Abstract
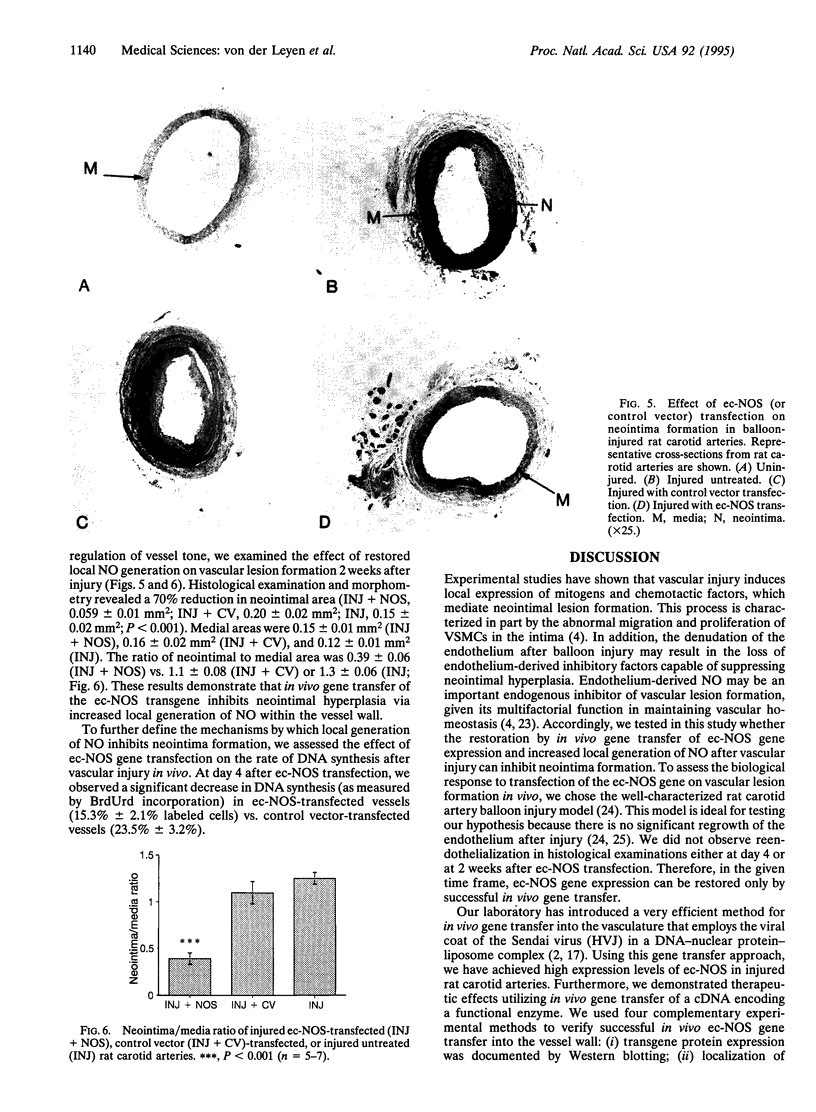
It is postulated that vascular disease involves a disturbance in the homeostatic balance of factors regulating vascular tone and structure. Recent developments in gene transfer techniques have emerged as an exciting therapeutic option to treat vascular disease. Several studies have established the feasibility of direct in vivo gene transfer into the vasculature by using reporter genes such as beta-galactosidase or luciferase. To date no study has documented therapeutic effects with in vivo gene transfer of a cDNA encoding a functional enzyme. This study tests the hypothesis that endothelium-derived nitric oxide is an endogenous inhibitor of vascular lesion formation. After denudation by balloon injury of the endothelium of rat carotid arteries, we restored endothelial cell nitric oxide synthase (ec-NOS) expression in the vessel wall by using the highly efficient Sendai virus/liposome in vivo gene transfer technique. ec-NOS gene transfection not only restored NO production to levels seen in normal untreated vessels but also increased vascular reactivity of the injured vessels. Neointima formation at day 14 after balloon injury was inhibited by 70%. These findings provide direct evidence that NO is an endogenous inhibitor of vascular lesion formation in vivo (by inhibiting smooth muscle cell proliferation and migration) and suggest the possibility of ec-NOS transfection as a potential therapeutic approach to treat neointimal hyperplasia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush P. A., Gonzalez N. E., Ignarro L. J. Biosynthesis of nitric oxide and citrulline from L-arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun. 1992 Jul 15;186(1):308–314. doi: 10.1016/s0006-291x(05)80808-3. [DOI] [PubMed] [Google Scholar]
- Cayatte A. J., Palacino J. J., Horten K., Cohen R. A. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994 May;14(5):753–759. doi: 10.1161/01.atv.14.5.753. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Cooke J. P., Singer A. H., Tsao P., Zera P., Rowan R. A., Billingham M. E. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992 Sep;90(3):1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Johnson G., 3rd, Tsao P. S., Mulloy D., Lefer A. M. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Pharmacol Exp Ther. 1990 Jan;252(1):35–41. [PubMed] [Google Scholar]
- Joly G. A., Schini V. B., Vanhoutte P. M. Balloon injury and interleukin-1 beta induce nitric oxide synthase activity in rat carotid arteries. Circ Res. 1992 Aug;71(2):331–338. doi: 10.1161/01.res.71.2.331. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993 Jul;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita R., Gibbons G. H., Ellison K. E., Nakajima M., Zhang L., Kaneda Y., Ogihara T., Dzau V. J. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita R., Gibbons G. H., Kaneda Y., Ogihara T., Dzau V. J. Novel in vitro gene transfer method for study of local modulators in vascular smooth muscle cells. Hypertension. 1993 Jun;21(6 Pt 2):894–899. doi: 10.1161/01.hyp.21.6.894. [DOI] [PubMed] [Google Scholar]
- Naruse K., Shimizu K., Muramatsu M., Toki Y., Miyazaki Y., Okumura K., Hashimoto H., Ito T. Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arterioscler Thromb. 1994 May;14(5):746–752. doi: 10.1161/01.atv.14.5.746. [DOI] [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991 Dec 15;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Nakane M., Buttery L. D., Martinez A., Springall D., Polak J. M., Förstermann U., Murad F. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am J Physiol. 1993 Nov;265(5 Pt 1):C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakugi H., Jacob H. J., Krieger J. E., Ingelfinger J. R., Pratt R. E. Vascular injury induces angiotensinogen gene expression in the media and neointima. Circulation. 1993 Jan;87(1):283–290. doi: 10.1161/01.cir.87.1.283. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Scherer-Singler U., Vincent S. R., Kimura H., McGeer E. G. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 1983 Nov;9(3):229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Catovsky S., Schray-Utz B., Busse R., Vanhoutte P. M. Insulin-like growth factor I inhibits induction of nitric oxide synthase in vascular smooth muscle cells. Circ Res. 1994 Jan;74(1):24–32. doi: 10.1161/01.res.74.1.24. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Schini V. B., Elizondo E., Junquero D. C., Vanhoutte P. M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Effects on cell proliferation. Circ Res. 1992 Nov;71(5):1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Watkins R. W., Pula K., Cook J., Hoos L., McLeod R., Prioli N. A., Davis H. R., Jr NG-nitro-L-arginine methyl ester does not affect balloon catheter-induced intimal hyperplasia in rats. Biochem Biophys Res Commun. 1993 Nov 30;197(1):304–309. doi: 10.1006/bbrc.1993.2476. [DOI] [PubMed] [Google Scholar]