Abstract
Background and Objectives
Low level laser (light) therapy (LLLT) has been demonstrated to promote hair growth in males. A double-blind randomized controlled trial was undertaken to define the safety and physiologic effects of LLLT on females with androgenic alopecia.
Methods
Forty-seven females (18–60 years old, Fitzpatrick I–IV, and Ludwig–Savin Baldness Scale I-2, I-3, I-4, II-1, II-2 baldness patterns) were recruited. A transition zone scalp site was selected; hairs were trimmed to 3 mm height; the area was tattooed and photographed. The active group received a “TOPHAT655” unit containing 21, 5 mW diode lasers (655 ± 5 nm) and 30 LEDS (655 ± 20 nm), in a bicycle-helmet like apparatus. The placebo group unit appeared identical, containing incandescent red lights. Patients treated at home every other day × 16 weeks (60 treatments, 67 J/cm2 irradiance/25 minute treatment, 2.9 J dose), with follow up and photography at 16 weeks. A masked 2.85 cm2 photographic area was evaluated by another blinded investigator. The primary endpoint was the percent increase in hair counts from baseline.
Results
Forty-two patients completed the study (24 active, 18 sham). No adverse events or side effects were reported. Baseline hair counts were 228.2 ± 133.4 (N = 18) in the sham and 209.6 ± 118.5 (N = 24) in the active group (P = 0.642). Post Treatment hair counts were 252.1 ± 143.3 (N = 18) in the sham group and 309.9 ± 166.6 (N = 24) in the active group (P = 0.235). The change in hair counts over baseline was 23.9 ± 30.1 (N = 18) in the sham group and 100.3 ± 53.4 (N = 24) in the active group (P < 0.0001). The percent hair increase over the duration of the study was 11.05 ± 48.30 (N = 18) for the sham group and 48.07 ± 17.61 (N = 24) for the active group (P < 0.001). This demonstrates a 37% increase in hair growth in the active treatment group as compared to the placebo group.
Conclusions
LLLT of the scalp at 655 nm significantly improved hair counts in women with androgenetic alopecia at a rate similar to that observed in males using the same parameters. Lasers Surg. Med. 46:601–607, 2014. © 2014 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals, Inc.
Keywords: alopecia, clinical research, hair, human, laser, LED, low level laser therapy (LLLT), photobiomodulation, RCT
INTRODUCTION
Endre Mester first observed that mice treated with lasers during experiments investigating the potential carcinogenic effects of laser exposure regrew hair in shaved areas significantly faster than unexposed mice in 1967 1,2. Other investigators subsequently observed that some patients exhibited paradoxical hair growth at the periphery of areas treated with lasers for hair removal or adjacent to lesions treated with laser sources 3–5. These seminal observations stimulated others to investigate the potential effects and applications of low level laser (light) therapy (LLLT) in male and female pattern androgenetic alopecia 6–15.
We have previously reported the results of the male arm of a randomized controlled trial that was undertaken to define the safety and physiologic effects that occur when the hair follicle and surrounding tissue structures of the human scalp are exposed to LLLT using a bicycle helmet type device fitted with an array of laser and LED light sources operating at 655 nm 16. This laser system meets the requirements of an FDA Class 3R laser product, and as a non-medical laser system (RDW). The LED components are non-classified light sources when marketed for cosmetic applications, as is the case here. The device was granted an FDA 510k clearance for the treatment of males with Hamilton–Norwood IIa-V, or frontal patterns of hair loss, in patients with Fitzpatrick I–IV skin types based on the results for the male cohort of that trial 16,17.
The present investigation reports the results obtained for the female cohort of subjects treated under the TH655 study protocol.
MATERIALS AND METHODS
A clinical study was conducted as per the IRB approved TH655 protocol (Essex IRB, Lebanon, NJ). The trial was registered on www.ClinicalTrials.gov and was assigned the identifier NCT01437163. Forty-seven healthy female volunteers 18–60 years old were recruited at two IRB approved treatment sites.
Informed consent was obtained, and each female subject was screened to verify that she met the inclusion and exclusion criteria for the study. History and physical examinations were conducted. All 47 women had Fitzpatrick skin types I–IV and Ludwig–Savin Baldness Scale I–II (L-S I-2, I-3, I-4, II-1, II-2) baldness patterns. An area of scalp was selected in a transition zone at the vertex of the scalp at a site determined by the investigator. The hairs within the selected site were trimmed to a maximum height of 3 mm in area that was approximately 2.5 cm in diameter. The area was marked with a medical tattoo using green ink using aseptic technique.
