarizations are abundant in the guinea pig, a comparable paucity of AH neurons has also been reported in the pig ileum.11 Thus, further studies will be required to establish interspecies differences.
Lucifer Yellow has been previously used to fill human myenteric neurons,1 but this tracer increases noise and sometimes blocks fine-tipped microelectrodes and is problematic for routine use.12 Carboxyfluorescein affects the resistance and noise of micropipettes much less8,12 and has been used previously to record neurons in the mammalian central nervous system.13 It has also been used in recordings from smooth muscle cells and enteric neurons in the guinea pig intestines, allowing dye coupling to be quantified.8 It is affordable and non-cytotoxic, although one study has suggested that it may reduce resting membrane potential slightly.14 Quantifying this effect will require considerably larger samples than were possible in the course of this study.
Funding
This study is supported by Australian National Health & Medical Research Council project grant 1032414 and a small project grant from BioLED research unit, Victoria University.
Disclosure
The authors of this manuscript do not have any potential conflicts to disclose.
Author Contribution
SEC performed experiments, analyzed data, and drafted the manuscript; VJ processed samples for immunohistochemistry and captured images; SJHB and KN developed the concept and edited manuscript.
References
- 1.Brookes SJ, Ewart WR, Wingate DL. Intracellular recordings from myenteric neurones in the human colon. The Journal of Physiology. 1987;390:305–18. doi: 10.1113/jphysiol.1987.sp016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruyama T. Two types of spike generation of human Auerbach's plexus cells in culture. Neurosci Lett. 1981;25:143–8. doi: 10.1016/0304-3940(81)90322-0. [DOI] [PubMed] [Google Scholar]
- 3.Schemann M, Michel K, Peters S, Bischoff SC, Neunlist M. Cutting-edge technology. III. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;282:G919–25. doi: 10.1152/ajpgi.00043.2002. [DOI] [PubMed] [Google Scholar]
- 4.Vignali S, Peter N, Ceyhan G, Demir IE, Zeller F, Senseman D, Michel K, Schemann M. Recordings from human myenteric neurons using voltage-sensitive dyes. J Neurosci Methods. 2010;192:240–8. doi: 10.1016/j.jneumeth.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Cirillo C, Tack J, Vanden Berghe P. Nerve activity recordings in routine human intestinal biopsies. Gut. 2013;62:227–35. doi: 10.1136/gutjnl-2011-301777. [DOI] [PubMed] [Google Scholar]
- 6.Nurgali K. Plasticity and ambiguity of the electrophysiological phenotypes of enteric neurons. Neurogastroenterol Motil. 2009;21:903–13. doi: 10.1111/j.1365-2982.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 7.Carbone SE, Dinning PG, Costa M, Spencer NJ, Brookes SJH, Wattchow DA. Ascending excitatory neural pathways modulate slow phasic myogenic contractions in the isolated human colon. Neurogastroenterol Motil. 2013;25:670–6. doi: 10.1111/nmo.12129. [DOI] [PubMed] [Google Scholar]
- 8.Carbone SE, Wattchow DA, Spencer NJ, Brookes SJH. Loss of responsiveness of circular smooth muscle cells from the guinea pig ileum is associated with changes in gap junction coupling. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1434–44. doi: 10.1152/ajpgi.00376.2011. [DOI] [PubMed] [Google Scholar]
- 9.Brehmer A. Structure of enteric neurons. Adv Anat Embryol Cell Biol. 2006;186:1–91. [PubMed] [Google Scholar]
- 10.Furness JB. The Enteric Nervous System. Oxford: Blackwell Publishing; 2006. [Google Scholar]
- 11.Cornelissen W, De Laet A, Kroese AB, Van Bogaert PP, Scheuermann DW, Timmermans JP. Electrophysiological features of morphological Dogiel type II neurons in the myenteric plexus of pig small intestine. J Neurophysiol. 2000;84:102–11. doi: 10.1152/jn.2000.84.1.102. [DOI] [PubMed] [Google Scholar]
- 12.Hanani M. Lucifer yellow - an angel rather than the devil. J Cell Mol Med. 2012;16:22–31. doi: 10.1111/j.1582-4934.2011.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao G, Barnes CA, McNaughton BL. Intracellular fluorescent staining with carboxyfluorescein: a rapid and reliable method for quantifying dye-coupling in mammalian central nervous system. J Neurosci Methods. 1986;16:251–63. doi: 10.1016/0165-0270(86)90050-6. [DOI] [PubMed] [Google Scholar]
- 14.Beggs JM, Kairiss EW. Electrophysiology and morphology of neurons in rat perirhinal cortex. Brain Res. 1994;665:18–32. doi: 10.1016/0006-8993(94)91147-9. [DOI] [PubMed] [Google Scholar]