The site was then photographed using a custom camera apparatus that consisted of a Canon Rebel T3i 18 Megapixel camera system (Canon USA, Melville, NY) equipped with a Tamron 60 mm f/2 Macro lens with 1:1 magnification (Tamron USA, Commack, NY). A 55 mm Lens attachment ring was used to affix a Promaster RL60 LED Ring Light (Promaster, Inc, Fairfield, CT). The camera system was mounted to a custom Stand-off device which was manually positioned onto the scalp surface by the investigator each time photographs were taken. Images were taken positioning the tattoo in the center of the frame. These baseline images were coded and then forwarded to the photographic consultant. The photographic consultant verified that the images were of acceptable quality and processed the images for transmission to the investigator responsible for conducting the hair counts. The transmitted images were masked using a black mask to produce a 1.9 cm diameter circle centered on the tattoo, which provided a consistent 2.85 cm2 area for hair counts. Neither the photographic consultant nor the investigator performing the hair counts was aware of the identity of the subject or the subjects' study group assignment.
Subjects were randomly assigned to active treatment or placebo treatment groups. Each subject received a numbered “TOPHAT655” unit (Apira Science, Inc, Boca Raton, FL) which was distributed to her by the Project Manager, who also provided instructions for the care and use of the device. The patients, the treating physicians, the photographic consultant, and the investigator performing the hair counts were not aware whether the device was a therapeutic (active) device or a functioning placebo (sham). The investigational devices did not have corporate logos or other identifiers, with the exception of a study investigational device number. A serial number was assigned to each helmet, which was recorded in a device log that contained the reference code for placebo and actual test unit. This log was not revealed to any investigator, subject, office staff, hair counter or sponsor employee.
The active treatment group received a “TOPHAT 655” unit containing 21, 5 mW laser diodes and 30 LEDS both operating at 655 nm (655 ± 5 nm and 655 ± 20 nm, respectively) and providing constant illumination over the scalp under the apparatus. Each subject self-treated at home for 25 minutes per treatment session every other day for 16 weeks (60 treatments, 67 J/cm2 delivered irradiance, and 2.9 J per treatment session).
The sham group received a unit that was identical in appearance and function to the laser group devices, with the exception that the light sources were incandescent wheat lights that were painted red to mimic the appearance and configuration of the functioning device. Each subject in the sham group self-treated at home for 25 minutes/treatment, every other day for 16 weeks (60 treatments). Incandescent sources were substituted 1:1 for each laser diode and LED source position on the sham helmet's interior.
The light output of the active treatment and sham treatment devices was determined using an Ophir Nova Display Power Meter equipped with a Model 30A-P-R-SH detector head (Ophir-Spiricon, LLC, Logan, UT). The active devices delivered an energy density of 67 J/cm2 at 655 nm per 25 minute treatment session at the level of the scalp. The placebo units delivered no measurable light at scalp level. The active device design was such that constant illumination was delivered over the areas of the scalp covered by the device.
The operating temperatures of the active and placebo devices were matched and were measured using a Klein Tools Model IR 3000 Thermometer (Klein Tools, Lincolnshire, IL). The temperature of the units was 27.8 ± 0.3°C at the level of the electronics and 22.2 ± 0.3°C on the interior surface of the helmet.
Study treatments were self-administered as follows: The subject's head was self-positioned within the helmet, until a sensor triggered the start of therapy. There was no contact between the subject and the light-emitting device; only the light reaches the subject scalp. Treatment duration was set to 25 minutes. The lasers and LEDs automatically shut off after the treatment session was complete. All device function was controlled by a hand set that was actuated by the user subject once the power cord was plugged into a standard 120 volt outlet and the start button was pressed. All other functions were pre-programmed and automatic. A full set of user instructions accompanied each helmet. There was no pre or post treatment care required, only that subjects' hair must be clean and not contain spray or gel fixative agents. No safety eyewear was required during the treatment sessions. A complete demonstration of the proper use of the helmet was provided to each subject at the time the test units were distributed. Periodic subject monitoring was conducted by telephone. Subjects were queried relative to their use of the device and for any possible side effects or adverse events.
The subjects returned at 16 weeks for follow up and post treatment photography of the previously marked area. The area was again trimmed and photographed using the same apparatus and photographic conditions as at the initial (baseline) visit. The images were processed, transmitted and analyzed in the same fashion as was the case for the pretreatment photographs.
One pre-treatment (baseline) and one post-treatment image were counted for each subject. The number of terminal hairs present in the masked area was counted and recorded.
Data analysis was conducted by a consulting statistician, who was provided the raw data and who was blinded as to identify the subjects and their individual treatments. The primary endpoint for evaluation was the percent increase in hair counts from baseline at the end of 16 weeks of treatment. The percent increase from baseline is to be obtained by the following formula:
A data pooling analysis was done to determine whether there was a site by treatment interaction in the percent increase. An analysis of variance was done with only site, treatment group, and site by treatment group interaction in the model and the interaction was not statistically significant. The data were pooled across both sites to arrive at an estimate of the effect for the primary endpoint. Univariate tests comparing the Sham and Active treatment groups were by Wilcoxon rank-sum tests, and an unequal variance t-test was performed.
RESULTS AND STATISTICAL ANALYSIS
Study Site Subject Distribution
The study was a blinded multicenter study. The study subjects were allocated to Active Treatment or Sham on a 1:1 basis at each of two study sites. The distribution of study subjects by random treatment assignment and study site are given in Table1.
Table 1.
Subjects, Treatment Assignments, and Study Sites
| Site | Sham (Placebo) | Active Treatment | Total |
|---|---|---|---|
| 1 | 6 | 7 | 13 |
| 2 | 12 | 17 | 29 |
| Total | 18 | 24 | 42 |
A total of 47 patients were enrolled in the study and completed baseline screening and photography. However, three subjects at site one and two subjects from site two withdrew from the study prior to the initiation of treatment. Thus there were 24 active treatment and 18 sham subjects available for analysis at the end of the study after 16 weeks of treatment.
There were no reported side effects or adverse events reported by any subject or site at any time during the conduct of the study.
Baseline Demographic Characteristics
There was information gathered on three important demographic characteristics, subject age, subject Fitzpatrick Skin Type, and Ludwig–Savin Baldness Scale. The results of these characteristics by treatment group are presented in the Table2.
Table 2.
Baseline Demographic Characteristics by Treatment Group
| Characteristic | Sham (Placebo) | Active Treatment | P-value |
|---|---|---|---|
| Age | 0.068 | ||
| Mean (SD) N | 51.00 (7.05) 18 | 46.29 (9.22) 24 | |
| Med (Min, Max) | 53 (33, 60) | 49 (26, 58) | |
| Fitzpatrick Skin Type | 0.582 | ||
| I n (%) | 3 (22.22) | 4 (16.67) | |
| II n (%) | 3 (16.67) | 6 (25.00) | |
| III n (%) | 12 (61.11) | 12 (50.00) | |
| IV n (%) | 0 (0.00) | 2 (8.33) | |
| Ludwig-Savin Baldness Scale | 0.858 | ||
| I n (%) | 7 (33.33) | 11 (45.83) | |
| II n (%) | 11 (66.67) | 13 (54.17) |
Note that age was not statistically significant by treatment group nor was it significant by study site (P = 0.0320). Neither Fitzpatrick skin type nor the Ludwig–Savin Baldness Scale differed by treatment group. Both study sites differed by Fitzpatrick Skin Type (P < 0.001) and by Ludwig–Savin Baldness Scale (P < 0.001).
Hair Counts and Photography
Photographs of the selected scalp site were taken prior to any treatment (baseline) and the same site was again photographed after the final treatment had been performed (post-treatment).
Examples of baseline (pre treatment) and final (post treatment) images are presented in Figures 1 and 2. Figure 1 demonstrates the results for typical patients in the placebo or sham group. Note that there is only a slight change present in the images taken at 16 weeks as compared to the baseline images. Figure 2 demonstrates baseline and final images for typical subjects in the active treatment group. A significant increase in the number of terminal hairs present is evident in the 16 week photographs compared to baseline. The diameter of the hairs present in the sample areas was not measured.
Fig 1.
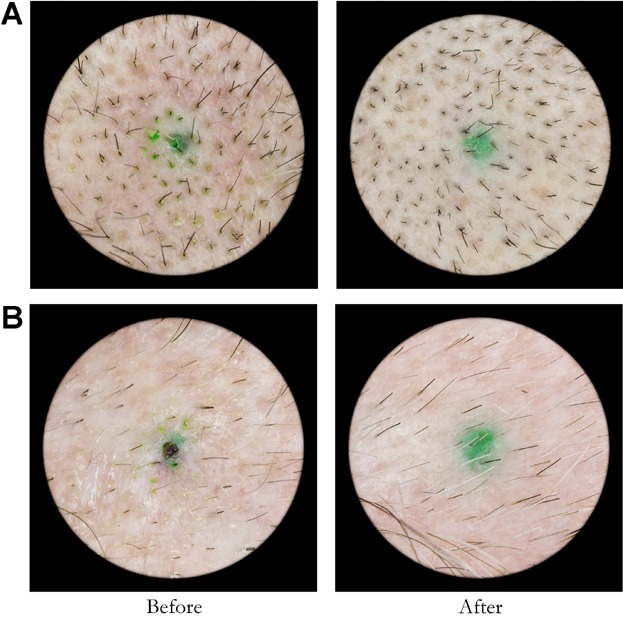
Sham treatment group subject pre and post treatment image examples. Hair counts for subject A were 151 at baseline and 166 post treatment. Hair counts for subject B were 41 at baseline and 44 post treatment.
Fig 2.
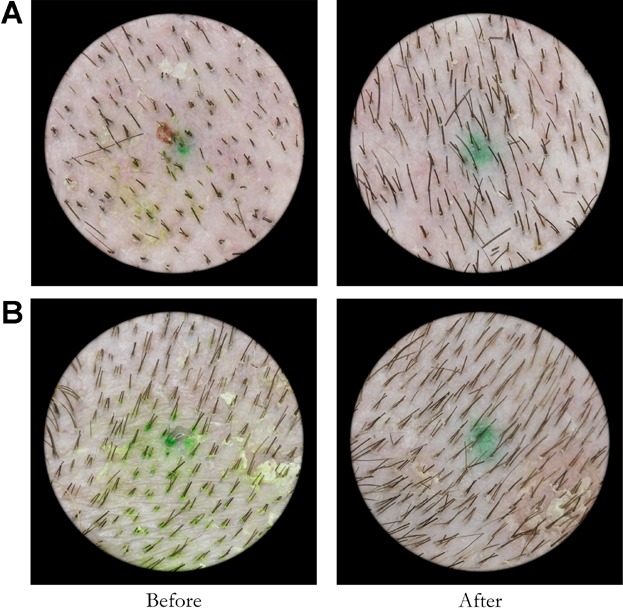
Active treatment group subject pre and post treatment image examples. Hair counts for subject A were 153 at baseline and 221 post treatment. Hair counts for subject B were 108 at baseline and 209 post treatment.
Baseline Hair Counts
The analyses reported below were conducted in Minitab 16 (Minitab, Inc, State College, PA). The raw data for these analyses appear in Appendix 1.
The baseline hair counts by treatment group and study site are presented in Table3. While the two study sites differ in the absolute values for the mean baseline hair counts, there was no statistical difference between the mean hair counts in the active and sham group subjects at the particular study center. An analysis of variance was done with only site, treatment group, and site by treatment group interaction in the model and the interaction was not statistically significant (P = 0.812).The study site was used as a possible covariate in the multivariable analyses performed below.
Table 3.
Baseline Hair Counts of Vertex Scalp Site
| Site | Sham (Placebo), Mean (SD) N, Med (Min, Max) | Active Treatment, Mean (SD) N, Med (Min, Max) | P-value |
|---|---|---|---|
| 1 | 317.5 (174.1) 6, 277 (130, 560) | 335.4 (144.6) 7, 260.0 (244, 599) | 0.846a |
| 2 | 183.5 (84.9) 12, 201.5 (41, 327) | 157.8 (50.5) 17, 152.0 (53, 234) | 0.361 |
| P-Value | 0.125a | 0.019a | — |
Two-sided unequal variance t-test.
Primary Analysis
The primary endpoint was the percent increase in hair counts from baseline at the end of 16 weeks of treatment (60 treatments). The percent increase from baseline was obtained for each subject by using the formula above.
A data pooling analysis was done to determine if there was a site by treatment interaction in the percent increase. If the interaction between site and treatment was significant with a P < 0.15, there would be evidence of a site by treatment interaction that would require weighting the site results to get an estimate of the study effect. An analysis of variance was done with only site, treatment group, and site by treatment group interaction in the model and the interaction was not statistically significant (P = 0.812). Thus the data were pooled across both sites to arrive at an estimate of the effect for the primary endpoint.
Univariate tests comparing the Sham and Active Treatment groups were intended to be by Wilcoxon rank-sum tests unless the variance between the two groups was statistically significantly different. In that case, the comparison was to be conducted by an unequal variance t-test. The results of the pooled data analysis appear in Table4.
Table 4.
Baseline Hair Counts, End of Study Hair Counts, and Percent Increase by Treatment Group
| Variable | Sham (Placebo), Mean (SD) N, Med (Min, Max) | Active Treatment, Mean (SD) N, Med (Min, Max) | P-value |
|---|---|---|---|
| Baseline | 228.2 (133.4) 18, 216.5 (41, 560) | 209.6 (118.5) 24, 187.5 (53, 599) | 0.642a |
| Post Treatment | 252.1 (143.3) 18, 248.0 (44, 636) | 309.9 (166.6) 24, 270.5 (57, 829) | 0.235a |
| Difference from Baseline | 23.9 (30.1) 18, 15.5 (-23, 108) | 100.3 (53.4) 24, 91.0 (4, 230) | <0.0001a |
| Percent Increase | 11.05 (48.30) 18, 10.15 (-4.66, 43.20) | 48.07 (17.61) 24, 45.58 (7.55, 93.52) | <0.001a |
Two-sided unequal variance t-test.
These results indicate that the univariate result comparing the increase in hair counts was statistically significant (P = 0.001). Low level laser treatment for 16 weeks increased mean hair counts by about 37% relative to sham treatment using the study device and the study treatment parameters. A multivariable analysis accounting for baseline differences in hair counts by study site indicates that the percent increase by treatment adjusted for study site indicate that the study site had a non-significant impact on the percent (P = 0.218). Therefore the study site differences in baseline counts did not modify the effect of treatment on the percent increase in hair counts after treatment. A second supportive multivariable analysis used baseline count as a covariate and in that analysis, the baseline term was not significant (P = 0.627), treatment was highly significant (P < 0.0001), but study site was not statistically significant (P = 0.219). Further, when age, Fitzpatrick type and Ludwig–Savin scale were included in a third sensitivity model, none were statistically significant with P-values of 0.901, 0.939, and 0.538, respectively. Thus, the univariate result is confirmed by the multivariable analysis with active LLLT treatment as the only significant term in the model (P < 0.001).
DISCUSSION
Treatment of androgenetic alopecia with LLLT has been studied in humans and in animal models using a variety of light sources, wavelengths and treatment parameters 6–9,11,12,14–16,18. We previously reported the results of the TH655 RCT using the so-called TOPHAT 655 device in males with androgenetic alopecia 16.
The present study details the results of the female arm of the same study protocol, which was initiated and completed after the male study was concluded. These investigations employed a randomized, double-blind design and used a true placebo via a helmet identical in appearance to the active device, with incandescent sources that glowed red but did not deliver measurable light to the subject's scalp and which operated at a temperature of 22.2 ± 0.3°C. Neither the active nor the sham devices delivered thermal energy to the scalp. Treatments were passive and did not depend on the user for delivery, aside from the subject being required to place the unit on the scalp and activate the controller.
Increases in hair counts were also observed in the sham or placebo group in the present study as was also the case in the earlier male cohort 16. These observations may represent a true placebo effect, since the sham device did not deliver thermal energy or measurable light at scalp level. However, seasonal variations in hair growth or other factors could be the basis for this observation.
Avci et al. recently reviewed the use of LLLT for the treatment of hair loss 18. They note that phototherapy is assumed to stimulate anagen re-entry in telogen hair follicles, prolong the duration of the anagen phase, increase the rates of proliferation in active anagen hair follicles and prevent premature catagen development 18. They discuss several possible mechanisms for the photobiomodulation effect observed in these cases 18.
One such theory is that LLLT, particularly at wavelengths in the red range as was used in this investigation, affects the functioning of the stem cells that cause hair growth 16,18. LLLT activates cytochrome c oxidase and increases mitochondrial electron transport 19–27, which leads to an increase in ATP and subsequent reversal of hair follicles from the dormant telogen stage of growth, to the active growth or anagen stage 6,7,9,11,13,14,16,18.
There is a growing body of evidence that the use of LLLT for the purpose of promoting hair growth is both safe and effective in both men and women. The optimal wavelengths and treatment parameters for treatment of alopecia remain indeterminate at this time. There is a need to conduct further studies in order to determine the potential role for near infrared and/or combinations of wavelengths as well as to investigate the effects of parameters such as coherence, pulsing and treatment frequency on clinical outcomes. The present study was not designed to investigate alternative treatment regimes or parameters. It was designed to evaluate the safety and effectiveness of a particular device designed for home use with specific parameters on the treatment of women with androgenetic alopecia.
We have demonstrated that the use of low level laser therapy at 655 nm applied to the scalp every other day for 16 weeks (60 treatments) via the TOPHAT 655 device resulted in a significant improvement in women who used the device. There was a 37% increase in terminal hair counts in the active treatment group as compared to the control (sham) group (P < 0.001) in 18–60 year old female subjects with I-2, I-3, I-4, II-1, or II-2 Ludwig–Savin baldness patterns and Fitzpatrick I–IV Skin Types. These results mirror those of the previously reported male trial which demonstrated a 35% increase in males with Hamilton–Norwood IIa-V baldness patterns and Type I–IV Fitzpatrick Skin Types 16.
Similarly, the female subjects were able to conduct the treatments at home and were able to apply and use the device as directed without any side effects or adverse events being reported at any time during the conduct of the study. This indicates that the device is safe for the unsupervised environment of home use and that the therapy is easily managed by both men and women using this device.
SUMMARY
The present study demonstrates that that low level laser (light) treatment of the scalp every other day for 16 weeks using the TOPHAT 655 device is a safe and effective treatment for androgenic alopecia in healthy women between the ages of 18–60 with Fitzpatrick Skin Types I–IV and Ludwig–Savin Baldness Scale I-2–II-2 baldness patterns. Subjects receiving LLLT at 655 nm achieved a 37% increase in hair counts as compared to sham treated control patients in this multicenter RCT. These results are similar to those reported in an earlier study using the same device in males with alopecia.
Appendix A.
Raw Hair Counts by Study Site and Treatment Group.
| Subjecta | Site | Treatment | Age (yrs) | Fitzpatrick Skin Type | Ludwig Savin Scale | BaselineHair Count | Posttrtb | Diffc | Pct_basd |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Active | 43 | 1 | I | 483 | 687 | 204 | 42.236 |
| 2* | 1 | — | 27 | 1 | II | ||||
| 3 | 1 | Sham | 57 | 3 | I | 292 | 297 | 5 | 1.712 |
| 4* | 1 | — | 45 | 1 | I | ||||
| 5 | 1 | Sham | 44 | 2 | I | 494 | 471 | −23 | −4.656 |
| 6 | 1 | Active | 52 | 1 | I | 245 | 333 | 88 | 35.918 |
| 7 | 1 | Active | 57 | 1 | I | 244 | 358 | 114 | 46.721 |
| 8* | 1 | — | 49 | 3 | I | ||||
| 9 | 1 | Sham | 57 | 1 | II | 130 | 150 | 20 | 15.385 |
| 10 | 1 | Active | 50 | 1 | II | 249 | 334 | 85 | 34.137 |
| 11 | 1 | Sham | 33 | 1 | I | 560 | 636 | 76 | 13.571 |
| 12 | 1 | Sham | 58 | 3 | II | 262 | 311 | 49 | 18.702 |
| 13 | 1 | Active | 52 | 3 | II | 268 | 450 | 182 | 67.910 |
| 14 | 1 | Active | 52 | 2 | I | 260 | 354 | 94 | 36.154 |
| 15 | 1 | Active | 44 | 2 | I | 599 | 829 | 230 | 38.397 |
| 16 | 1 | Sham | 53 | 1 | II | 167 | 170 | 3 | 1.796 |
| 17 | 2 | Active | 44 | 3 | I | 228 | 375 | 147 | 64.474 |
| 18 | 2 | Active | 51 | 3 | II | 234 | 385 | 151 | 64.530 |
| 19 | 2 | Active | 50 | 3 | II | 145 | 221 | 76 | 52.414 |
| 20 | 2 | Active | 47 | 3 | I | 182 | 276 | 94 | 51.648 |
| 21 | 2 | Active | 33 | 3 | II | 153 | 221 | 68 | 44.444 |
| 22 | 2 | Active | 26 | 3 | II | 192 | 263 | 71 | 36.979 |
| 23 | 2 | Active | 56 | 3 | II | 148 | 203 | 55 | 37.162 |
| 24 | 2 | Active | 45 | 2 | I | 108 | 209 | 101 | 93.519 |
| 25 | 2 | Active | 44 | 3 | II | 53 | 57 | 4 | 7.547 |
| 26 | 2 | Active | 38 | 2 | II | 144 | 230 | 86 | 59.722 |
| 27 | 2 | Active | 51 | 3 | II | 152 | 265 | 113 | 74.342 |
| 28 | 2 | Active | 58 | 2 | II | 110 | 139 | 29 | 26.364 |
| 29 | 2 | Active | 53 | 3 | II | 225 | 340 | 115 | 51.111 |
| 30 | 2 | Active | 58 | 3 | I | 97 | 146 | 49 | 50.515 |
| 31 | 2 | Sham | 60 | 3 | I | 41 | 44 | 3 | 7.317 |
| 32 | 2 | Sham | 51 | 3 | I | 224 | 248 | 24 | 10.714 |
| 33 | 2 | Sham | 59 | 3 | II | 116 | 140 | 24 | 20.690 |
| 34 | 2 | Sham | 45 | 2 | II | 209 | 249 | 40 | 19.139 |
| 35 | 2 | Sham | 46 | 3 | I | 327 | 342 | 15 | 4.587 |
| 36 | 2 | Sham | 54 | 3 | II | 250 | 358 | 108 | 43.200 |
| 37 | 2 | Sham | 53 | 3 | II | 135 | 149 | 14 | 10.370 |
| 38 | 2 | Sham | 42 | 3 | II | 232 | 248 | 16 | 6.897 |
| 39* | 2 | — | 20 | 3 | I | ||||
| 40 | 2 | Sham | 53 | 3 | II | 262 | 270 | 8 | 3.053 |
| 41 | 2 | Sham | 52 | 3 | I | 61 | 60 | −1 | −1.639 |
| 42 | 2 | Active | 28 | 4 | I | 204 | 328 | 124 | 60.784 |
| 43 | 2 | Sham | 55 | 2 | II | 151 | 166 | 15 | 9.934 |
| 44* | 2 | — | 27 | 3 | II | ||||
| 45 | 2 | Sham | 46 | 3 | II | 194 | 229 | 35 | 18.041 |
| 46 | 2 | Active | 31 | 4 | I | 183 | 264 | 81 | 44.262 |
| 47 | 2 | Active | 48 | 2 | II | 124 | 171 | 47 | 37.903 |
Patient numbers were grouped for convenience not by order of presentation or randomization.
Psttrt is the hair count after 16 weeks of treatment.
Diff = Psttrt – Baseline Hair Count.
Pct_bas is the percent hair increase (decrease) at 16 weeks as a percent of baseline.
Five subjects withdrew from the study after enrollment and prior to treatment.
REFERENCES
- 1.Mester E, Szende B, Gärtner P. Die Wirkung der Laserstrahlen auf den Haarwwuchs der Maus. Rad Biol Ther; 1967;9/5:621–626. [PubMed] [Google Scholar]
- 2.Mester E, Szende B, Tota JG. Effect of laser on hair growth in mice. Kiserl Orvostud. 1967;19:628–631. [Google Scholar]
- 3.Bernstein EF. Hair growth induced by diode laser treatment. Dermatol Surg. 2005;31:584–586. doi: 10.1111/j.1524-4725.2005.31168. [DOI] [PubMed] [Google Scholar]
- 4.Lolis MS, Marmur ES. Paradoxical effects of hair removal systems: A review. J Cosmet Dermatol. 2006;5:274–276. doi: 10.1111/j.1473-2165.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Willey A, Torrontegui J, Azpiazu J, Landa N. Hair stimulation following laser and intense pulsed light photo- epilation: Review of 543 cases and ways to manage it. Lasers Surg Med. 2007;39:297–301. doi: 10.1002/lsm.20485. [DOI] [PubMed] [Google Scholar]
- 6.Avram MR, Leonard RT, Jr, Epstein ES, Williams JL, Bauman AJ. The current role of laser/light sources in the treatment of male and female pattern hair loss. J Cosmet Laser Ther. 2007;9:27–28. doi: 10.1080/14764170601134479. [DOI] [PubMed] [Google Scholar]
- 7.Avram MR, Rogers NE. The use of low-level light for hair growth: Part I. J Cosmet Laser Ther. 2009;11:110–117. doi: 10.1080/14764170902842531. [DOI] [PubMed] [Google Scholar]
- 8.Stillman L. Reply to: The use of low-level light for hair growth: Part I. J Cosmet Laser Ther. 2010;12:116. doi: 10.3109/14764171003674448. [DOI] [PubMed] [Google Scholar]
- 9.Bouzari N, Firooz AR. Lasers may induce terminal hair growth. Dermatol Surg. 2006;32:460. doi: 10.1111/j.1524-4725.2006.32092.x. [DOI] [PubMed] [Google Scholar]
- 10.Chung PS, Kim YC, Chung MS, Jung SO, Ree CK. The effect of low-power laser on the murine hair growth. J Korean Soc Plastic Reconstruct Surg. 2004;31:1–8. [Google Scholar]
- 11.Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Invest. 2009;29:283–292. doi: 10.2165/00044011-200929050-00001. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki M, Miura Y, Tsuboi R, Ogawa H. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Intl J Dermatol. 2003;42:738–740. doi: 10.1046/j.1365-4362.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 13.Shukla S, Sahu K, Verma Y, Rao KD, Dube A, Gupta PK. Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol and Physiol. 2010;23:79–85. doi: 10.1159/000265678. [DOI] [PubMed] [Google Scholar]
- 14.Satino JL, Markou M. Hair regrowth and increased hair tensile strength using the HairMax LaserComb for low-level laser therapy. Int J Cosmet Surg Aesth Dermatol. 2003;5:113–117. [Google Scholar]
- 15.Trelles MA, Mayayo E, Cisneros JL. Tratamiento de la alopecia areata con laser HeNe. Investigacion Y Clinica Laser. 1984;1:15–17. [Google Scholar]
- 16.Lanzafame RJ, Blanche RR, Bodian AB, Chiacchierini RP, Fernandez-Obregon Kazmirek ER. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45(8):487–495. doi: 10.1002/lsm.22173. [DOI] [PubMed] [Google Scholar]
- 17. http://www.accessdata.fda.gov/cdrh_docs/pdf12/k122248.pdf accessed 5/1/14.
- 18.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-Level Laser (Light) Therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 20.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997;66(6):866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 21.Karu TI. The science of low power laser therapy. London Gordon and Breach Sci. Publ. 1998:14–33. 53–94, 95–121. [Google Scholar]
- 22.Karu TI. Primary and secondary mechanisms of action of visible to near–IR radiation on cells. J. Photochem. Photobiol, B. 1998;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 23.Vladimiorv IA, Klebanov GI, Borisenko GG, Osipov AN. Molecular and cellular mechanisms of the low intensity laser radiation effect. Biofizika. 2004;49(2):339–350. [PubMed] [Google Scholar]
- 24.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4(5–6):559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Karu TI. Low power laser therapy. In: Vo-Dinh T, editor. Biomedical Photonics Handbook. 48. CRC Press; 2003. pp. 1–25. [Google Scholar]
- 26.Liu TCY, Jiao JL, Xu XY, Liu XG, Deng SX, Liu SH. Photobiomodulation: Phenomenology and its mechanism. SPIE Proc. 2004;5632:185–191. [Google Scholar]
- 27.Hamblin MR, Demidova TN. Mechanisms of low level light therapy. SPIE Proc. 2006;6140:1–12. [Google Scholar]


